
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Marek Samec | + 1374 word(s) | 1374 | 2021-06-25 09:51:16 | | | |
| 2 | Lily Guo | Meta information modification | 1374 | 2021-07-08 07:58:15 | | |
Video Upload Options
Metabolic reprogramming characterized by alterations in nutrient uptake and critical molecular pathways associated with cancer cell metabolism represents a fundamental process of malignant transformation. Melatonin (N-acetyl-5-methoxytryptamine) is a hormone secreted by the pineal gland. Melatonin primarily regulates circadian rhythms but also exerts anti-inflammatory, anti-depressant, antioxidant and anti-tumor activities. Concerning cancer metabolism, melatonin displays significant anticancer effects via the regulation of key components of aerobic glycolysis, gluconeogenesis, the pentose phosphate pathway (PPP) and lipid metabolism. Melatonin treatment affects glucose transporter (GLUT) expression, glucose-6-phosphate dehydrogenase (G6PDH) activity, lactate production and other metabolic contributors. Moreover, melatonin modulates critical players in cancer development, such as HIF-1 and p53. Taken together, melatonin has notable anti-cancer effects at malignancy initiation, progression and metastasing. Further investigations of melatonin impacts relevant for cancer metabolism are expected to create innovative approaches supportive for the effective prevention and targeted therapy of cancers.
1. Introduction
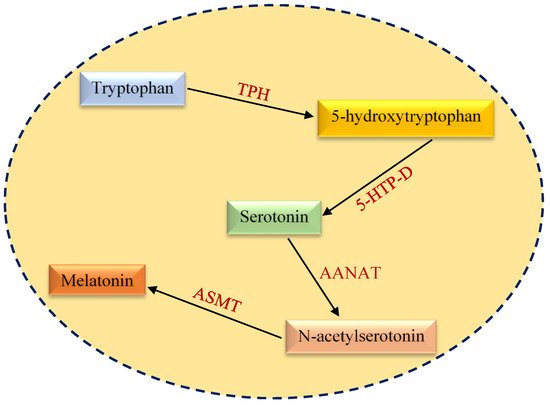
2. Structural and Functional Aspects of Melatonin
3. Expert Recommendations in the Framework of Predictive, Preventive and Personalized (3P) Medicine
4. Conclusions
References
- Allison, K.E.; Coomber, B.L.; Bridle, B.W. Metabolic reprogramming in the tumour microenvironment: A hallmark shared by cancer cells and t lymphocytes. Immunology 2017, 152, 175–184.
- Sun, X.; Wang, M.; Wang, M.; Yu, X.; Guo, J.; Sun, T.; Li, X.; Yao, L.; Dong, H.; Xu, Y. Metabolic reprogramming in triple-negative breast cancer. Front. Oncol. 2020, 10, 428.
- Giannattasio, S.; Mirisola, M.G.; Mazzoni, C. Editorial: Cell stress, metabolic reprogramming, and cancer. Front. Oncol. 2018, 8, 236.
- Phan, L.M.; Yeung, S.-C.J.; Lee, M.-H. Cancer metabolic reprogramming: Importance, main features, and potentials for precise targeted anti-cancer therapies. Cancer Biol. Med. 2014, 11, 1–19.
- Mayo, J.C.; Cernuda, R.; Quiros, I.; Rodriguez, P.; Garcia, J.I.; Hevia, D.; Sainz, R.M. Understanding the role of melatonin in cancer metabolism. Melatonin Res. 2019, 2, 76–104.
- Hansen, M.V.; Andersen, L.T.; Madsen, M.T.; Hageman, I.; Rasmussen, L.S.; Bokmand, S.; Rosenberg, J.; Gögenur, I. Effect of melatonin on depressive symptoms and anxiety in patients undergoing breast cancer surgery: A randomized, double-blind, placebo-controlled trial. Breast Cancer Res. Treat. 2014, 145, 683–695.
- Kostoglou-Athanassiou, I. Therapeutic applications of melatonin. Ther. Adv. Endocrinol. Metab. 2013, 4, 13–24.
- Li, Y.; Li, S.; Zhou, Y.; Meng, X.; Zhang, J.-J.; Xu, D.-P.; Li, H.-B. Melatonin for the prevention and treatment of cancer. Oncotarget 2017, 8, 39896–39921.
- Mociková-Kalická, K.; Bojková, B.; Adámeková, E.; Mníchová-Chamilová, M.; Kubatka, P.; Ahlersová, E.; Ahlers, I. Preventive effect of indomethacin and melatonin on 7,12-dimethybenz/a/anthracene-induced mammary carcinogenesis in female sprague-dawley rats. A preliminary report. Folia Biol. 2001, 47, 75–79.
- Orendáš, P.; Kubatka, P.; Bojková, B.; Kassayová, M.; Kajo, K.; Výbohová, D.; Kružliak, P.; Péč, M.; Adamkov, M.; Kapinová, A.; et al. Melatonin potentiates the anti-tumour effect of pravastatin in rat mammary gland carcinoma model. Int. J. Exp. Pathol. 2014, 95, 401–410.
- Bojková, B.; Kubatka, P.; Qaradakhi, T.; Zulli, A.; Kajo, K. Melatonin may increase anticancer potential of pleiotropic drugs. Int. J. Mol. Sci. 2018, 19, 3910.
- Reiter, R.J.; Sharma, R.; Rosales-Corral, S. Anti-warburg effect of melatonin: A proposed mechanism to explain its inhibition of multiple diseases. Int. J. Mol. Sci. 2021, 22, 764.
- Kubatka, P.; Zubor, P.; Busselberg, D.; Kwon, T.K.; Adamek, M.; Petrovic, D.; Opatrilova, R.; Gazdikova, K.; Caprnda, M.; Rodrigo, L.; et al. Melatonin and breast cancer: Evidences from preclinical and human studies. Crit. Rev. Oncol. Hematol. 2018, 122, 133–143.
- Zhao, Y.; Ren, J.; Hillier, J.; Jones, M.; Lu, W.; Jones, E.Y. Structural characterization of melatonin as an inhibitor of the wnt deacylase notum. J. Pineal. Res. 2020, 68, e12630.
- Rani, P.; Pal, D.; Hegde, R.R.; Hashim, S.R. Acetamides: Chemotherapeutic agents for inflammation-associated cancers. J. Chemother. 2016, 28, 255–265.
- Tordjman, S.; Chokron, S.; Delorme, R.; Charrier, A.; Bellissant, E.; Jaafari, N.; Fougerou, C. Melatonin: Pharmacology, functions and therapeutic benefits. Curr. Neuropharmacol. 2017, 15, 434–443.
- Zhao, D.; Yu, Y.; Shen, Y.; Liu, Q.; Zhao, Z.; Sharma, R.; Reiter, R.J. Melatonin synthesis and function: Evolutionary history in animals and plants. Front. Endocrinol. 2019, 10, 249.
- Saha, S.; Singh, K.M.; Gupta, B.B.P. Melatonin synthesis and clock gene regulation in the pineal organ of teleost fish compared to mammals: Similarities and differences. Gen. Comp. Endocrinol. 2019, 279, 27–34.
- Benyassi, A.; Schwartz, C.; Coon, S.L.; Klein, D.C.; Falcón, J. Melatonin synthesis: Arylalkylamine N-acetyltransferases in trout retina and pineal organ are different. Neuroreport 2000, 11, 255–258.
- Low, M.J. Neuroendocrinology. In Williams Textbook of Endocrinology, 13th ed.; Melmed, S., Polonsky, K.S., Larsen, P.R., Kronenberg, H.M., Eds.; Elsevier: Philadelphia, PA, USA, 2016; pp. 109–175. ISBN 978-0-323-29738-7.
- Cook, J.S.; Sauder, C.L.; Ray, C.A. Melatonin differentially affects vascular blood flow in humans. Am. J. Physiol. Heart Circ. Physiol. 2011, 300, H670–H674.
- Reiter, R.J. Circadian and non-circadian melatonin: Influences on glucose metabolism in cancer cells. J. Curr. Sci. Technol. 2020, 10, 85–98.
- Hardeland, R. Chronobiology of melatonin beyond the feedback to the suprachiasmatic nucleus—Consequences to melatonin dysfunction. Int. J. Mol. Sci. 2013, 14, 5817–5841.
- Dubocovich, M.L. Melatonin receptors: Role on sleep and circadian rhythm regulation. Sleep Med. 2007, 8 (Suppl. S3), 34–42.
- Poza, J.J.; Pujol, M.; Ortega-Albás, J.J.; Romero, O. Melatonin in sleep disorders. Neurología 2020.
- Ashrafizadeh, M.; Najafi, M.; Kavyiani, N.; Mohammadinejad, R.; Farkhondeh, T.; Samarghandian, S. Anti-inflammatory activity of melatonin: A focus on the role of NLRP3 inflammasome. Inflammation 2021.
- Karaaslan, C.; Suzen, S. Antioxidant properties of melatonin and its potential action in diseases. Curr. Top Med. Chem. 2015, 15, 894–903.
- Ahmadi, Z.; Ashrafizadeh, M. Melatonin as a potential modulator of Nrf2. Fundam. Clin. Pharmacol. 2020, 34, 11–19.
- Abadi, S.H.M.H.; Shirazi, A.; Alizadeh, A.M.; Changizi, V.; Najafi, M.; Khalighfard, S.; Nosrati, H. The effect of melatonin on superoxide dismutase and glutathione peroxidase activity, and malondialdehyde levels in the targeted and the non-targeted lung and heart tissues after irradiation in xenograft mice colon cancer. Curr. Mol. Pharmacol. 2018, 11, 326–335.
- Pohanka, M. Impact of melatonin on immunity: A review. Cent. Eur. J. Med. 2013, 8, 369–376.
- Maestroni, G.J.M. Melatonin and the immune system therapeutic potential in cancer, viral diseases, and immunodeficiency states. In The Pineal Gland and Cancer: Neuroimmunoendocrine Mechanisms in Malignancy; Bartsch, C., Bartsch, H., Blask, D.E., Cardinali, D.P., Hrushesky, W.J.M., Mecke, D., Eds.; Springer: Berlin/Heidelberg, Germany, 2001; pp. 384–394. ISBN 978-3-642-59512-7.
- Miller, S.C.; Pandi, P.S.R.; Esquifino, A.I.; Cardinali, D.P.; Maestroni, G.J.M. The role of melatonin in immuno-enhancement: Potential application in cancer. Int. J. Exp. Pathol. 2006, 87, 81–87.
- Campos, L.A.; Bueno, C.; Barcelos, I.P.; Halpern, B.; Brito, L.C.; Amaral, F.G.; Baltatu, O.C.; Cipolla-Neto, J. Melatonin therapy improves cardiac autonomic modulation in pinealectomized patients. Front. Endocrinol. 2020, 11, 239.
- Wang, Y.; Wang, P.; Zheng, X.; Du, X. Therapeutic strategies of melatonin in cancer patients: A systematic review and meta-analysis. OncoTargets Ther. 2018, 11, 7895–7908.
- Di Bella, G.; Mascia, F.; Gualano, L.; Di Bella, L. Melatonin anticancer effects: Review. Int. J. Mol. Sci. 2013, 14, 2410–2430.
- Wang, T.; Liu, B.; Guan, Y.; Gong, M.; Zhang, W.; Pan, J.; Liu, Y.; Liang, R.; Yuan, Y.; Ye, L. Melatonin inhibits the proliferation of breast cancer cells induced by bisphenol a via targeting estrogen receptor-related pathways. Thorac. Cancer 2018, 9, 368–375.
- Hill, S.M.; Frasch, T.; Xiang, S.; Yuan, L.; Duplessis, T.; Mao, L. Molecular mechanisms of melatonin anticancer effects. Integr. Cancer Ther. 2009, 8, 337–346.
- Rodriguez, C.; Martín, V.; Herrera, F.; García-Santos, G.; Rodriguez-Blanco, J.; Casado-Zapico, S.; Sánchez-Sánchez, A.M.; Suárez, S.; Puente-Moncada, N.; Anítua, M.J.; et al. Mechanisms involved in the pro-apoptotic effect of melatonin in cancer cells. Int. J. Mol. Sci. 2013, 14, 6597–6613.
- Moretti, R.M.; Marelli, M.M.; Maggi, R.; Dondi, D.; Motta, M.; Limonta, P. Antiproliferative action of melatonin on human prostate cancer LNCaP cells. Oncol. Rep. 2000, 7, 347–351.
- Gatti, G.; Lucini, V.; Dugnani, S.; Calastretti, A.; Spadoni, G.; Bedini, A.; Rivara, S.; Mor, M.; Canti, G.; Scaglione, F.; et al. Antiproliferative and pro-apoptotic activity of melatonin analogues on melanoma and breast cancer cells. Oncotarget 2017, 8, 68338–68353.
- Cheng, J.; Yang, H.-L.; Gu, C.-J.; Liu, Y.-K.; Shao, J.; Zhu, R.; He, Y.-Y.; Zhu, X.-Y.; Li, M.-Q. Melatonin restricts the viability and angiogenesis of vascular endothelial cells by suppressing HIF-1α/ROS/VEGF. Int. J. Mol. Med. 2019, 43, 945–955.
- Mortezaee, K.; Potes, Y.; Mirtavoos-Mahyari, H.; Motevaseli, E.; Shabeeb, D.; Musa, A.E.; Najafi, M.; Farhood, B. Boosting immune system against cancer by melatonin: A mechanistic viewpoint. Life Sci. 2019, 238, 116960.
- Koklesova, L.; Samec, M.; Liskova, A.; Zhai, K.; Büsselberg, D.; Giordano, F.A.; Kubatka, P.; Golunitschaja, O. Mitochondrial impairments in aetiopathology of multifactorial diseases: Common origin but individual outcomes in context of 3P medicine. EPMA J. 2021, 1–14.
- Qian, S.; Golubnitschaja, O.; Zhan, X. Chronic inflammation: Key player and biomarker-set to predict and prevent cancer development and progression based on individualized patient profiles. EPMA J. 2019, 10, 365–381.
- Ma, S.; Zhu, L.; Fan, X.; Luo, T.; Liu, D.; Liang, Z.; Hu, X.; Shi, T.; Tan, W.; Wang, Z. Melatonin derivatives combat with inflammation-related cancer by targeting the main culprit STAT3. Eur. J. Med. Chem. 2021, 211, 113027.
- Abolhasanpour, N.; Alihosseini, S.; Golipourkhalili, S.; Badalzadeh, R.; Mahmoudi, J.; Hosseini, L. Insight into the effects of melatonin on endoplasmic reticulum, mitochondrial function, and their cross-talk in the stroke. Arch. Med. Res. 2021.
- Polivka, J.; Polivka, J.; Pesta, M.; Rohan, V.; Celedova, L.; Mahajani, S.; Topolcan, O.; Golubnitschaja, O. Risks associated with the stroke predisposition at young age: Facts and hypotheses in light of individualized predictive and preventive approach. EPMA J. 2019, 10, 81–99.
- Ferracioli-Oda, E.; Qawasmi, A.; Bloch, M.H. Meta-analysis: Melatonin for the treatment of primary sleep disorders. PLoS ONE 2013, 8, e63773.
- Abdelgadir, I.S.; Gordon, M.A.; Akobeng, A.K. Melatonin for the management of sleep problems in children with neurodevelopmental disorders: A systematic review and meta-analysis. Arch. Dis. Child. 2018, 103, 1155–1162.
- Cho, J.H.; Bhutani, S.; Kim, C.H.; Irwin, M.R. Anti-inflammatory effects of melatonin: A systematic review and meta-analysis of clinical trials. Brain Behav. Immun. 2021, 93, 245–253.
- Proietti, S.; Cucina, A.; Minini, M.; Bizzarri, M. Melatonin, mitochondria, and the cancer cell. Cell. Mol. Life Sci. 2017, 74, 4015–4025.
- Reiter, R.J.; Rosales-Corral, S.A.; Tan, D.-X.; Acuna-Castroviejo, D.; Qin, L.; Yang, S.-F.; Xu, K. Melatonin, a full service anti-cancer agent: Inhibition of initiation, progression and metastasis. Int. J. Mol. Sci. 2017, 18, 843.
- Borin, T.F.; Arbab, A.S.; Gelaleti, G.B.; Ferreira, L.C.; Moschetta, M.G.; Jardim-Perassi, B.V.; Iskander, A.; Varma, N.R.S.; Shankar, A.; Coimbra, V.B.; et al. Melatonin decreases breast cancer metastasis by modulating rho-associated kinase protein-1 expression. J. Pineal Res. 2016, 60, 3–15.
- Glenister, R.; McDaniel, K.; Francis, H.; Venter, J.; Jensen, K.; Dusio, G.; Glaser, S.; Meng, F.; Alpini, G. Therapeutic actions of melatonin on gastrointestinal cancer development and progression. Transl. Gastrointest. Cancer 2013, 2, 110–120.
- Reiter, R.J.; Sharma, R.; Ma, Q.; Rorsales-Corral, S.; de Almeida Chuffa, L.G. Melatonin inhibits warburg-dependent cancer by redirecting glucose oxidation to the mitochondria: A mechanistic hypothesis. Cell. Mol. Life Sci. 2020, 77, 2527–2542.
- Wang, S.-W.; Tai, H.-C.; Tang, C.-H.; Lin, L.-W.; Lin, T.-H.; Chang, A.-C.; Chen, P.-C.; Chen, Y.-H.; Wang, P.-C.; Lai, Y.-W.; et al. Melatonin impedes prostate cancer metastasis by suppressing MMP-13 expression. J. Cell. Physiol. 2021, 236, 3979–3990.
- Zharinov, G.M.; Bogomolov, O.A.; Chepurnaya, I.V.; Neklasova, N.Y.; Anisimov, V.N. Melatonin increases overall survival of prostate cancer patients with poor prognosis after combined hormone radiation treatment. Oncotarget 2020, 11, 3723–3729.
- Shen, D.; Ju, L.; Zhou, F.; Yu, M.; Ma, H.; Zhang, Y.; Liu, T.; Xiao, Y.; Wang, X.; Qian, K. The inhibitory effect of melatonin on human prostate cancer. Cell. Commun. Signal. 2021, 19, 34.
- Blask, D.E.; Dauchy, R.T.; Dauchy, E.M.; Mao, L.; Hill, S.M.; Greene, M.W.; Belancio, V.P.; Sauer, L.A.; Davidson, L. Light exposure at night disrupts host/cancer circadian regulatory dynamics: Impact on the warburg effect, lipid signaling and tumor growth prevention. PLoS ONE 2014, 9, e102776.
- Hevia, D.; Gonzalez-Menendez, P.; Fernandez-Fernandez, M.; Cueto, S.; Rodriguez-Gonzalez, P.; Garcia-Alonso, J.I.; Mayo, J.C.; Sainz, R.M. Melatonin decreases glucose metabolism in prostate cancer cells: A 13C stable isotope-resolved metabolomic study. Int. J. Mol. Sci. 2017, 18, 1620.
- Sanchez-Sanchez, A.M.; Antolin, I.; Puente-Moncada, N.; Suarez, S.; Gomez-Lobo, M.; Rodriguez, C.; Martin, V. Melatonin cytotoxicity is associated to warburg effect inhibition in ewing sarcoma cells. PLoS ONE 2015, 10, e0135420.
- Mi, L.; Kuang, H. Melatonin regulates cisplatin resistance and glucose metabolism through hippo signaling in hepatocellular carcinoma cells. Cancer Manag. Res. 2020, 12, 1863–1874.
- He, M.; Zhou, C.; Lu, Y.; Mao, L.; Xi, Y.; Mei, X.; Wang, X.; Zhang, L.; Yu, Z.; Zhou, Z. Melatonin antagonizes nickel-induced aerobic glycolysis by blocking ROS-mediated HIF-1 α/MiR210/ISCU axis activation. Oxid. Med. Cell. Longev. 2020, 2020, 5406284.
- Puente-Moncada, N.; Turos-Cabal, M.; Sánchez-Sánchez, A.M.; Antolín, I.; Herrera, F.; Rodriguez-Blanco, J.; Duarte-Olivenza, C.; Rodriguez, C.; Martín, V. Role of glucose metabolism in the differential antileukemic effect of melatonin on wild-type and FLT3-ITD mutant cells. Oncol. Rep. 2020, 44, 293–302.
- Hevia, D.; González-Menéndez, P.; Quiros-González, I.; Miar, A.; Rodríguez-García, A.; Tan, D.-X.; Reiter, R.J.; Mayo, J.C.; Sainz, R.M. Melatonin uptake through glucose transporters: A new target for melatonin inhibition of cancer. J. Pineal Res. 2015, 58, 234–250.
- Dauchy, R.T.; Hoffman, A.E.; Wren-Dail, M.A.; Hanifin, J.P.; Warfield, B.; Brainard, G.C.; Xiang, S.; Yuan, L.; Hill, S.M.; Belancio, V.P.; et al. Daytime blue light enhances the nighttime circadian melatonin inhibition of human prostate cancer growth. Comp. Med. 2015, 65, 473–485.
- Xiang, S.; Dauchy, R.T.; Hauch, A.; Mao, L.; Yuan, L.; Wren, M.A.; Belancio, V.P.; Mondal, D.; Frasch, T.; Blask, D.E.; et al. Doxorubicin resistance in breast cancer is driven by light at night-induced disruption of the circadian melatonin signal. J. Pineal Res. 2015, 59, 60–69.
- Dauchy, R.T.; Xiang, S.; Mao, L.; Brimer, S.; Wren, M.A.; Yuan, L.; Anbalagan, M.; Hauch, A.; Frasch, T.; Rowan, B.G.; et al. Circadian and melatonin disruption by exposure to light at night drives intrinsic resistance to tamoxifen therapy in breast cancer. Cancer Res. 2014, 74, 4099–4110.
- Chuffa, L.G.A.; Lupi Júnior, L.A.; Seiva, F.R.F.; Martinez, M.; Domeniconi, R.F.; Pinheiro, P.F.F.; Dos Santos, L.D.; Martinez, F.E. Quantitative proteomic profiling reveals that diverse metabolic pathways are influenced by melatonin in an in vivo model of ovarian carcinoma. J. Proteome Res. 2016, 15, 3872–3882.
- Mao, L.; Dauchy, R.T.; Blask, D.E.; Dauchy, E.M.; Slakey, L.M.; Brimer, S.; Yuan, L.; Xiang, S.; Hauch, A.; Smith, K.; et al. Melatonin suppression of aerobic glycolysis (Warburg effect), survival signalling and metastasis in human leiomyosarcoma. J. Pineal Res. 2016, 60, 167–177.
- Janssens, J.P.; Schuster, K.; Voss, A. Preventive, predictive, and personalized medicine for effective and affordable cancer care. EPMA J. 2018, 9, 113–123.
- Golubnitschaja, O. Paradigm change from curative to predictive medicine: Novel strategic trends in Europe. Croat. Med. J. 2009, 50, 596–597.
- Liskova, A.; Samec, M.; Koklesova, L.; Giordano, F.A.; Kubatka, P.; Golubnitschaja, O. Liquid biopsy is instrumental for 3PM dimensional solutions in cancer management. J. Clin. Med. 2020, 9, 2749.
- Hu, R.; Wang, X.; Zhan, X. Multi-parameter systematic strategies for predictive, preventive and personalised medicine in cancer. EPMA J. 2013, 4, 2.
- Crigna, A.T.; Samec, M.; Koklesova, L.; Liskova, A.; Giordano, F.A.; Kubatka, P.; Golubnitschaja, O. Cell-free nucleic acid patterns in disease prediction and monitoring—Hype or hope? EPMA J. 2020, 11, 603–627.
- Kunin, A.; Sargheini, N.; Birkenbihl, C.; Moiseeva, N.; Fröhlich, H.; Golubnitschaja, O. Voice perturbations under the stress overload in young individuals: Phenotyping and suboptimal health as predictors for cascading pathologies. EPMA J. 2020, 517–527.
- Goncharenko, V.; Bubnov, R.; Polivka, J.; Zubor, P.; Biringer, K.; Bielik, T.; Kuhn, W.; Golubnitschaja, O. Vaginal dryness: Individualised patient profiles, risks and mitigating measures. EPMA J. 2019, 10, 73–79.




