
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Tatsuhiro Ayabe | + 4373 word(s) | 4373 | 2021-06-03 10:27:21 | | | |
| 2 | Vivi Li | Meta information modification | 4373 | 2021-07-09 04:57:49 | | |
Video Upload Options
In this entry, we introduce the effects and underlying mechanisms of hop-derived bitter acids found in beer. Iso-α-acids (IAAs), the main bitter components of beer, enhance hippocampus-dependent memory and prefrontal cortex-associated cognitive function via dopamine neurotransmission activation. Matured hop bitter acids (MHBAs), oxidized components with β-carbonyl moieties derived from aged hops, also enhance memory functions via norepinephrine neurotransmission-mediated mechanisms. Furthermore, the effects of both IAAs and MHBAs are attenuated by vagotomy, suggesting that these bitter acids enhance cognitive function via vagus nerve stimulation. Moreover, supplementation with IAAs attenuates neuroinflammation and cognitive impairments in various rodent models of neurodegeneration including Alzheimer’s disease. Daily supplementation with hop-derived bitter acids (e.g., 35 mg/day of MHBAs) may be a safe and effective strategy to stimulate the vagus nerve and thus enhance cognitive function.
1. Introduction
2. Iso-α-Acids (IAAs)
2.1. Characterization of IAAs
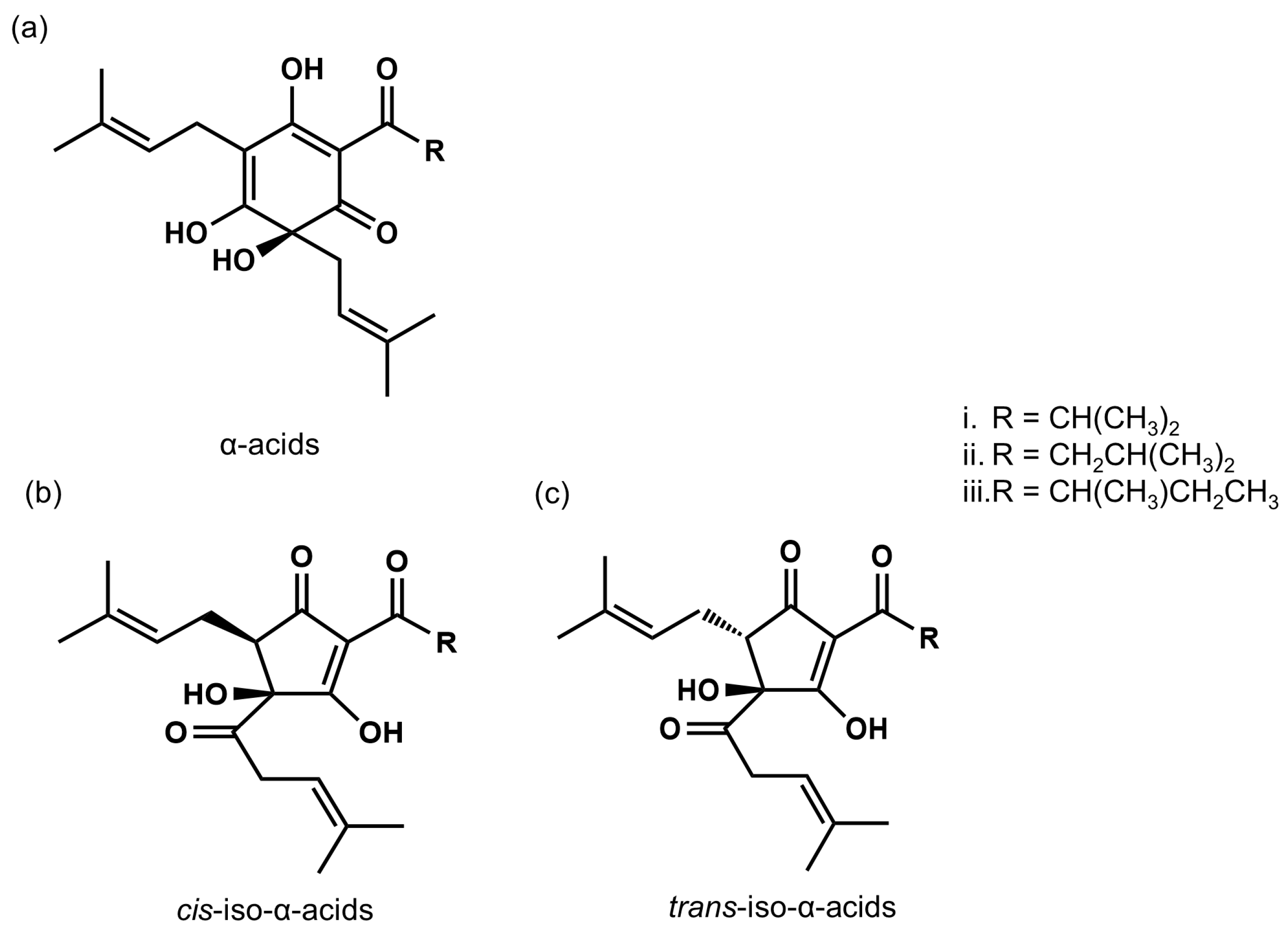
2.2. IAAs Prevent Type II Diabetes, Lipid Metabolism, and Obesity-Induced Cognitive Decline
2.3. IAAs Enhance Cognitive Function via Activation of the Vagus Nerve and Dopamine Signaling
3. Matured Hop Bitter Acids (MHBAs)
3.1. Characterization of MHBAs
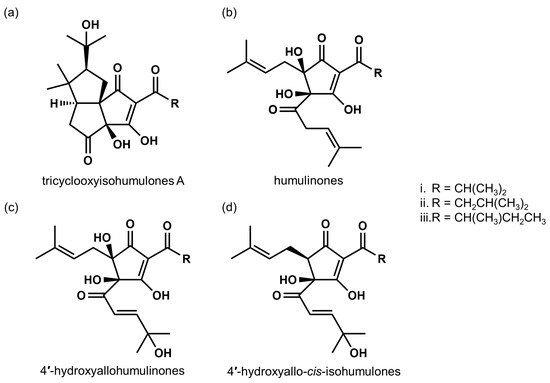
3.2. MHBAs Improve Lipid Metabolism and Obesity-Induced Cognitive Decline
3.3. MHBAs Enhance Cognitive Function via Activation of the Vagus Nerve and Norepinephrine Signaling
4. Potential for Hop-Derived Bitter Acids in Alzheimer’s Disease Treatment
References
- Livingston, G.; Sommerlad, A.; Orgeta, V.; Costafreda, S.G.; Huntley, J.; Ames, D.; Ballard, C.; Banerjee, S.; Burns, A.; Cohen-Mansfield, J. Dementia prevention, intervention, and care. Lancet 2017, 390, 2673–2734.
- Neafsey, E.J.; Collins, M.A. Moderate alcohol consumption and cognitive risk. Neuropsychiatr. Dis. Treat. 2011, 7, 465–484.
- Xu, W.; Wang, H.; Wan, Y.; Tan, C.; Li, J.; Tan, L.; Yu, J.T. Alcohol consumption and dementia risk: A dose-response meta-analysis of prospective studies. Eur. J. Epidemiol. 2017, 32, 31–42.
- Bastianetto, S.; Menard, C.; Quirion, R. Neuroprotective action of resveratrol. Biochim. Biophys. Acta 2015, 1852, 1195–1201.
- Turner, R.S.; Thomas, R.G.; Craft, S.; van Dyck, C.H.; Mintzer, J.; Reynolds, B.A.; Brewer, J.B.; Rissman, R.A.; Raman, R.; Aisen, P.S. A randomized, double-blind, placebo-controlled trial of resveratrol for Alzheimer disease. Neurology 2015, 85, 1383–1391.
- Sawda, C.; Moussa, C.; Turner, R.S. Resveratrol for Alzheimer’s disease. Ann. N. Y. Acad. Sci. 2017, 1403, 142–149.
- Biendl, M.; Pinzl, C. Hops and health. MBAA TQ 2009, 46, 1–7.
- Intelmann, D.; Batram, C.; Kuhn, C.; Haseleu, G.; Meyerhof, W.; Hofmann, T. Three TAS2R Bitter Taste Receptors Mediate the Psychophysical Responses to Bitter Compounds of Hops (Humulus lupulus L.) and Beer. Chemosens. Percept. 2009, 2, 118–132.
- Kowaka, M.; Kokubo, E. Composition of bitter substances of hops and characteristics of beer bitterness. J. Am. Soc. Brew. Chem. 1977, 35, 16–21.
- Kunimune, T.; Shellhammer, T.H. Foam-stabilizing effects and cling formation patterns of iso-alpha-acids and reduced iso-alpha-acids in lager beer. J. Agric. Food Chem. 2008, 56, 8629–8634.
- Simpson, W.J.; Smith, A.R. Factors affecting antibacterial activity of hop compounds and their derivatives. J. Appl. Bacteriol. 1992, 72, 327–334.
- Schurr, B.C.; Hahne, H.; Kuster, B.; Behr, J.; Vogel, R.F. Molecular mechanisms behind the antimicrobial activity of hop iso-alpha-acids in Lactobacillus brevis. Food Microbiol. 2015, 46, 553–563.
- Taniguchi, Y.; Matsukura, Y.; Taniguchi, H.; Koizumi, H.; Katayama, M. Development of preparative and analytical methods of the hop bitter acid oxide fraction and chemical properties of its components. Biosci. Biotechnol. Biochem. 2015, 79, 1684–1694.
- Biessels, G.J.; Staekenborg, S.; Brunner, E.; Brayne, C.; Scheltens, P. Risk of dementia in diabetes mellitus: A systematic review. Lancet Neurol. 2006, 5, 64–74.
- Gudala, K.; Bansal, D.; Schifano, F.; Bhansali, A. Diabetes mellitus and risk of dementia: A meta-analysis of prospective observational studies. J. Diabetes Investig. 2013, 4, 640–650.
- Xu, W.; Atti, A.; Gatz, M.; Pedersen, N.; Johansson, B.; Fratiglioni, L. Midlife overweight and obesity increase late-life dementia risk: A population-based twin study. Neurology 2011, 76, 1568–1574.
- Waldstein, S.; Katzel, L. Interactive relations of central versus total obesity and blood pressure to cognitive function. Int. J. Obes. 2006, 30, 201.
- Raji, C.A.; Ho, A.J.; Parikshak, N.N.; Becker, J.T.; Lopez, O.L.; Kuller, L.H.; Hua, X.; Leow, A.D.; Toga, A.W.; Thompson, P.M. Brain structure and obesity. Hum. Brain Mapp. 2010, 31, 353–364.
- Spencer, S.J.; D’Angelo, H.; Soch, A.; Watkins, L.R.; Maier, S.F.; Barrientos, R.M. High-fat diet and aging interact to produce neuroinflammation and impair hippocampal-and amygdalar-dependent memory. Neurobiol. Aging 2017, 58, 88–101.
- Yajima, H.; Ikeshima, E.; Shiraki, M.; Kanaya, T.; Fujiwara, D.; Odai, H.; Tsuboyama-Kasaoka, N.; Ezaki, O.; Oikawa, S.; Kondo, K. Isohumulones, bitter acids derived from hops, activate both peroxisome proliferator-activated receptor alpha and gamma and reduce insulin resistance. J. Biol. Chem. 2004, 279, 33456–33462.
- Gross, B.; Pawlak, M.; Lefebvre, P.; Staels, B. PPARs in obesity-induced T2DM, dyslipidaemia and NAFLD. Nat. Rev. Endocrinol. 2017, 13, 36–49.
- Lehmann, J.M.; Lenhard, J.M.; Oliver, B.B.; Ringold, G.M.; Kliewer, S.A. Peroxisome proliferator-activated receptors alpha and gamma are activated by indomethacin and other non-steroidal anti-inflammatory drugs. J. Biol. Chem. 1997, 272, 3406–3410.
- Lehmann, J.M.; Moore, L.B.; Smith-Oliver, T.A.; Wilkison, W.O.; Willson, T.M.; Kliewer, S.A. An antidiabetic thiazolidinedione is a high affinity ligand for peroxisome proliferator-activated receptor gamma (PPAR gamma). J. Biol. Chem. 1995, 270, 12953–12956.
- Yajima, H.; Noguchi, T.; Ikeshima, E.; Shiraki, M.; Kanaya, T.; Tsuboyama-Kasaoka, N.; Ezaki, O.; Oikawa, S.; Kondo, K. Prevention of diet-induced obesity by dietary isomerized hop extract containing isohumulones, in rodents. Int. J. Obes. 2005, 29, 991–997.
- Miura, Y.; Hosono, M.; Oyamada, C.; Odai, H.; Oikawa, S.; Kondo, K. Dietary isohumulones, the bitter components of beer, raise plasma HDL-cholesterol levels and reduce liver cholesterol and triacylglycerol contents similar to PPARalpha activations in C57BL/6 mice. Br. J. Nutr. 2005, 93, 559–567.
- Obara, K.; Mizutani, M.; Hitomi, Y.; Yajima, H.; Kondo, K. Isohumulones, the bitter component of beer, improve hyperglycemia and decrease body fat in Japanese subjects with prediabetes. Clin. Nutr. 2009, 28, 278–284.
- Ayabe, T.; Ohya, R.; Kondo, K.; Ano, Y. Iso-alpha-acids, bitter components of beer, prevent obesity-induced cognitive decline. Sci. Rep. 2018, 8, 4760.
- Ano, Y.; Hoshi, A.; Ayabe, T.; Ohya, R.; Uchida, S.; Yamada, K.; Kondo, K.; Kitaoka, S.; Furuyashiki, T. Iso-alpha-acids, the bitter components of beer, improve hippocampus-dependent memory through vagus nerve activation. FASEB J. 2019, 33, 4987–4995.
- Yamada, K.; Uchida, S.; Takahashi, S.; Takayama, M.; Nagata, Y.; Suzuki, N.; Shirakura, S.; Kanda, T. Effect of a centrally active angiotensin-converting enzyme inhibitor, perindopril, on cognitive performance in a mouse model of Alzheimer’s disease. Brain Res. 2010, 1352, 176–186.
- Arunrungvichian, K.; Boonyarat, C.; Fokin, V.V.; Taylor, P.; Vajragupta, O. Cognitive improvements in a mouse model with substituted 1,2,3-triazole agonists for nicotinic acetylcholine receptors. ACS Chem. Neurosci. 2015, 6, 1331–1340.
- Bristow, L.J.; Easton, A.E.; Li, Y.W.; Sivarao, D.V.; Lidge, R.; Jones, K.M.; Post-Munson, D.; Daly, C.; Lodge, N.J.; Gallagher, L.; et al. The Novel, Nicotinic Alpha7 Receptor Partial Agonist, BMS-933043, Improves Cognition and Sensory Processing in Preclinical Models of Schizophrenia. PLoS ONE 2016, 11, e0159996.
- Cohen, S.J.; Stackman, R.W., Jr. Assessing rodent hippocampal involvement in the novel object recognition task. A review. Behav. Brain Res. 2015, 285, 105–117.
- Ayabe, T.; Ohya, R.; Ano, Y. Hop-Derived Iso-alpha-Acids in Beer Improve Visual Discrimination and Reversal Learning in Mice as Assessed by a Touch Panel Operant System. Front. Behav. Neurosci. 2019, 13, 67.
- Nithianantharajah, J.; McKechanie, A.G.; Stewart, T.J.; Johnstone, M.; Blackwood, D.H.; St Clair, D.; Grant, S.G.; Bussey, T.J.; Saksida, L.M. Bridging the translational divide: Identical cognitive touchscreen testing in mice and humans carrying mutations in a disease-relevant homologous gene. Sci. Rep. 2015, 5, 14613.
- Gilbert, C.D.; Sigman, M.; Crist, R.E. The neural basis of perceptual learning. Neuron 2001, 31, 681–697.
- Bussey, T.J.; Saksida, L.M. Memory, perception, and the ventral visual-perirhinal-hippocampal stream: Thinking outside of the boxes. Hippocampus 2007, 17, 898–908.
- Kehagia, A.A.; Murray, G.K.; Robbins, T.W. Learning and cognitive flexibility: Frontostriatal function and monoaminergic modulation. Curr. Opin. Neurobiol. 2010, 20, 199–204.
- Klanker, M.; Feenstra, M.; Denys, D. Dopaminergic control of cognitive flexibility in humans and animals. Front. Neurosci. 2013, 7, 201.
- Kita, M.; Yoshida, S.; Kondo, K.; Yamakawa, Y.; Ano, Y. Effects of iso-alpha-acids, the hop-derived bitter components in beer, on the MRI-based Brain Healthcare Quotient in healthy middle-aged to older adults. Neuropsychopharmacol. Rep. 2019, 39, 273–278.
- Nemoto, K.; Oka, H.; Fukuda, H.; Yamakawa, Y. MRI-based Brain Healthcare Quotients: A bridge between neural and behavioral analyses for keeping the brain healthy. PLoS ONE 2017, 12, e0187137.
- Huang, Y.Y.; Kandel, E.R. D1/D5 receptor agonists induce a protein synthesis-dependent late potentiation in the CA1 region of the hippocampus. Proc. Natl. Acad. Sci. USA 1995, 92, 2446–2450.
- Xing, B.; Kong, H.; Meng, X.; Wei, S.G.; Xu, M.; Li, S.B. Dopamine D1 but not D3 receptor is critical for spatial learning and related signaling in the hippocampus. Neuroscience 2010, 169, 1511–1519.
- da Silva, W.C.; Kohler, C.C.; Radiske, A.; Cammarota, M. D1/D5 dopamine receptors modulate spatial memory formation. Neurobiol. Learn. Mem. 2012, 97, 271–275.
- Shinohara, R.; Taniguchi, M.; Ehrlich, A.T.; Yokogawa, K.; Deguchi, Y.; Cherasse, Y.; Lazarus, M.; Urade, Y.; Ogawa, A.; Kitaoka, S.; et al. Dopamine D1 receptor subtype mediates acute stress-induced dendritic growth in excitatory neurons of the medial prefrontal cortex and contributes to suppression of stress susceptibility in mice. Mol. Psychiatry 2018, 23, 1717–1730.
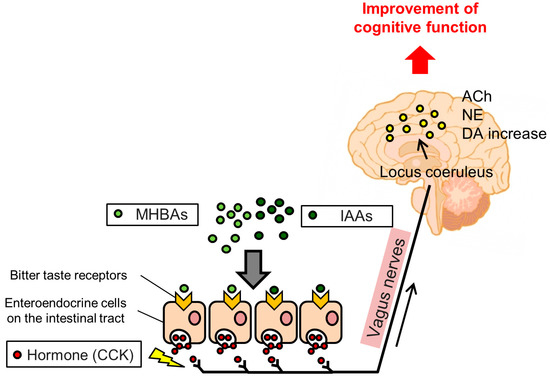
- Egan, J.M.; Margolskee, R.F. Taste cells of the gut and gastrointestinal chemosensation. Mol. Interv. 2008, 8, 78–81.
- Wu, S.V.; Rozengurt, N.; Yang, M.; Young, S.H.; Sinnett-Smith, J.; Rozengurt, E. Expression of bitter taste receptors of the T2R family in the gastrointestinal tract and enteroendocrine STC-1 cells. Proc. Natl. Acad. Sci. USA 2002, 99, 2392–2397.
- Chen, M.C.; Wu, S.V.; Reeve, J.R., Jr.; Rozengurt, E. Bitter stimuli induce Ca2+ signaling and CCK release in enteroendocrine STC-1 cells: Role of L-type voltage-sensitive Ca2+ channels. Am. J. Physiol. Cell Physiol. 2006, 291, C726–C739.
- Kempadoo, K.A.; Mosharov, E.V.; Choi, S.J.; Sulzer, D.; Kandel, E.R. Dopamine release from the locus coeruleus to the dorsal hippocampus promotes spatial learning and memory. Proc. Natl. Acad. Sci. USA 2016, 113, 14835–14840.
- Takeuchi, T.; Duszkiewicz, A.J.; Sonneborn, A.; Spooner, P.A.; Yamasaki, M.; Watanabe, M.; Smith, C.C.; Fernandez, G.; Deisseroth, K.; Greene, R.W.; et al. Locus coeruleus and dopaminergic consolidation of everyday memory. Nature 2016, 537, 357–362.
- Taniguchi, Y.; Matsukura, Y.; Ozaki, H.; Nishimura, K.; Shindo, K. Identification and quantification of the oxidation products derived from alpha-acids and beta-acids during storage of hops (Humulus lupulus L.). J. Agric. Food Chem. 2013, 61, 3121–3130.
- Morimoto-Kobayashi, Y.; Ohara, K.; Takahashi, C.; Kitao, S.; Wang, G.; Taniguchi, Y.; Katayama, M.; Nagai, K. Matured Hop Bittering Components Induce Thermogenesis in Brown Adipose Tissue via Sympathetic Nerve Activity. PLoS ONE 2015, 10, e0131042.
- Yamazaki, T.; Morimoto-Kobayashi, Y.; Koizumi, K.; Takahashi, C.; Nakajima, S.; Kitao, S.; Taniguchi, Y.; Katayama, M.; Ogawa, Y. Secretion of a gastrointestinal hormone, cholecystokinin, by hop-derived bitter components activates sympathetic nerves in brown adipose tissue. J. Nutr. Biochem. 2019, 64, 80–87.
- Morimoto-Kobayashi, Y.; Ohara, K.; Ashigai, H.; Kanaya, T.; Koizumi, K.; Manabe, F.; Kaneko, Y.; Taniguchi, Y.; Katayama, M.; Kowatari, Y.; et al. Matured hop extract reduces body fat in healthy overweight humans: A randomized, double-blind, placebo-controlled parallel group study. Nutr. J. 2016, 15, 25.
- Suzuki, S.; Yamazaki, T.; Takahashi, C.; Kaneko, Y.; Morimoto-Kobayashi, Y.; Katayama, M. The relationship between the effect of matured hop extract and physical activity on reducing body fat: Re-analysis of data from a randomized, double-blind, placebo-controlled parallel group study. Nutr. J. 2018, 17, 98.
- Ayabe, T.; Ohya, R.; Ano, Y. Iso-alpha-acids and matured hop bitter acids in beer improve obesity-induced cognitive impairment. Biosci. Biotechnol. Biochem. 2019, 83, 1937–1945.
- Ayabe, T.; Ohya, R.; Taniguchi, Y.; Shindo, K.; Kondo, K.; Ano, Y. Matured Hop-Derived Bitter Components in Beer Improve Hippocampus-Dependent Memory Through Activation of the Vagus Nerve. Sci. Rep. 2018, 8, 15372.
- Mello-Carpes, P.B.; da Silva de Vargas, L.; Gayer, M.C.; Roehrs, R.; Izquierdo, I. Hippocampal noradrenergic activation is necessary for object recognition memory consolidation and can promote BDNF increase and memory persistence. Neurobiol. Learn. Mem. 2016, 127, 84–92.
- Mello-Carpes, P.B.; Izquierdo, I. The Nucleus of the Solitary Tract → Nucleus Paragigantocellularis → Locus Coeruleus → CA1 region of dorsal hippocampus pathway is important for consolidation of object recognition memory. Neurobiol. Learn. Mem. 2013, 100, 56–63.
- Groves, D.A.; Brown, V.J. Vagal nerve stimulation: A review of its applications and potential mechanisms that mediate its clinical effects. Neurosci. Biobehav. Rev. 2005, 29, 493–500.
- Hays, S.A.; Rennaker, R.L.; Kilgard, M.P. Targeting plasticity with vagus nerve stimulation to treat neurological disease. Prog. Brain Res. 2013, 207, 275–299.
- Haam, J.; Yakel, J.L. Cholinergic modulation of the hippocampal region and memory function. J. Neurochem. 2017, 142, 111–121.
- Fukuda, T.; Ayabe, T.; Ohya, R.; Ano, Y. Matured hop bitter acids improve spatial working and object recognition memory via nicotinic acetylcholine receptors. Psychopharmacology 2019, 236, 2847–2854.
- Fukuda, T.; Obara, K.; Saito, J.; Umeda, S.; Ano, Y. Effects of hop bitter acids, bitter components in beer, on cognition in healthy adults: A randomized controlled trial. J. Agric. Food Chem. 2020, 68, 206–212.
- Alvarez, J.A.; Emory, E. Executive function and the frontal lobes: A meta-analytic review. Neuropsychol. Rev. 2006, 16, 17–42.
- MacLeod, C.M.; MacDonald, P.A. Interdimensional interference in the Stroop effect: Uncovering the cognitive and neural anatomy of attention. Trends Cog. Sci. 2000, 4, 383–391.
- Fukuda, T.; Ohya, R.; Kobayashi, K.; Ano, Y. Matured Hop Bitter Acids in Beer Improve Lipopolysaccharide-Induced Depression-Like Behavior. Front. Neurosci. 2019, 13, 41.
- Almaguer, C.; Gastl, M.; Arendt, E.K.; Becker, T. Comparative study of the contribution of hop (Humulus lupulus L.) hard resins extracted from different hop varieties to beer quality parameters. J. Am. Soci. Brew. Chem. 2015, 73, 115–123.
- Steenbergen, L.; Sellaro, R.; Stock, A.K.; Verkuil, B.; Beste, C.; Colzato, L.S. Transcutaneous vagus nerve stimulation (tVNS) enhances response selection during action cascading processes. Eur. Neuropsychopharmacol. 2015, 25, 773–778.
- Chunchai, T.; Samniang, B.; Sripetchwandee, J.; Pintana, H.; Pongkan, W.; Kumfu, S.; Shinlapawittayatorn, K.; KenKnight, B.H.; Chattipakorn, N.; Chattipakorn, S.C. Vagus Nerve Stimulation Exerts the Neuroprotective Effects in Obese-Insulin Resistant Rats, Leading to the Improvement of Cognitive Function. Sci. Rep. 2016, 6, 26866.
- Liu, A.F.; Zhao, F.B.; Wang, J.; Lu, Y.F.; Tian, J.; Zhao, Y.; Gao, Y.; Hu, X.J.; Liu, X.Y.; Tan, J.; et al. Effects of vagus nerve stimulation on cognitive functioning in rats with cerebral ischemia reperfusion. J. Transl. Med. 2016, 14, 101.
- Suzuki, S.; Morimoto-Kobayashi, Y.; Takahashi, C.; Taniguchi, Y.; Katayama, M. Genetic, acute and subchronic toxicity studies of matured hop extract produced by extraction from heat-treated hops. J. Toxicol. Sci. 2018, 43, 473–484.
- Glenner, G.G.; Wong, C.W. Alzheimer’s disease: Initial report of the purification and characterization of a novel cerebrovascular amyloid protein. Biochem. Biophys. Res. Commun. 2012, 425, 534–539.
- Querfurth, H.W.; LaFerla, F.M. Alzheimer’s disease. N. Engl. J. Med. 2010, 362, 329–344.
- Amor, S.; Puentes, F.; Baker, D.; van der Valk, P. Inflammation in neurodegenerative diseases. Immunology 2010, 129, 154–169.
- Heneka, M.T. Inflammasome activation and innate immunity in Alzheimer’s disease. Brain Pathol. 2017, 27, 220–222.
- Yan, Y.; Jiang, W.; Liu, L.; Wang, X.; Ding, C.; Tian, Z.; Zhou, R. Dopamine controls systemic inflammation through inhibition of NLRP3 inflammasome. Cell 2015, 160, 62–73.
- Agarwal, S.; Yadav, A.; Chaturvedi, R.K. Peroxisome proliferator-activated receptors (PPARs) as therapeutic target in neurodegenerative disorders. Biochem. Biophys. Res. Commun. 2017, 483, 1166–1177.
- Ano, Y.; Dohata, A.; Taniguchi, Y.; Hoshi, A.; Uchida, K.; Takashima, A.; Nakayama, H. Iso-alpha-acids, Bitter Components of Beer, Prevent Inflammation and Cognitive Decline Induced in a Mouse Model of Alzheimer’s Disease. J. Biol. Chem. 2017, 292, 3720–3728.
- Ano, Y.; Takaichi, Y.; Uchida, K.; Kondo, K.; Nakayama, H.; Takashima, A. Iso-alpha-Acids, the Bitter Components of Beer, Suppress Microglial Inflammation in rTg4510 Tauopathy. Molecules 2018, 23, 3133.
- Ising, C.; Venegas, C.; Zhang, S.; Scheiblich, H.; Schmidt, S.V.; Vieira-Saecker, A.; Schwartz, S.; Albasset, S.; McManus, R.M.; Tejera, D.; et al. NLRP3 inflammasome activation drives tau pathology. Nature 2019, 575, 669–673.
- Bakker, A.; Krauss, G.L.; Albert, M.S.; Speck, C.L.; Jones, L.R.; Stark, C.E.; Yassa, M.A.; Bassett, S.S.; Shelton, A.L.; Gallagher, M. Reduction of hippocampal hyperactivity improves cognition in amnestic mild cognitive impairment. Neuron 2012, 74, 467–474.
- Ano, Y.; Yoshikawa, M.; Takaichi, Y.; Michikawa, M.; Uchida, K.; Nakayama, H.; Takashima, A. Iso-alpha-Acids, Bitter Components in Beer, Suppress Inflammatory Responses and Attenuate Neural Hyperactivation in the Hippocampus. Front. Pharm. 2019, 10, 81.
- Wang, T.; Nowrangi, D.; Yu, L.; Lu, T.; Tang, J.; Han, B.; Ding, Y.; Fu, F.; Zhang, J.H. Activation of dopamine D1 receptor decreased NLRP3-mediated inflammation in intracerebral hemorrhage mice. J. Neuroinflammation 2018, 15, 2.
- Ano, Y.; Ohya, R.; Kondo, K.; Nakayama, H. Iso-alpha-acids, Hop-Derived Bitter Components of Beer, Attenuate Age-Related Inflammation and Cognitive Decline. Front. Aging Neurosci. 2019, 11, 16.
- Bondareff, W.; Mountjoy, C.Q.; Roth, M. Loss of neurons of origin of the adrenergic projection to cerebral cortex (nucleus locus ceruleus) in senile dementia. Neurology 1982, 32, 164–168.
- Heneka, M.T.; Ramanathan, M.; Jacobs, A.H.; Dumitrescu-Ozimek, L.; Bilkei-Gorzo, A.; Debeir, T.; Sastre, M.; Galldiks, N.; Zimmer, A.; Hoehn, M.; et al. Locus ceruleus degeneration promotes Alzheimer pathogenesis in amyloid precursor protein 23 transgenic mice. J. Neurosci. 2006, 26, 1343–1354.
- Heneka, M.T.; Nadrigny, F.; Regen, T.; Martinez-Hernandez, A.; Dumitrescu-Ozimek, L.; Terwel, D.; Jardanhazi-Kurutz, D.; Walter, J.; Kirchhoff, F.; Hanisch, U.K.; et al. Locus ceruleus controls Alzheimer’s disease pathology by modulating microglial functions through norepinephrine. Proc. Natl. Acad. Sci. USA 2010, 107, 6058–6063.
- Vonck, K.; Raedt, R.; Naulaerts, J.; De Vogelaere, F.; Thiery, E.; Van Roost, D.; Aldenkamp, B.; Miatton, M.; Boon, P. Vagus nerve stimulation 25 years later! What do we know about the effects on cognition? Neurosci. Biobehav. Rev. 2014, 45, 63–71.




