
Video Upload Options
liquid crystal (LC) state, specific orientations and alignments of LC molecules produce outstanding anisotropy in structure and properties, followed by diverse optoelectronic functions.
1. Supramolecular Liquid Crystals
When alkali metal cations and several types of strong and highly selective ligands were discovered in 1960s, supramolecular chemistry began to grow into an independent discipline [1][2][3]. In 1987, Lehn defined supramolecular chemistry as the science which focuses on the structure and function of supramolecular systems formed by intermolecular forces of two or more compounds [4].
In 1927, E. Bradfield and B. Jones found the first supramolecular LC structure generated by hydrogen bonding between the carboxyl groups of benzoic acid derivatives [5][1]. Obviously, supramolecular LC is totally different from traditional covalent-bonded LC molecules, and should be viewed as an LC composite system based on intermolecular non-covalent interactions, including hydrogen bonds (H-bonds), halogen bonds, van der Waals force, electrostatic interaction, conjugation effect, hydrophobic interaction and so on. To date, supramolecular LC has gradually developed into a mature and widely applied discipline for lots of aspects (e.g., nanowire [6][7][8][9], templates [10], LC physical gel [11] and electro-optic materials [12][13]).
Compared with covalent bonds, non-covalent interactions are reversible and highly responsive to external physical or chemical stimuli (e.g., heat and solvent). H-bonds, π−π stacking, van der Waals forces and other non-covalent interactions have lower energy, and is easy enough to induce assembly and disassembly when using to an external stimulus. A supramolecular fibrous LC network featuring heat recovery was employed to construct soft materials like physical gels [11][12].
Many supramolecular LC systems were formed between LC molecules, units or other components, and appeared in different new phases [13][14][15][16]. Different from conventional LC, these highly ordered supramolecular architectures would be paid much attention due the response ability, not the nature of LC (e.g., phase transition, thermal stability and spatial order). Therefore, supramolecular LC formed by non-covalent interactions provided a new route to design multifunctional and practical materials [17][18][19][20][21].
In supramolecular LC systems, H-bonds are a general option to position different components in a certain arrangement with enhanced intermolecular binding strength [13][22]. For aromatic LCs, π−π conjugation can also cause these molecules to form well-organized supramolecular assemblies [23][24][25]. Other non-covalent interactions, like halogen bonds, ionic bonds and π−π conjugation, have their own unique way of constructing multifunctional LC supramolecules [26][27][28][29][30]. When combined with electron-donating groups or systems, LCs containing an electrophilic halogen atom can act as a acceptor to form halogen-bonded supramolecular LCs [29][31][32][33]. As for ionic LCs, they were formed by the self-assembly of ions in solution, and behaved with different self-assemblies and LC phases upon different solvent inductions [30][34].
Besides, non-covalent interactions can even boost inorganic materials with LC molecules forming nanoparticle–LC systems. Not surprisingly, graphene, carbon nanotubes and other multifunctional carbon nanomaterials containing large π-conjugated structures are easily assembled with LC molecules. For example, the nematic matrix, like 5CB and 8CB, can align carbon nanotubes to form long-range orientational ordered structures with a grooved surface or magnetic or electric field [35][36]. Additionally, carbon nanotubes can modify the LC’s anisotropy in optic and dielectric anisotropy [37][38][39]. Monolayer graphene flakes can provide the parameter of orientational order to enhance the dielectric anisotropy in the nematic LC of MLC-15600-100 [40]. It has also been reported that the presence of graphene flakes can accelerate the electro-optic response in a nematic LC [41]. H-bonds were also used as a linker to combine LC molecules and nanoparticles into a supramolecular whole [42]. For example, highly specific H-bonding interactions improved the dispersibility and compatibility of the ZrO2 nanoparticles in supramolecular nematic LC nanocomposites [43].
2. Hydrogen-Bonded Supramolecular Liquid Crystal
H-bonds are an ideal non-covalent interaction for the construction of supramolecular architectures [20][44][45][46][47][48]. Once a donor (D) with an available acidic hydrogen atom interacts with an acceptor (A) carrying available non-bonding electron lone pairs, H-bonds arise and, therefore, are endowed with high selectivity and directionality [20]. Importantly, H-bonds are easily affected by solvents, salts, ions, temperature and so on, so they are viewed as key to the controllability of H-bonded supramolecular systems. As a tool to assemble supramolecular architectures, H-bonding can bring positive effects to LC, e.g., to generate new phases, expand the phase temperature range, improve thermal stability and so on.
As for H-bonded supramolecular LC (see Figure 1), the common structures are formed through benzoic acid, carboxylic acids, pyridyl and others [49][50]. In the first example of H-bonded supramolecular LC complexes, the LC state was formed by intermolecular H-bonds within benzoic acid derivatives [51]. According to the number of hydrogen bonds, H-bonded supramolecular LCs are classified into two categories, single H-bonded [52][53] (see Figure 1a) and multiple H-bonded ones [54][55][56] (see Figure 1b). Furthermore, especially for LC polymers, side-chain (see Figure 1c) and main-chain H-bonded supramolecular LCs (see Figure 1d) are the most common. In addition, there are some hybrid and network H-bonded supramolecular liquid crystals [57][58][59][60]. Figure 2 shows the formation and brief classification of H-bonded supramolecular LCs.
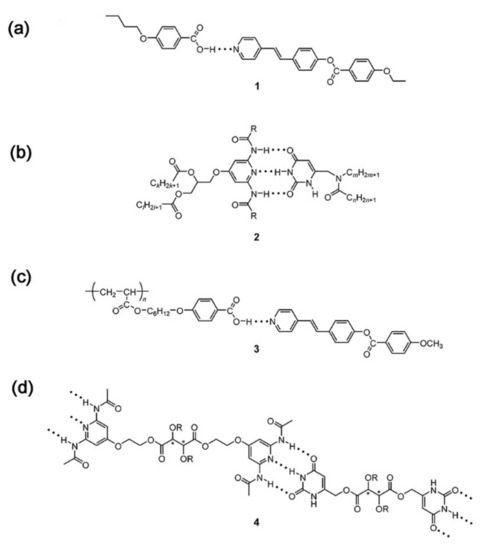
Figure 1. Supramolecular hydrogen-bonded liquid crystal (LC) molecules: (a) Low molecular weight complex by Kato and Fre’chet in 1989. Reprinted with permission from reference [61]. Copyright (1989) American Chemical Society. (b) Low molecular weight complex by Lehn and coworkers in 1989. Reprinted with permission from reference [62]. Copyright (1989) Royal Society of Chemistry. (c) Side-chain polymer by Kato and Fre’chet in 1989. Reprinted with permission from reference [63]. Copyright (1989) American Chemical Society. (d) Main-chain polymeric complex by Lehn and coworkers in 1990. Reprinted with permission from reference [64]. Copyright (1990) John Wiley and Sons.
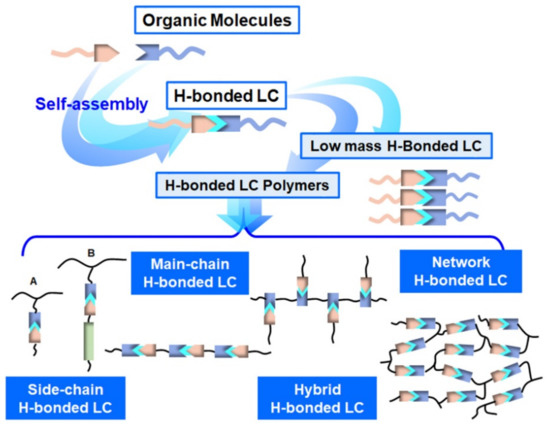
Figure 2. Classification of H-bonded supramolecular LCs.
Complex 1 (see Figure 1a) consists of 4-butoxybenzoic acid (4BA) and trans-4-[(4-ethoxybenzoyl)oxy]-4’-stilbazole (2Sz) with one H-bond, featuring sematic and nematic phases. The mesomorphic range of Complex 1 was extended to 102 °C, and a new smectic phase appeared between 136 °C and 160 °C, but each component showed only a nematic phase [61]. Complex 2 (see Figure 1b) was formed with three parallel H-bonds between the uracil and 2,6-diaminopyridine groups, with a columnar hexagonal LC mesophase [62]. There was no mesomorphic phase of these pure compounds. Fortunately, the mesophase became observable only if long enough aliphatic chains were introduced into the uracil derivatives. Side-chain polyacrylate was used as framework to build up a special supramolecular LC through hydrogen bonds between its pendant pentoxybenzoic acid groups and guest stilbazole ester (see Figure 1c) [63]. The mesomorphic range of the mixture with the determined ratio (polyacrylate: stilbazole ester = 1:1) reached 112 °C, while the corresponding ranges of each component were 15 and 48 °C, respectively. It is likely that this strong enhancement on the mesophase is due to the formation of hydrogen-bonded complexes. On the other hand, supramolecular main-chain “polymers” were obtained with triply hydrogen-bonded complementary pairs between uracil and 2,6-diacylamino-pyridine (see Figure 1d) [64]. Under different conditions, these 1:1 mixtures showed special optical textures (e.g., stretched and helically wound fibers).
One of the obvious traits of these supramolecular H-bonded mesogens is high thermal stability. The stabilization effect greatly contributes to the ordered condensed state of hydrogen-bonded supramolecular LCs. Another merit is the unique reversibility during dynamic formation and breaking, compared with covalent-bonded LC molecules. Both of them provide a specific idea for designing new LC structures [65][14]. Naturally, H-bonded supramolecular LCs have been rapidly developed into a flexible, facile and cost-effective approach for novel smart materials [66][67][68].
In this review article, we describe topics on the recent progress of H-bonded supramolecular liquid crystal, which consists of assemblies among organic LC molecules, or between inorganic materials and organic LC molecules. We will discuss molecular structures and the relationships between molecular self-assembled structures and functions, especially the formation, mechanism and advantage of hydrogen bonds. In addition, we focus on some H-bonded LC systems which showed a new LC phase, wider phase transition temperature, better mechanical properties, “reversible” deformation behaviors, wider reflection area or other better performance properties with some external stimuli, for example, light, pH, thermality and humidity.
References
- Dietrich, B.; Lehn, J.M.; Sauvage, J.P. Diaza-Polyoxa-Macrocycles et Macrobicycles. Tetrahedron Lett. 1969, 10, 2885–2888.
- Dietrich, B.; Lehn, J.M.; Sauvage, J.P. Cryptates—XI:Complexes macrobicycliques, formation, structure, proprietes. Tetrahedron 1973, 29, 1647–1658.
- Truter, M.R. Structures of Organic Complexes with Alkali Metal Ions; Springer: Berlin/Heidelberg, Germany, 2006.
- Lehn, J.-M. Supramolecular Chemistry—Scope and Perspectives Molecules, Supermolecules, and Molecular Devices (Nobel Lecture). Angew. Chem. Int. Ed. 1988, 27, 89–112.
- Weiss, R.; Metz, B.; Moras, D. Crystal structures of two barium cryptates. J. Am. Chem. Soc. 1971, 93, 1806–1808.
- Chen, X.; Chen, L.; Yao, K.; Chen, Y. Self-assembly of diblock polythiophenes with discotic liquid crystals on side chains for the formation of a highly ordered nanowire morphology. ACS Appl. Mater. Interfaces 2013, 5, 8321–8328.
- Guan, S.; Wen, W.; Yang, Z.; Chen, A. Liquid Crystalline Nanowires by Polymerization Induced Hierarchical Self-Assembly. Macromolecules 2019, 53, 465–472.
- Park, J.H.; Kim, K.H.; Park, Y.W.; Lagerwall, J.P.; Scalia, G. Ultralong Ordered Nanowires from the Concerted Self-Assembly of Discotic Liquid Crystal and Solvent Molecules. Langmuir 2015, 31, 9432–9440.
- Dhakal, N.P.; Jiang, J.; Guo, Y.; Peng, C. Self-Assembly of Aqueous Soft Matter Patterned by Liquid-Crystal Polymer Networks for Controlling the Dynamics of Bacteria. ACS Appl. Mater. Interfaces 2020, 12, 13680–13685.
- Todisco, M.; Fraccia, T.P.; Smith, G.P.; Corno, A.; Bethge, L.; Klussmann, S.; Paraboschi, E.M.; Asselta, R.; Colombo, D.; Zanchetta, G.; et al. Nonenzymatic Polymerization into Long Linear RNA Templated by Liquid Crystal Self-Assembly. ACS Nano 2018, 12, 9750–9762.
- Chen, J.W.; Huang, C.C.; Chao, C.Y. Supramolecular Liquid-Crystal Gels Formed by Polyfluorene-Based π-Conjugated Polymer for Switchable Anisotropic Scattering Device. ACS Appl. Mater. Interfaces 2014, 6, 6757–6764.
- Foelen, Y.; Van Der Heijden, D.A.C.; Del Pozo, M.; Lub, J.; Bastiaansen, C.W.; Schenning, A.P.H.J. An Optical Steam Sterilization Sensor Based On a Dual-Responsive Supramolecular Cross-Linked Photonic Polymer. ACS Appl. Mater. Interfaces 2020, 12, 16896–16902.
- Alnoman, R.B.; Ahmed, H.A.; Hagar, M.; Abu Al-Ola, K.A.; Alrefay, B.S.; Haddad, B.A.; Albalawi, R.F.; Aljuhani, R.H.; Aloqebi, L.D.; Alsenani, S.F. Induced Phases of New H-bonded Supramolecular Liquid Crystal Complexes; Mesomorphic and Geometrical Estimation. Molecules 2020, 25, 1549.
- Nafee, S.S.; Ahmed, H.A.; Hagar, M. New architectures of supramolecular H-bonded liquid crystal complexes based on dipyridine derivatives. Liq. Cryst. 2020, 47, 1–14.
- Sales, E.S.; Dos Santos, G.M.; Mandle, R.J.; Costa, W.C.; Bechtold, I.H.; Goncalves, I.L.; Eifler-Lima, V.L.; Merlo, A.A. Insight into Out-of-Layer Fluctuations in the Smectic A Stability of 3,5-Diarylisoxazole Liquid Crystals. Chemphyschem 2020, 21, 1408–1419.
- Scholte, A.; Hauche, S.; Wagner, M.; Prehm, M.; Poppe, S.; Chen, C.; Liu, F.; Zeng, X.; Ungar, G.; Tschierske, C. A Self-Assembled Liquid Crystal Honeycomb of Highly Stretched (3-1-1)-Hexagons. Chem. Commun. (Camb. Engl.) 2020, 65, 62–65.
- Sun, H.J.; Zhang, S.; Percec, V. From Structure to Function via Complex Supramolecular Dendrimer Systems. Chem. Soc. Rev. 2015, 44, 3900–3923.
- Salikolimi, K.; Sudhakar, A.A.; Ishida, Y. Functional Ionic Liquid Crystals. Langmuir 2020, 36, 11702–11731.
- Saccone, M.; Catalano, L. Halogen Bonding beyond Crystals in Materials Science. J. Phys. Chem. B 2019, 123, 9281–9290.
- Gonzaález-Rodriíguez, D.; Schenning, A.P.H.J. Hydrogen-bonded Supramolecular π-Functional Materials†. Chem. Mater. 2011, 23, 310–325.
- Fox, J.D.; Rowan, S.J. Supramolecular Polymerizations and Main-Chain Supramolecular Polymers. Macromolecules 2009, 42, 6823–6835.
- Paterson, D.A.; Martínez-Felipe, A.; Jansze, S.M.; Marcelis, A.T.; Storey, J.M.; Imrie, C.T. New insights into the liquid crystal behaviour of hydrogen-bonded mixtures provided by temperature-dependent FTIR spectroscopy. Liq. Cryst. 2015, 42, 1–12.
- Sakurai, T.; Yoneda, S.; Sakaguchi, S.; Kato, K.; Takata, M.; Seki, S. Donor/Acceptor Segregated π-Stacking Arrays by Use of Shish-Kebab-Type Polymeric Backbones: Highly Conductive Discotic Blends of Phthalocyaninatopolysiloxanes and Perylenediimides. Macromolecules 2017, 50, 9265–9275.
- De, J.; Bala, I.; Gupta, S.P.; Pandey, U.K.; Pal, S.K. High Hole Mobility and Efficient Ambipolar Charge Transport in Heterocoronene-Based Ordered Columnar Discotics. J. Am. Chem. Soc. 2019, 141, 18799–18805.
- Yardley, R.E.; Paquette, J.A.; Taing, H.; Gaebler, H.M.; Eichhorn, S.H.; Hamilton, I.P.; Maly, K.E. Stabilization of Columnar Liquid Crystal Phases via Arene-Perfluoroarene Interactions. Organ. Lett. 2019, 21, 10102–10105.
- Saccone, M.; Palacio, F.F.; Cavallo, G.; Dichiarante, V.; Virkki, M.; Terraneo, G.; Priimagi, A.; Metrangolo, P. Photoresponsive ionic liquid crystals assembled via halogen bond: En route towards light-controllable ion transporters. Faraday Discuss. 2017, 203, 407–422.
- Vapaavuori, J.; Siiskonen, A.; Dichiarante, V.; Forni, A.; Saccone, M.; Pilati, T.; Pellerin, C.; Shishido, A.; Metrangolo, P.; Priimagi, A. Supramolecular control of liquid crystals by doping with halogen-bonding dyes. RSC Adv. 2017, 7, 40237–40242.
- Alaasar, M.; Poppe, S.; Tschierske, C. Photoresponsive halogen bonded polycatenar liquid crystals. J. Mol. Liq. 2019, 277, 233–240.
- Wang, H.; Bisoyi, H.K.; Urbas, A.M.; Bunning, T.J.; Li, Q. The Halogen Bond: An Emerging Supramolecular Tool in the Design of Functional Mesomorphic Materials. Chemistry 2019, 25, 1369–1378.
- Goossens, K.; Lava, K.; Bielawski, C.W.; Binnemans, K. Ionic Liquid Crystals: Versatile Materials. Chem. Rev. 2016, 116, 4643–4807.
- Cavallo, G.; Metrangolo, P.; Milani, R.; Pilati, T.; Priimagi, A.; Resnati, G.; Terraneo, G. The Halogen Bond. Chem. Rev. 2016, 116, 2478–2601.
- Gilday, L.C.; Robinson, S.W.; Barendt, T.A.; Langton, M.J.; Mullaney, B.R.; Beer, P.D. Halogen Bonding in Supramolecular Chemistry. Chem. Rev. 2015, 115, 7118–7195.
- Metrangolo, P.; Meyer, F.; Pilati, T.; Resnati, G.; Terraneo, G. Halogen bonding in supramolecular chemistry. Angew. Chem. Int. Ed. Engl. 2008, 47, 6114–6127.
- Li, S.; Saielli, G.; Wang, Y. Aggregation Behavior of Dihexadecylviologen Bistriflimide Ionic Liquid Crystal in Different Solvents: Influence of Polarity and Concentration. Phys. Chem. Chem. Phys. 2018, 20, 22730–22738.
- Lynch, M.D.; Patrick, D.L. Organizing Carbon Nanotubes with Liquid Crystals. Nano Lett. 2002, 2, 1197–1201.
- Selmani, S.; Hawes, G.F.; Schipper, D.J. Liquid-Crystal Phase Optimization Using the Alignment Relay Technique for the Deposition of Single-Walled Carbon Nanotubes. ACS Appl. Nano Mater. 2020, 3, 2118–2122.
- Tie, W.W.; Bhattacharyya, S.S.; Zheng, Z.; Cho, K.J.; Kim, T.H.; Lim, Y.J.; Lee, S.H. Electric field assisted-unidirectional hybrid films of carbon nanotubes and liquid crystal polymer for light modulation. Liq. Cryst. 2020, 47, 317–329.
- Shukla, R.K.; Chaudhary, A.; Bubnov, A.; Hamplova, V.; Raina, K.K. Electrically switchable birefringent self-assembled nanocomposites: Ferroelectric liquid crystal doped with the multiwall carbon nanotubes. Liq. Cryst. 2020, 47, 1379–1389.
- Shukla, R.K.; Chaudhary, A.; Bubnov, A.; Raina, K.K. Multi-walled carbon nanotubes-ferroelectric liquid crystal nanocomposites: Effect of cell thickness and dopant concentration on electro-optic and dielectric behaviour. Liq. Cryst. 2018, 45, 1672–1681.
- Basu, R.; Kinnamon, D.; Garvey, A. Nano-electromechanical rotation of graphene and giant enhancement in dielectric anisotropy in a liquid crystal. Appl. Phys. Lett. 2015, 106, 201909.
- Basu, R.; Garvey, A.; Kinnamon, D. Effects of graphene on electro-optic response and ion-transport in a nematic liquid crystal. J. Appl. Phys. 2015, 117, 074301.
- Roohnikan, M.; Toader, V.; Rey, A.; Reven, L. Hydrogen-Bonded Liquid Crystal Nanocomposites. Langmuir 2016, 32, 8442–8450.
- Roohnikan, M.; Lindner-D’Addario, M.; Toader, V.; Rey, A.; Tan, D.; Friščić, T.; Reven, L. Mechanochemical nanoparticle functionalization for liquid crystal nanocomposites based on COOH-pyridine heterosynthons. J. Mater. Chem. C 2018, 6, 1789–1796.
- Kato, T.; Kihara, H.; Kumar, U.; Uryu, T.; Fréchet, J.M. Aufbau eines fliissigkristallinen Polymernetzwerks durch Selbstorganisation uber intermolekulare Wasserstoffbriickenbindungen. Angew. Chem. 1994, 106, 1728–1730.
- Kato, T.; Fréchet, J.M.J. Hydrogen bonding and the self-assembly of supramolecular liquid-crystalline materials. Macromol. Symp. 1995, 98, 311–326.
- Kato, T.; Kato, T.; Fréchet, J.M.J.; Uryu, T.; Kaneuchi, F.; Jin, C.; Fréchet, J.M.J. Hydrogen-bonded liquid crystals built from hydrogen-bonding donors and acceptors Infrared study on the stability of the hydrogen bond between carboxylic acid and pyridyl moieties. Liq. Cryst. 2011, 33, 1429–1437.
- Prins, L.J.; Reinhoudt, D.N.; Timmerman, P. Noncovalent synthesis using hydrogen bonding. Angew. Chem. Int. Ed. 2001, 40, 2382–2426.
- Kumar, U.; Kato, T.; Frechet, J.M. Use of intermolecular hydrogen bonding for the induction of liquid crystallinity in the side chain of polysiloxanes. J. Am. Chem. Soc. 1992, 114, 6630–6639.
- Paleos, C.M.; Tsiourvas, D. Thermotropic Liquid Crystals Formed by Intermolecular Hydrogen Bonding Interactions. Angew. Chem. Int. Ed. 1995, 34, 1696–1911.
- Pfletscher, M.; Hölscher, S.; Wölper, C.; Mezger, M.; Giese, M. Structure–Property Relationships in Hydrogen-Bonded Liquid Crystals. Chem. Mater. 2017, 29, 8462–8471.
- Paleos, C.M.; Tsiourvas, D. Supramolecular hydrogen-bonded liquid crystals. Liq. Cryst. 2001, 28, 1127–1161.
- Didehban, K.; Namazi, H.; Entezami, A.A. Dendrimer-based hydrogen-bonded liquid crystalline complexes: Synthesis and characterization. Eur. Polym. J. 2009, 45, 1836–1844.
- Ruokolainen, J.; Tanner, J.; Ikkala, O. Direct Imaging of Self-Organized Comb Copolymer-like Systems Obtained by Hydrogen Bonding: Poly(4-vinylpyridine)-4-Nonadecylphenol. Macromolecules 1998, 31, 3532–3536.
- Pisupati, S.; Kumar, P.A.; Pisipati, V.G.K.M. Induced crystal G phase through intermolecular hydrogen bonding II. Influence of alkyl chain length of n-alkyl p-hydroxybenzoates on thermal and phase behaviour. Liq. Cryst. 2010, 27, 665–669.
- Kanie, K.; Nishii, M.; Yasuda, T.; Taki, T.; Ujiie, S.; Kato, T. Self-assembly of thermotropic liquid-crystalline folic acid derivatives: Hydrogen-bonded complexes forming layers and columns. J. Mater. Chem. 2001, 11, 2875–2886.
- Kanie, K.; Yasuda, T.; Kato, T.; Ujiie, S. Thermotropic liquid-crystalline folic acid derivatives: Supramolecular discotic and smectic aggregation. Chem. Commun. 2000, 19, 1899–1900.
- Ihata, O.; Yokota, H.; Kanie, K.; Ujiie, S.; Kato, T. Induction of mesophases through the complexation between benzoic acids with lateral groups and polyamides containing a 2,6-diaminopyridine moiety. Liq. Cryst. 2010, 27, 69–74.
- Kato, T.; Kihara, H.; Kumar, U.; Uryu, T.; Frkhet, J.M.J. A Liquid-Crystalline Polymer Network Built by Molecular Self-Assembly through Intermolecular Hydrogen Bonding. Angew. Chem. Int. Ed. 1994, 33, 1644–1645.
- Kato, T.; Ihata, O.; Ujiie, S.; Tokita, M.; Watanabe, J. Self-Assembly of Liquid-Crystalline Polyamide Complexes through the Formation of Double Hydrogen Bonds between a 2,6-Bis(amino)pyridine Moiety and Benzoic Acids. Macromolecules 1998, 31, 3551–3555.
- Kihara, H.; Kato, T.; Uryu, T.; Fréchet, J.M.J. Supramolecular Liquid-Crystalline Networks Built by Self-Assembly of Multifunctional Hydrogen-Bonding Molecules. Chem. Mater. 1996, 8, 961–968.
- Kato, T.; Frechet, J.M. New approach to mesophase stabilization through hydrogen bonding molecular interactions in binary mixtures. J. Am. Chem. Soc. 1989, 111, 8533–8534.
- Brienne, M.-J.Î.; Gabard, J.; Lehn, J.-M.; Stibor, I. Macroscopic expression of molecular recognition. Supramolecular liquid crystalline phases induced by association of complementary heterocyclic components. J. Chem. Soc. Chem. Commun. 1989, 24, 1868–1870.
- Kato, T.; Frechet, J.M.J. Stabilization of a liquid-crystalline phase through noncovalent interaction with a polymer side chain. Macromolecules 1989, 22, 3818–3819.
- Fouquey, C.; Lehn, J.; Levelut, A. Molecular Recognition Directed Self-Assembly of Supramolecular Liquid Crystalline Polymers from Complementary Chiral Components. Adv. Mater. 1990, 2, 254–257.
- Goor, O.; Hendrikse, S.I.S.; Dankers, P.Y.W.; Meijer, E.W. From supramolecular polymers to multi-component biomaterials. Chem. Soc. Rev. 2017, 46, 6621–6637.
- Roohnikan, M.; Ebrahimi, M.; Ghaffarian, S.R.; Tamaoki, N. Supramolecular self-assembly of a novel hydrogen-bonded cholesteric liquid crystal exhibiting macromolecular behaviour. Liq. Cryst. 2013, 40, 314–320.
- Kishikawa, K.; Furukawa, Y.; Watanabe, T.; Kohri, M.; Taniguchi, T.; Kohmoto, S. Hydrogen bond network-stabilisation of blue phases by addition of a chiral N-(10-hydroxydecyl)succinimide derivative and alkane diols. Liq. Cryst. 2017, 44, 1332–1339.
- Wei, C.-L.; Chen, T.-C.; Raghunath, P.; Lin, M.-C.; Lin, H.-C. Hydrogen-bonded effects on supramolecular blue phase liquid crystal dimeric complexes. RSC Adv. 2015, 5, 54629–54637.




