Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Oyime Aula | + 3273 word(s) | 3273 | 2021-06-30 03:25:20 | | | |
| 2 | Peter Tang | Meta information modification | 3273 | 2021-07-07 07:36:34 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Aula, O. Schistosomiasis. Encyclopedia. Available online: https://encyclopedia.pub/entry/11758 (accessed on 13 January 2026).
Aula O. Schistosomiasis. Encyclopedia. Available at: https://encyclopedia.pub/entry/11758. Accessed January 13, 2026.
Aula, Oyime. "Schistosomiasis" Encyclopedia, https://encyclopedia.pub/entry/11758 (accessed January 13, 2026).
Aula, O. (2021, July 07). Schistosomiasis. In Encyclopedia. https://encyclopedia.pub/entry/11758
Aula, Oyime. "Schistosomiasis." Encyclopedia. Web. 07 July, 2021.
Copy Citation
Schistosomiasis is a common neglected tropical disease of impoverished people and livestock in many developing countries in tropical Africa, the Middle East, Asia, and Latin America.
schistosomiasis
Schistosoma haematobium
Schistosoma mansoni
sub-Saharan Africa
Africa
1. Life Cycle of Schistosoma sp.
Schistosomes have a complex life cycle involving both intermediate gastropod hosts and a definitive mammalian host (Figure 1). Unlike other trematode species, Schistosoma spp. are dioecious (separate male and female worms) which undergo sexual reproduction in the mammalian definitive host. Schistosome eggs are produced and excreted into the environement via the faeces (S. mansoni) or urine (S. haematobium). Miracidia are released when the eggs come in contact with water and infect the snail host. There, miracidia develops into mother sporocysts and undergo asexual reproduction to produce daughter sporocysts which produce cecariae. Infected snails shed cercariae into the water and upon locating a suitable definitive host, penetrate the skin, transform into schistosomula and migrate through the circulatory system to the lungs, heart and liver where they mature into adult worms (Figure 1). The adult worms then exit the liver and pair up to mate in the mesenteric vessels of the bowel (S. mansoni, S. intercalatum) or bladder (S. haematobium).
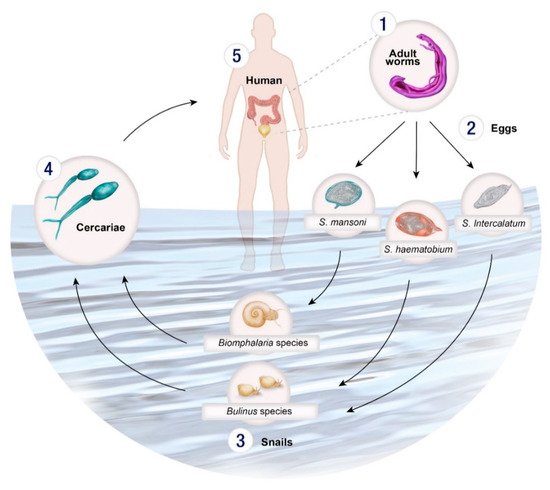
Figure 1. Life cycle of Schistosoma spp. (1) Male and female adult worms reproduce sexually in the venous system of the bladder (S. haematobium) or the bowel (S. mansoni, S. intercalatum, S. guineensis) producing eggs which are excreted in urine or via faeces, or are retained in body tissues, such as the liver. (2) The eggs hatch upon contact with water releasing miracidia which then penetrate a specific intermediate molluscan host. (3) Within the snail host, the miracidia develop into sporocysts and asexually reproduce daughter sporocysts which in turn produce cercariae. (4) The cercariae emerge from the snail and directly penetrate the skin of the (5) human host and transform into schistosomula. The schistosomula migrate via the circulatory system to the lungs and then the heart before arriving in the liver where they mature. Once mature the adult worms exit the liver and pair up to mate in the mesenteric vessels of the bowel bowel (S. mansoni, S. intercalatum, S. guineensis) or bladder (S. haematobium).
A proportion of the eggs are carried by the bloodstream to other areas of the body where they can become lodged in tissues, and trigger an inflammatory response, causing acute or chronic disease. (Figure 1). Schistosomes have an average life span of 3–10 years but can live up to 40 years in their human hosts in permanent copulation [1][2].
The control and elimination of schistosomiasis requires interruption of a complex pathway of transmission governed by the interplay of humans, intermediate host snails and human–water contact patterns. The snail hosts are crucial for determining the range of schistosomiasis and are responsible for the focal nature of the disease (i.e., highly variable infection prevalence and intensity even within a small area such as from one village to another). Two genera of snails (Bulinus and Biomphalaria) are responsible for the distribution of schistosomes in Africa. These molluscs can be an important focus of control efforts involving environmental modification (e.g., digging water drainage ditches or tunnels to flood and bury the snail habitats to disrupt snail habitats), or through the use of chemicals, such as niclosamide [3][4]. Concerning, however, are the detrimental impacts that such chemicals can have on the environment including the general pollution they cause and being toxic to larger animals such as fish [5][6].
2. Clinical Presentation of Schistosomiasis in Africa
Schistosome infection has three distinct disease phases beginning with an initial dermatitis reaction following skin penetration by the cercariae resulting in an allergic inflammatory maculopapular lesion [7]. The infection may then proceed to a symptomatic acute schistosomiasis stage also known as Katayama fever or Katayama syndrome. Acute schistosomiasis is rarely reported in individuals living in areas endemic for S. mansoni or S. haematobium. One possible explanation for this being that in-utero sensitisation might decrease the severity of common symptoms of Katayama syndrome in chronically exposed individuals resulting in lowered immune responsiveness to schistosome antigens in infants born to infected mothers; it may also be equally likely that cases from endemic areas are simply unrecognised or under-reported [7][8]. The most common symptoms of acute schistosomiasis include prolonged fever, weakness, vomiting, nausea, diarrhoea, malaise and rapid weight loss [9][10]. The third and final disease stage, chronic schistosomiasis, occurs when eggs are deposited in various body tissues, commonly affecting the liver, bladder and urogenital system, and less commonly in the central nervous system [9][11]. Adult worms can avoid detection by the immune system by camouflaging their outer layer with host antigens and tegmental shedding and are able to reside for long periods in their hosts [12]. In contrast, schistosome eggs are fully exposed to the immune system, and this results in the formation of granulomatous and fibrotic lesions around the eggs in various tissues resulting in necrosis, ulceration and bleeding that can have long-term detrimental effects [9][10][13][14]. Chronic schistosomiasis is associated with hepatosplenomegaly, portal fibrosis and, in the case of S. haematobium, haematuria (blood in urine), ureter fibrosis, and squamous cell carcinoma of the urinary bladder [9].
S. mansoni is the leading cause of intestinal schistosomiasis in Africa. Around 50% of eggs deposited by adult worms are retained in the liver, causing chronic disease [15]. Pathogenesis due to S. intercalatum is less severe than S. mansoni and S. haematobium and most infected patients, particularly children, do not show symptoms of the disease [16].
2.1. Female Genital Schistosomiasis
Female genital schistosomiasis (FGS) is characterised by the presence of S. haematobium eggs in the vagina and cervix and affects up to 20 million women in sub-Saharan Africa and the Middle East [17][18]. The eggs penetrate the urogenital system, causing uterine enlargement, menstrual disorders, cervicitis and infertility [19]. Schistosomiasis in pregnant women presents with symptoms ranging from anaemia during pregnancy to newborns with low birth weight, and increased infant and maternal mortality rates [20][21][22][23]. Urogenital schistosomiasis has also been linked with increased risk of HIV infection in women resulting from the production of genital mucosal lesions surrounding the eggs [24][25]. The immune response caused by S. haematobium infection leads to chronic inflammatory granulomatous lesions, genital epithelial bleeding and sandy patches in the cervix and vagina that, if left untreated, can become an easy entry point for the HIV virus, as well as leading to infertility [1][24][26][27][28][29]. Concurrent infection with HIV and S. haematobium leads to increased disease pathology while HIV infection may lead to an increased chance of contracting schistosomiasis [24][30][31]. Schistosomiasis has also been suspected of increasing disease progression and death in HIV patients by increasing the HIV RNA load in the blood plasma [32][33]. More than 70% of HIV infections worldwide occur in sub-Saharan Africa and thus HIV remains a major health challenge in Africa and an important confounding factor for schistosome infection. Diagnosis of FGS can be challenging due to different transformation stages of the S. haematobium parasite and immune response in the affected tissues. In cases where the infected patient is asymptomatic, the disease may be mistaken for sexually transmitted diseases (STDs) or cervical cancer [34][18][35]. Stigma against STDs can lead to misdiagnosis, or reluctance of young women to present to a doctor when experiencing clinical symptoms of FGS.
2.2. Primary and Secondary Infertility in S. haematobium Infections
Infertility is the inability to become pregnant after regular and unprotected sexual intercourse for more than one year [36]. It can be diagnosed as either primary–where the woman has never conceived, or secondary-when the woman has experienced previous labour. Suspected cases of infertility resulting from S. haematobium infection in the genital tracts have been widely reported in Africa [37][38][39][40][41][42][43]. While the presence of an adult worm infection in the urogenital system is generally asymptomatic, the deposition of S. haematobium eggs along the urogenital tract, including the cervix and vagina, trigger a hypersensitive immune response, causing scarring and fibrosis in the genital tract, ovaries and fallopian tubes. The eggs may appear as papillary white lesions [37][38], causing thickening, nodular lesions and adhesions which eventually lead to obstruction and blockage of the fallopian tubes. The resulting fibrosis and blockage is suspected to lead to infertility. A case study in Nigeria reported the inability of a woman to get pregnant with her second child despite having a regular menstrual cycle; tuboplasty (surgery undertaken to restore the functionality and integrity of the fallopian tubes) revealed lesions and blockage of the patient’s fallopian tubes due to the presence of S. haematobium eggs [38]. Urogenital schistosomiasis has also been linked to ectopic pregnancies as a result of blockage of the fallopian tubes [39][40]. Patients can recover with administration of PZQ if the infection is treated sufficiently early [38][39].
2.3. Male Genital Schistosomiasis
Male genital schistosomiasis (MGS) was first reported in 1911 [44] and is described as the presence of schistosome (S. haematobium) eggs in the male genital organs and fluids. The awareness and severity of this disease especially in endemic areas is often overlooked and underreported as it can be misdiagnosed as a sexually transmitted infection (STI) [31][45][46]. Symptoms of this disease include painful urination, painful ejaculation, irregular ejaculations, hermatospermia, prostatitis, epididymitis (inflammation of the epididymitis at the back of the testicles), which could mimic tuberculosis and associated funiculitis, erectile dysfunction, enlarged genital organs and infertility [31][47][48][49].
2.4. Bladder Cancer in S. haematobium Infections
Globally, 275,000 people are diagnosed with bladder cancer annually and 108,000 people die of the disease. Bladder cancer caused by translational cell carcinoma (TCC) occurs in industrialised and developing countries not endemic for urogenital schistosomiasis, while bladder cancer caused by squamous cell carcinoma (SCC) is a long-term sequela of chronic infection and occurs in many parts of Africa plagued with urogenital schistosomiasis [50][51]. Bladder cancer is one of the foremost serious complications of chronic S. haematobium infection and it is estimated that the schistosome-associated bladder cancer incidence is 3–4 cases per 100,000 infections [52].
TCC arises from the transitional epithelium lining of the bladder and presents in its early stage as a painless haematuria. In contrast, a squamous hyperplasia (usually not present in a normal urothelium which is a highly specialized epithelium lining the lower urinary tract) gives rise to SCC due to injuries caused by the immunological responses to deposited eggs in the bladder [53]. This is followed by painful haematuria, chronic inflammation and necroturia [53]. SCC often presents with symptoms only at a late stage and can be challenging to treat by surgery or with chemotherapy.
A study in Egypt reported that 82% of patients with SCC had S. haematobium eggs lodged in their bladder wall and infected individuals had the tendency to develop cancer at a younger age than uninfected individuals [54]. Kitinya et al. [55] reported that of 172 individuals with bladder cancer in Egypt over a nine year period (1971–1980), 72% were SCC cases. Similarly, a study in Northern Tanzania reported 46% of SCC patients had S. haematobium eggs in the tumour tissues [55]. Another study from Angola, situated in the western part of Western Africa, reported a >70% (215/300) S. haematobium prevalence with 3 of the infected patients having calcified bladders and one SCC case was recorded [56]. A decrease in the prevalence of urogenital schistosomiasis in Egypt has seen a decline in the SCC and an increase in the median age of infected individuals with bladder cancer [57].
The mechanisms associated with S. haematobium and the development of SCC are largely unknown although the carcinogenic process appears to be closely related to tissue inflammation [58]. Botelho et al. [59] described the relationship between S. haematobium and cancer by exposing normal epithelial cells (Chinese Hamster Ovary (CHO) cells) in culture with S. haematobium total antigen and observed increased cell proliferation, decreased apoptosis, migration, invasion and tumourigenesis [59][60]. This suggested that S. haematobium has the ability to induce the formation of cancer-like cells [59]. Furthermore, S. haematobium exposed cells injected into mice with no immune system resulted in the development of tumours similar to those found in bladder cancer [61].
3. Treatment and Control
Preventive chemotherapy (PC) through MDA with PZQ is the cornerstone of the treatment and control of schistosomiasis in endemic regions of Africa. PZQ has been proven a safe and effective oral drug active against adult worms of all Schistosoma species [62][63], although its mechanism of action is still not fully understood. However, it cannot be used for chemoprophylaxis due to its short half-life, and it is ineffective against migrating schistosomula [64]. Corticosteroids, in addition to PZQ, are effective as an adjuvant when patients present with Katayama syndrome, usually within two months of exposure to cercariae [8][65] to suppress immunological reactions and prevent acute disease. Other drugs that proved effective for the treatment of schistosomiasis include oxamniquine for S. mansoni, and metrifonate for S. haematobium [9][10] but these are either no longer readily available or have been withdrawn due to unacceptable toxicity. Co-infections of Schistosoma spp. and soil-transmitted helminths (STH) are common in many endemic areas in Africa, and as such, combination PC with both PZQ and albendazole is recommended by WHO [66] particularly for SAC and other high risk groups.
PZQ is given to SAC between the ages of 5 and 15 years who have the highest infection rates and are more readily reached through school programs. PC is usually carried out by firstly assessing the prevalence of the disease which determines the frequency of treatment in that area [67]. For example, areas showing disease prevalence with 50% or more usually should receive a single annual treatment while areas with 10% prevalence will receive triennial treatment [67]. As of 2019, 57.1% (61.8 million) SAC who require treatment have received PZQ [68].
Re-infection remains a major challenge to control efforts in Africa due to a number of factors including: high levels of infection prevalence and intensity, poor or non-compliance of PZQ treatment and low coverage, recontacting contaminated water as a result of daily activities and seasonal factors. Hence, a multifaceted intervention approach will be needed to move from wide-spread control to elimination including: snail control; treatment; effective risk mapping and epidemiological surveillance; accurate diagnostics; improved access to clean water, sanitation and hygiene (WASH); and public health education to bring about behavioural changes to prevent infection and reinfection [69][70][71][72][73]. These integrated approaches, together with the development and deployment of future anti-schistosome vaccines effective in humans (albeit no schistosomiasis vaccine has yet been accepted for public use) will contribute greatly to reducing and interrupting transmission in endemic areas leading to eventual elimination [74][75]. Another challenge to be faced is climate change and the resultant elevated temperatures which may increase the geographical distribution of the parasite through expansion of suitable environments for snails into higher altitudes and into further locations in Africa currently unaffected by the disease. While most studies focus on increasing temperature, it has been shown that snails and schistosomes within their hosts survive during the winter months and produce viable cercariae that complete their life cycles when optimal temperature is reached [76]. Furthermore, snails from temperate region demonstrate better resistance to harsh winter conditions than tropical snails [76].
4. Diagnosis
There are a number of approaches used for schistosomiasis diagnosis and schistosome detection. The standard method used in Africa is the detection of eggs in urine or stool by microscopy [77][78], although a number of immunological [79][80][81][82][83][84][85] and molecular [86][87][88][89][90][91][92][93] diagnostic assays have been developed with some deployed in Africa. Polymerase chain reaction (PCR) and quantitative PCR (qPCR)-based molecular methods are now increasingly being employed for diagnosis in high-resource settings globally but they are expensive, take time, require a significant laboratory infrastructure and training which hampers their current use in low socio-economic endemic field settings. Isothermal amplification detection (IAD) methods can overcome some of these obstacles including the limitations of costly thermal cyclers required for the PCR-based detection of parasite DNA in stool or urine. IAD assays work similar to that of conventional PCR in that they utilise DNA or RNA polymerase in the extension of target-specific primers. However, isothermal amplification facilitates amplification without the repeated cycles of denaturation and annealing required for PCR. The most established IAD method for schistosome detection is loop-mediated isothermal amplification (LAMP) [94][95][96][97][98][99][100][101][102][103]. Other isothermal methods for parasite detection include helicase-dependent isothermal amplification (HDA) [104], recombinase polymerase amplification (RPA) [105] and nucleic acid sequence-based amplification (NASBA) [106], but only RPA has been applied in the detection of Schistosoma spp. [107][108][109][110].
As indicated earlier, the microscopic detection of eggs in urine (S. haematobium) and faeces (S. mansoni) is the most commonly used method for the diagnosis of schistosomiasis. The Kato-Katz (KK) method is used to detect S. mansoni eggs in faeces, while urine microscopy, preceeded by urine filtration, is used to identify S. haematobium infections [77][78]. S. haematobium eggs were identified in the semen of fishermen as part of a cross-sectional study along the southwestern shoreline of Lake Malawi in Sub-Saharan Africa, suggestive of high lodgement of eggs in the reproductive organs of men [49]. The precise origin of eggs found in semen is unresolved but they may have originated in the bladder, carried with drops of urine through the urethra and released with the semen [31]. Eggs of S. haematobium can be readily detected by light microscopy, and cell-free circulating schistosome DNA has been detected in semen several weeks after a single dose of PZQ [111].
In addition to egg detection, active infections can be detected from worm-derived circulating anodic antigens (CAAs) and circulating cathodic antigens (CCAs) in serum and urine using enzyme-linked immunosorbent assay (ELISA) or monoclonal- antibody-based lateral flow tests [112][113]. These detection methods have the ability to detect infection before the worms begin producing eggs, [114][115][116]. However, they do not discriminate between past, active or re-infections, especially in endemic areas where patients can remain seropositive several years after treatment [114][117].
Haematuria and proteinuria reagent strip testing can also be used as indirect diagnostic methods for S. haematobium infection [118]. Strip testing has previously been shown to provide sensitivity and specificity levels of 75% and 87%, respectively, for detection of S. haematobium [119] and has been suggested as an alternative form of diagnostic to the usual urine microscopy. Strip testing may be useful in sub-Saharan Africa due to its substantially higher sensitivity than microscopic methods and ease of storage of the strips [112]. However, the strip tests also detect haematuria not associated with S. haematobium infection, and the method exhibits poor sensitivity in detecting egg-positive urine post-treatment and low-intensity infections [112].
4.1. Environmental Monitoring
As indicated earlier, schistosomiasis is a highly focal disease with transmission being highly dependent on the presence of fresh water and appropriate snail intermediate hosts, as well as water contact activities by humans who become infected. The risk of infection is dependent on seasonal changes in snail populations, water levels, infection rates and cercarial output. Flooding events may also cause temporarily higher rates of infection in human communities. Information on snail hosts and the distribution of cercariae are important tools in the control and elimination of schistosomiasis [58][109][120].
Molecular xenomonitoring is a useful disease surveillance tool for the detection of infection rates in field population of snails and could be useful in identifying infection risk areas to help guide intervention measures for schistosomiasis control and elimination [121][122][123][124]. There are a number of methods used for xenomonitoring including sentinel mice which have been used to identify transmission sites in natural water bodies in China; however, this process is time-consuming and expensive [122][125]. Morphological identification of miracidia and cercariae collected from water sources can be inaccurate due to disintegration of the larvae and misidentification of human and non-human cercariae that co-exist in most endemic areas; the latter issue is also applicable to identifying cercariae from infected snails [126] (Figure 1).
PCR-based detection methods have been developed that detect cercariae in water samples and schistosome species in snail intermediate snail hosts, and these have proved useful in identifying and monitoring schistosomiasis transmission areas in Africa [122][127]. An example is the DraI PCR, which has been used to monitor snail transmission S. haematobium cercariae in Morocco [128]. The DraI ribosomal sequence is specific to the S. haematobium group and can detect low amounts of DNA due to its abundant sequences in the S. haematobium genome [129][130][131][132]. Another example is a two-step multiplex PCR approach which first identifies Schistosoma infected snails, followed by species-specific identification using internal transcribed spacer (ITS) rRNA primers [133][134].
References
- Colley, D.G.; Bustinduy, A.L.; Secor, W.E.; King, C.H. Human schistosomiasis. Lancet 2014, 383, 2253–2264.
- Chabasse, D.; Bertrand, G.; Leroux, J.P.; Gauthey, N.; Hocquet, P. Developmental bilharziasis caused by Schistosoma mansoni discovered 37 years after infestation. Bull. Soc. Pathol. Exot. Fil. 1985, 78, 643–647.
- McCullough, F.S.; Gayral, P.; Duncan, J.; Christie, J.D. Molluscicides in schistosomiasis control. Bull. World Health Organ. 1980, 58, 681–689.
- WHO. Field Use of Molluscicides in Schistosomiasis Control Programmes: An Operational Manual for Programme Managers; WHO: Geneva, Swizterland, 2017.
- Ekabo, O.A.; Farnsworth, N.R.; Henderson, T.O.; Mao, G.; Mukherjee, R. Antifungal and molluscicidal saponins from Serjania salzmanniana. J. Nat. Prod. 1996, 59, 431–435.
- Rocha-Filho, C.A.; Albuquerque, L.P.; Silva, L.R.; Silva, P.C.; Coelho, L.C.; Navarro, D.M.; Albuquerque, M.C.; Melo, A.M.; Napoleão, T.H.; Pontual, E.V.; et al. Assessment aof toxicity of Moringa oleifera flower extract to Biomphalaria glabrata, Schistosoma mansoni and Artemia salina. Chemosphere 2015, 132, 188–192.
- Gray, D.; Ross, A.; Li, Y.-S.; McManus, D. Diagnosis and management of schistosomiasis. Br. Med. J. 2011, 342, 1138.
- Ross, A.G.; Vickers, D.; Olds, G.R.; Shah, S.M.; McManus, D.P. Katayama syndrome. Lancet Infect. Dis. 2007, 7, 218–224.
- Gryseels, B.; Polman, K.; Clerinx, J.; Kestens, L. Human schistosomiasis. Lancet 2006, 368, 1106–1118.
- Ross, A.G.P.; Bartley, P.B.; Sleigh, A.C.; Olds, G.R.; Li, Y.; Williams, G.M.; McManus, D.P. Schistosomiasis. N. Engl. J. Med. 2002, 346, 1212–1220.
- Ross, A.G.; McManus, D.P.; Farrar, J.; Hunstman, R.J.; Gray, D.J.; Li, Y.S. Neuroschistosomiasis. J. Neurol. 2012, 259, 22–32.
- Gunn, A.; Pitt, S.J. Parasitology an Integrated Approach; John Wiley & Sons: Chichester, UK, 2012.
- Wynn, T.A.; Thompson, R.W.; Cheever, A.W.; Mentink-Kane, M.M. Immunopathogenesis of schistosomiasis. Immunol Rev. 2004, 201, 156–167.
- Burke, M.L.; Jones, M.K.; Gobert, G.N.; Li, Y.S.; Ellis, M.K.; McManus, D.P. Immunopathogenesis of human schistosomiasis. Parasite Immunol. 2009, 31, 163–176.
- Costain, A.H.; MacDonald, A.S.; Smits, H.H. Schistosome egg migration: Mechanisms, pathogenesis and host immune responses. Front. Immunol. 2018, 9, 3042.
- Almeda, J.; Corachan, M.; Sousa, A.; Ascaso, C.; Carvalho, J.M.; Rollinson, D.; Southgate, V.R. Schistosomiasis in the Republic of São Tomé and Principe: Human studies. Trans. R. Soc. Trop. Med. Hyg. 1994, 88, 406–409.
- Poggensee, G.; Feldmeier, H.; Krantz, I. Schistosomiasis of the female genital tract: Public health aspects. Parasitol. Today 1999, 15, 378–381.
- Christinet, V.; Lazdins-Helds, J.K.; Stothard, J.R.; Reinhard-Rupp, J. Female genital schistosomiasis (FGS): From case reports to a call for concerted action against this neglected gynaecological disease. Int. J. Parasitol. 2016, 46, 395–404.
- Nour, N.M. Schistosomiasis: Health effects on women. Rev. Obstet. Gynecol. 2010, 3, 28–32.
- Friedman, J.F.; Mital, P.; Kanzaria, H.K.; Olds, G.R.; Kurtis, J.D. Schistosomiasis and pregnancy. Trends Parasitol. 2007, 23, 159–164.
- Helling-Giese, G.; Kjetland, E.F.; Gundersen, S.G.; Poggensee, G.; Richter, J.; Krantz, I.; Feldmeier, H. Schistosomiasis in women: Manifestations in the upper reproductive tract. Acta Trop. 1996, 62, 225–238.
- Ajanga, A.; Lwambo, N.J.; Blair, L.; Nyandindi, U.; Fenwick, A.; Brooker, S. Schistosoma mansoni in pregnancy and associations with anaemia in Northwest Tanzania. Trans. R. Soc. Trop. Med. Hyg. 2006, 100, 59–63.
- Ben-Chetrit, E.; Lachish, T.; Mørch, K.; Atias, D.; Maguire, C.; Schwartz, E. Schistosomiasis in pregnant travelers: A case series. J. Travel Med. 2015, 22, 94–98.
- Downs, J.A.; Dupnik, K.M.; van Dam, G.J.; Urassa, M.; Lutonja, P.; Kornelis, D.; de Dood, C.J.; Hoekstra, P.; Kanjala, C.; Isingo, R.; et al. Effects of schistosomiasis on susceptibility to HIV-1 infection and HIV-1 viral load at HIV-1 seroconversion: A nested case-control study. PLoS Negl. Trop. Dis. 2017, 11, e0005968.
- Kjetland, E.F.; Leutscher, P.D.C.; Ndhlovu, P.D. A review of female genital schistosomiasis. Trends Parasitol. 2012, 28, 58–65.
- Kjetland, E.F.; Ndhlovu, P.D.; Gomo, E.; Mduluza, T.; Midzi, N.; Gwanzura, L.; Mason, P.R.; Sandvik, L.; Friis, H.; Gundersen, S.G. Association between genital schistosomiasis and HIV in rural Zimbabwean women. Aids 2006, 20, 593–600.
- Olusegun, A.F.; Ehis, O.C.; Richard, O. Proportion of urinary schistosomiasis among HIV-infected subjects in Benin City, Nigeria. Oman Med. J. 2011, 26, 175–177.
- Downs, J.A.; Mguta, C.; Kaatano, G.M.; Mitchell, K.B.; Bang, H.; Simplice, H.; Kalluvya, S.E.; Changalucha, J.M.; Johnson, W.D., Jr.; Fitzgerald, D.W. Urogenital schistosomiasis in women of reproductive age in Tanzania’s Lake Victoria region. Am. J. Trop. Med. Hyg. 2011, 84, 364–369.
- Feldmeier, H.; Krantz, I.; Poggensee, G. Female genital schistosomiasis as a risk-factor for the transmission of HIV. Int. J. STD AIDS 1994, 5, 368–372.
- Ndhlovu, P.D.; Mduluza, T.; Kjetland, E.F.; Midzi, N.; Nyanga, L.; Gundersen, S.G.; Friis, H.; Gomo, E. Prevalence of urinary schistosomiasis and HIV in females living in a rural community of Zimbabwe: Does age matter? Trans. R. Soc. Trop Med. Hyg. 2007, 101, 433–438.
- Leutscher, P.; Ramarokoto, C.E.; Reimert, C.; Feldmeier, H.; Esterre, P.; Vennervald, B.J. Community-based study of genital schistosomiasis in men from Madagascar. Lancet 2000, 355, 117–118.
- Wall, K.M.; Kilembe, W.; Vwalika, B.; Dinh, C.; Livingston, P.; Lee, Y.M.; Lakhi, S.; Boeras, D.; Naw, H.K.; Brill, I.; et al. Schistosomiasis is associated with incident HIV transmission and death in Zambia. PLoS Negl. Trop. Dis. 2018, 12, e0006902.
- Mbabazi, P.S.; Andan, O.; Fitzgerald, D.W.; Chitsulo, L.; Engels, D.; Downs, J.A. Examining the relationship between urogenital schistosomiasis and HIV infection. PLoS Negl. Trop. Dis. 2011, 5, e1396.
- Lothe, A.; Zulu, N.; Øyhus, A.O.; Kjetland, E.F.; Taylor, M. Treating schistosomiasis among South African high school pupils in an endemic area, a qualitative study. BMC Infect. Dis. 2018, 18, 239.
- Kjetland, E.F.; Hegertun, I.E.; Baay, M.F.; Onsrud, M.; Ndhlovu, P.D.; Taylor, M. Genital schistosomiasis and its unacknowledged role on HIV transmission in the STD intervention studies. Int. J. STD AIDS 2014, 25, 705–715.
- Zegers-Hochschild, F.; Adamson, G.D.; de Mouzon, J.; Ishihara, O.; Mansour, R.; Nygren, K.; Sullivan, E.; Vanderpoel, S. International Committee for Monitoring Assisted Reproductive Technology (ICMART) and the World Health Organization (WHO) revised glossary of ART terminology, 2009. Fertil. Steril. 2009, 92, 1520–1524.
- Bentefouet, T.L.; Thiam, M.; Gaye, A.M.; El Wardi, A.; Gueye, L.; Cisse, M.L. Case report of tubo-ovarian Bilharziasis presented with pelvic pain and secondary infertility. Case Rep. Images Obstet. Gynecol. 2017, 3, 19–22.
- Ogunniyi, S.O.; Nganwuchu, A.M.; Adenle, M.A.; Dare, F.O. Pregnancy following infertility due to pelvic schistosomiasis—A case report. West. Afr. J. Med. 1994, 13, 132–133.
- Aminu, M.B.; Abdullahi, K.; Dattijo, L.M. Tubal ectopic gestation associated with genital schistosomiasis: A case report. Afr. J. Reprod. Health La Rev. Afr. de la St. Reprod. 2014, 18, 144–146.
- Okonofua, F.E.; Ojo, O.S.; Odunsi, O.A.; Odesanmi, W.O. Ectopic pregnancy associated with tubal schistosomiasis in a Nigerian woman. Int. J. Gynaecol. Obstet. 1990, 32, 281–284.
- Woodall, P.A.; Kramer, M.R. Schistosomiasis and infertility in East Africa. Am. J. Trop. Med. Hyg. 2018, 98, 1137–1144.
- Kini, S.; Dayoub, N.; Raja, A.; Pickering, S.; Thong, J. Schistosomiasis-induced male infertility. BMJ Case Rep. 2009, 2009.
- Adisa, J.; Egbujo, E.M.; Yahaya, B.A.; Echejoh, G. Primary infertility associated with Schistosoma mansoni: A case report from the Jos plateau, North-Central Nigeria. Afr. Health Sci. 2012, 12, 563–565.
- Madden, F. Two rare manifestations of bilharziosis. Lancet 1911, 178, 754–755.
- Leutscher, P.D.; Ramarokoto, C.E.; Hoffmann, S.; Jensen, J.S.; Ramaniraka, V.; Randrianasolo, B.; Raharisolo, C.; Migliani, R.; Christensen, N. Coexistence of urogenital schistosomiasis and sexually transmitted infection in women and men living in an area where Schistosoma haematobium is endemic. Clin. Infect. Dis. 2008, 47, 775–782.
- Kayuni, S.; Lampiao, F.; Makaula, P.; Juziwelo, L.; Lacourse, E.J.; Reinhard-Rupp, J.; Leutscher, P.D.C.; Stothard, J.R. A systematic review with epidemiological update of male genital schistosomiasis (MGS): A call for integrated case management across the health system in sub-Saharan Africa. Parasite Epidemiol. Control. 2018, 4, e00077.
- Gelfand, M.; Ross, C.M.; Blair, D.M.; Castle, W.M.; Weber, M.C. Schistosomiasis of the male pelvic organs. Severity of infection as determined by digestion of tissue and histologic methods in 300 cadavers. Am. J. Trop. Med. Hyg. 1970, 19, 779–784.
- Vilana, R.; Corachan, M.; Gascon, J.; Valls, E.; Bru, C. Schistosomiasis of the male genital tract: Transrectal sonographic findings. J. Urol. 1997, 158, 1491–1493.
- Kayuni, S.A.; Lacourse, E.J.; Makaula, P.; Lampiao, F.; Juziwelo, L.; Fawcett, J.; Shaw, A.; Alharbi, M.H.; Verweij, J.J.; Stothard, J.R. Case report: Highlighting male genital schistosomiasis (MGS) in fishermen from the southwestern shoreline of Lake Malawi, Mangochi District. Am. J. Trop. Med. Hyg. 2019, 101, 1331.
- Abol-Enein, H. Infection: Is it a cause of bladder cancer? Scand. J. Urol. Nephrol. Suppl. 2008, 79–84.
- Heyns, C.F.; van der Merwe, A. Bladder cancer in Africa. Can. J. Urol. 2008, 15, 3899–3908.
- Shiff, C.; Veltri, R.; Naples, J.; Quartey, J.; Otchere, J.; Anyan, W.; Marlow, C.; Wiredu, E.; Adjei, A.; Brakohiapa, E.; et al. Ultrasound verification of bladder damage is associated with known biomarkers of bladder cancer in adults chronically infected with Schistosoma haematobium in Ghana. Trans. R. Soc. Trop. Med. Hyg. 2006, 100, 847–854.
- Michaud, D.S. Chronic inflammation and bladder cancer. Urol. Oncol. Semin. Orig. Investig. 2007, 25, 260–268.
- El-Bolkainy, M.N.; Mokhtar, N.M.; Ghoneim, M.A.; Hussein, M.H. The impact of schistosomiasis on the pathology of bladder carcinoma. Cancer 1981, 48, 2643–2648.
- Kitinya, J.N.; Laurèn, P.A.; Eshleman, L.J.; Paljärvi, L.; Tanaka, K. The incidence of squamous and transitional cell carcinomas of the urinary bladder in northern Tanzania in areas of high and low levels of endemic Schistosoma haematobium infection. Trans. R. Soc. Trop. Med. Hyg. 1986, 80, 935–939.
- Botelho, M.C.; Figueiredo, J.; Alves, H. Bladder cancer and urinary schistosomiasis in Angola. J. Nephrol. Res. 2015, 1, 22–24.
- Gouda, I.; Mokhtar, N.; Bilal, D.; El-Bolkainy, T.; El-Bolkainy, N.M. Bilharziasis and bladder cancer: A time trend analysis of 9843 patients. J. Egypt. Natl. Cancer Inst. 2007, 19, 158–162.
- McManus, D.P.; Dunne, D.W.; Sacko, M.; Utzinger, J.; Vennervald, B.J.; Zhou, X.N. Schistosomiasis. Nat. Rev. Dis. Primers 2018, 4, 13.
- Botelho, M.C.; Machado, J.; Da Costa, J. Schistosoma haematobium total antigen induces increased proliferation, migration and invasion, and decreases apoptosis of normal epithelial cells. Virulence 2010, 1, 84–87.
- Vennervald, B.J.; Polman, K. Helminths and malignancy. Parasite Immunol. 2009, 31, 686–696.
- Botelho, M.; Oliveira, P.; Gomes, J.; Gartner, F.; Lopes, C.; da Costa, J.M.C.; Machado, J.C. Tumourigenic effect of Schistosoma haematobium total antigen in mammalian cells. Int. J. Exp. Pathol. 2009, 90, 448–453.
- Doenhoff, M.J.; Cioli, D.; Utzinger, J. Praziquantel: Mechanisms of action, resistance and new derivatives for schistosomiasis. Curr. Opin. Infect. Dis. 2008, 21, 659–667.
- Cioli, D.; Pica-Mattoccia, L. Praziquantel. Parasitol Res. 2003, 90 (Suppl. S1), S3–S9.
- Utzinger, J.; Xiao, S.H.; Tanner, M.; Keiser, J. Artemisinins for schistosomiasis and beyond. Curr. Opin. Investig. Drugs 2007, 8, 105–116.
- Harries, A.D.; Cook, G.C. Acute schistosomiasis (Katayama fever): Clinical deterioration after chemotherapy. J. Infect. 1987, 14, 159–161.
- WHO. Preventive Chemotherapy in Human Helminthiasis: Coordinated Use of Anthelminthic Drugs in Control Interventions: A Manual for Health Professionals and Programme Managers. Schistosomiasis, A Major Public Health Problem. 2015. Available online: (accessed on 22 March 2019).
- WHO. Helminth Control in School Age Children A Guide for Control Managers Second Edition; World Heal Organisation: Geneva, Switzerland, 2011.
- WHO. Summary of global update on implementation of preventive chemotherapy against neglected tropical diseases in 2019/Resume des donnees mondiales actualisees sur la mise en oeuvre de la chimioprevention contre les maladies tropicales negligees en 2019. Wkly. Epidemiol. Rec. 2020, 95, 469.
- Cioli, D.; Pica-Mattoccia, L.; Basso, A.; Guidi, A. Schistosomiasis control: Praziquantel forever? Mol. Biochem. Parasitol. 2014, 195, 23–29.
- Prüss-Üstün, A.; Bos, R.; Gore, F.; Bartram, J. Safer Water, Better Health: Costs, Benefits and Sustainability of Interventions to Protect and Promote Health; World Health Organization: Geneva, Swizterland, 2008; p. 60.
- WHO. Neglected Tropical Diseases, Hidden Successes, Emerging Opportunities; World Health Organization: Geneva, Swizterland; 52p.
- WHO. Regional Strategic Plan for Neglected Tropical Diseases in the African Region 2014–2020; World Health Organization Regional Office for Africa: Brazzaville, Cango, 2013.
- Campbell, S.J.; Savage, G.B.; Gray, D.J.; Atkinson, J.A.; Soares Magalhães, R.J.; Nery, S.V.; McCarthy, J.S.; Velleman, Y.; Wicken, J.H.; Traub, R.J.; et al. Water, Sanitation, and Hygiene (WASH): A critical component for sustainable soil-transmitted helminth and schistosomiasis control. PLoS Negl. Trop. Dis. 2014, 8, e2651.
- Alsallaq, R.A.; Gurarie, D.; Ndeffo Mbah, M.; Galvani, A.; King, C. Quantitative assessment of the impact of partially protective anti-schistosomiasis vaccines. PLoS Negl. Trop. Dis. 2017, 11, e0005544.
- Tebeje, B.M.; Harvie, M.; You, H.; Loukas, A.; McManus, D.P. Schistosomiasis vaccines: Where do we stand? Parasites Vectors 2016, 9, 528.
- Mulero, S.; Rey, O.; Arancibia, N.; Mas-Coma, S.; Boissier, J. Persistent establishment of a tropical disease in Europe: The preadaptation of schistosomes to overwinter. Parasites Vectors 2019, 12, 379.
- Dazo, B.C.; Biles, J.E. Two new field techniques for detection and counting of Schistosoma haematobium eggs in urine samples, with an evaluation of both methods. Bull. World Health Organ. 1974, 51, 399–408.
- Teesdale, C.H.; Amin, M.A. A simple thick smear technique for the diagnosis of Schistosoma mansoni infection. Bull. World Health Organ. 1976, 54, 703–705.
- Lunde, M.N.; Ottesen, E.A. Enzyme-linked immunosorbent assay (ELISA) for detecting IgM and IgE antibodies in human schistosomiasis. Am. J. Trop Med. Hyg. 1980, 29, 82–85.
- Wen, L.Y.; Chen, J.H.; Ding, J.Z.; Zhang, J.F.; Lu, S.H.; Yu, L.L.; Shen, L.Y.; Wu, G.L.; Zhou, X.N.; Zheng, J. Evaluation on the applied value of the dot immunogold filtration assay (DIGFA) for rapid detection of anti-Schistosoma japonicum antibody. Acta Trop. 2005, 96, 142–147.
- Sarhan, R.M.; Aminou, H.A.; Saad, G.A.; Ahmed, O.A. Comparative analysis of the diagnostic performance of adult, cercarial and egg antigens assessed by ELISA, in the diagnosis of chronic human Schistosoma mansoni infection. Parasitol Res. 2014, 113, 3467–3476.
- Corstjens, P.L.; De Dood, C.J.; Kornelis, D.; Fat, E.M.; Wilson, R.A.; Kariuki, T.M.; Nyakundi, R.K.; Loverde, P.T.; Abrams, W.R.; Tanke, H.J.; et al. Tools for diagnosis, monitoring and screening of Schistosoma infections utilizing lateral-flow based assays and upconverting phosphor labels. Parasitology 2014, 141, 1841–1855.
- Coulibaly, J.T.; Knopp, S.; N’Guessan, N.A.; Silué, K.D.; Fürst, T.; Lohourignon, L.K.; Brou, J.K.; N’Gbesso, Y.K.; Vounatsou, P.; N’Goran, E.K.; et al. Accuracy of Urine Circulating Cathodic Antigen (CCA) Test for Schistosoma mansoni Diagnosis in Different Settings of Côte d’Ivoire. PLoS Negl. Trop. Dis. 2011, 5, e1384.
- Tchuem Tchuenté, L.-A.; Kueté Fouodo, C.J.; Kamwa Ngassam, R.I.; Sumo, L.; Dongmo Noumedem, C.; Kenfack, C.M.; Gipwe, N.F.; Nana, E.D.; Stothard, J.R.; Rollinson, D. Evaluation of Circulating Cathodic Antigen (CCA) Urine-Tests for Diagnosis of Schistosoma mansoni Infection in Cameroon. PLoS Negl. Trop. Dis. 2012, 6, e1758.
- Abdel-Fattah, M.; Al-Sherbiny, M.; Osman, A.; Charmy, R.; Tsang, V. Improving the detection limit of quantitative diagnosis of anti-S. haematobium antibodies using Falcon Assay Screening Test (FAST) ELISA by developing a new standard curve. Parasitol. Res. 2011, 108, 1457–1463.
- Gobert, G.N.; Chai, M.; Duke, M.; McManus, D.P. Copro-PCR based detection of Schistosoma eggs using mitochondrial DNA markers. Mol. Cell. Probes 2005, 19, 250–254.
- Lier, T.; Simonsen, T.; Haaheim, T.; Hjelmevoll, T.; Vennervald, T.; Johansen, T. Novel real-time PCR for detection of Schistosoma japonicum in stool. Southeast Asian J. Trop. Med. Public Health 2006, 37, 257–264.
- Pontes, L.; Dias-Neto, E.; Rabello, A. Detection by polymerase chain reaction of Schistosoma mansoni DNA in human serum and feces. Am. J. Trop. Med. Hyg. 2002, 66, 157–162.
- Sandoval, N.; Siles-Lucas, M.; Aban, J.L.; Pérez-Arellano, J.L.; Gárate, T.; Muro, A. Schistosoma mansoni: A diagnostic approach to detect acute schistosomiasis infection in a murine model by PCR. Exp. Parasitol. 2006, 114, 84–88.
- Sandoval, N.; Siles-Lucas, M.; Pérez-Arellano, J.L.; Carranza, C.; Puente, S.; López-Abán, J.; Muro, A. A new PCR-based approach for the specific amplification of DNA from different Schistosoma species applicable to human urine samples. Parasitology 2006, 133, 581–587.
- Suzuki, T.; Osada, Y.; Kumagai, T.; Hamada, A.; Okuzawa, E.; Kanazawa, T. Early detection of Schistosoma mansoni infection by touchdown PCR in a mouse model. Parasitol. Int. 2006, 55, 213–218.
- Weerakoon, K.G.; Gordon, C.A.; Williams, G.M.; Cai, P.; Gobert, G.N.; Olveda, R.M.; Ross, A.G.; Olveda, D.U.; McManus, D.P. Droplet digital PCR diagnosis of human schistosomiasis: Parasite cell-free DNA detection in diverse clinical samples. J. Infect. Dis. 2017, 216, 1611–1622.
- Weerakoon, K.G.; Gordon, C.A.; Cai, P.; Gobert, G.N.; Duke, M.; Williams, G.M.; McManus, D.P. A novel duplex ddPCR assay for the diagnosis of schistosomiasis japonica: Proof of concept in an experimental mouse model. Parasitology 2017, 144, 1005–1015.
- Notomi, T.; Okayama, H.; Masubuchi, H.; Yonekawa, T.; Watanabe, K.; Amino, N.; Hase, T. Loop-mediated isothermal amplification of DNA. Nucleic Acids Res. 2000, 28, E63.
- Xu, J.; Guan, Z.-X.; Zhao, B.; Wang, Y.-Y.; Cao, Y.; Zhang, H.-Q.; Zhu, X.-Q.; He, Y.-K.; Xia, C.-M. DNA detection of Schistosoma japonicum: Diagnostic validity of a lamp assay for low-intensity infection and effects of chemotherapy in humans (diagnostic validity of lamp in humans). PLoS Negl. Trop. Dis. 2015, 9, e0003668.
- Qin, Z.-Q.; Xu, J.; Feng, T.; Lv, S.; Qian, Y.-J.; Zhang, L.-J.; Li, Y.-L.; Lv, C.; Bergquist, R.; Li, S.-Z.; et al. Field Evaluation of a Loop-Mediated Isothermal Amplification (LAMP) Platform for the Detection of Schistosoma japonicum Infection in Oncomelania hupensis Snails. Trop. Med. Infect. Dis. 2018, 15, 124.
- Mwangi, I.N.; Agola, E.L.; Mugambi, R.M.; Shiraho, E.A.; Mkoji, G.M. Development and evaluation of a loop-mediated isothermal amplification assay for diagnosis of Schistosoma mansoni infection in faecal samples. J. Parasitol. Res. 2018, 2018, 1267826.
- Kumagai, T.; Furushima-Shimogawara, R.; Ohmae, H.; Wang, T.P.; Lu, S.; Chen, R.; Wen, L.; Ohta, N. Detection of early and single infections of Schistosoma japonicum in the intermediate host snail, Oncomelania hupensis, by PCR and loop-mediated isothermal amplification (LAMP) assay. Am. J. Trop. Med. Hyg. 2010, 83, 542–548.
- Hamburger, J.; Abbasi, I.; Kariuki, C.; Wanjala, A.; Mzungu, E.; Mungai, P.; Muchiri, E.; King, C.H. Evaluation of loop-mediated isothermal amplification suitable for molecular monitoring of schistosome-infected snails in field laboratories. Am. J. Trop. Med. Hyg. 2013, 88, 344–351.
- Gandasegui, J.; Fernández-Soto, P.; Muro, A.; Lopes de Melo, F.; Loyo, R.; de Souza Gomes, C. A field survey using LAMP assay for detection of Schistosoma mansoni in a low-transmission area of schistosomiasis in Umbuzeiro, Brazil: Assessment in human and snail samples. PLoS Negl. Trop. Dis. 2018, 12, e0006314.
- Gandasegui, J.; Fernandez-Soto, P.; Hernandez-Goenaga, J.; Lopez-Aban, J.; Vicente, B.; Muro, A. Biompha-lamp: A new rapid loop-mediated isothermal amplification assay for detecting Schistosoma mansoni in Biomphalaria glabrata snail host. PLoS Negl. Trop. Dis. 2016, 10, e0005225.
- Fernández-Soto, P.; Gandasegui Arahuetes, J.; Sánchez Hernández, A.; López Abán, J.; Vicente Santiago, B.; Muro, A. A Loop-Mediated Isothermal Amplification (LAMP) Assay for Early Detection of Schistosoma mansoni in Stool Samples: A Diagnostic Approach in a Murine Model (LAMP Assay for Early Detection of Schistosoma mansoni in Stool Samples). PLoS Negl. Trop. Dis. 2014, 8, e3126.
- Abbasi, I.; King, C.H.; Muchiri, E.M.; Hamburger, J. Detection of Schistosoma mansoni and Schistosoma haematobium DNA by loop-mediated isothermal amplification: Identification of infected snails from early prepatency. Am. J. Trop. Med. Hyg. 2010, 83, 427–432.
- Vincent, M.; Xu, Y.; Kong, H. Helicase-dependent isothermal DNA amplification. EMBO Rep. 2004, 5, 795–800.
- Piepenburg, O.; Williams, C.H.; Stemple, D.L.; Armes, N.A. DNA detection using recombination proteins. PloS Biol. 2006, 4, 1115.
- Compton, J. Nucleic acid sequence-based amplification. Nature 1991, 350, 91.
- Xing, W.; Yu, X.; Feng, J.; Sun, K.; Fu, W.; Wang, Y.; Zou, M.; Xia, W.; Luo, Z.; He, H.; et al. Field evaluation of a recombinase polymerase amplification assay for the diagnosis of Schistosoma japonicum infection in Hunan province of China. BMC Infect. Dis. 2017, 17, 164.
- Sun, K.; Xing, W.; Yu, X.; Fu, W.; Wang, Y.; Zou, M.; Luo, Z.; Xu, D. Recombinase polymerase amplification combined with a lateral flow dipstick for rapid and visual detection of Schistosoma japonicum. Parasit Vectors 2016, 9, 476.
- Rostron, P.; Pennance, T.; Bakar, F.; Rollinson, D.; Knopp, S.; Allan, F.; Kabole, F.; Ali, S.M.; Ame, S.M.; Webster, B.L. Development of a recombinase polymerase amplification (RPA) fluorescence assay for the detection of Schistosoma haematobium. Parasites Vectors 2019, 12, 514.
- Poulton, K.; Webster, B. Development of a lateral flow recombinase polymerase assay for the diagnosis of Schistosoma mansoni infections. Anal. Biochem. 2018, 546, 65–71.
- Kato-Hayashi, N.; Yasuda, M.; Yuasa, J.; Isaka, S.; Haruki, K.; Ohmae, H.; Osada, Y.; Kanazawa, T.; Chigusa, Y. Use of cell-free circulating schistosome DNA in serum, urine, semen, and saliva to monitor a case of refractory imported schistosomiasis hematobia. J. Clin. Microbiol. 2013, 51, 3435–3438.
- van Dam, G.J.; Wichers, J.H.; Ferreira, T.M.F.; Ghati, D.; van Amerongen, A.; Deelder, A.M. Diagnosis of schistosomiasis by reagent strip test for detection of circulating cathodic antigen. J. Clin. Microbiol. 2004, 42, 5458–5461.
- van Dam, G.J.; de Dood, C.J.; Lewis, M.; Deelder, A.M.; van Lieshout, L.; Tanke, H.J.; van Rooyen, L.H.; Corstjens, P.L. A robust dry reagent lateral flow assay for diagnosis of active schistosomiasis by detection of Schistosoma circulating anodic antigen. Exp. Parasitol. 2013, 135, 274–282.
- Grenfell, R.F.; Martins, W.; Drummond, S.C.; Antunes, C.M.; Voieta, I.; Otoni, A.; Oliveira, A.A.; Silva-Moraes, V.; Oliveira, E.R.; Oliveira, E.; et al. Acute schistosomiasis diagnosis: A new tool for the diagnosis of schistosomiasis in a group of travelers recently infected in a new focus of Schistosoma mansoni. Rev. Soc. Bras. Med. Trop. 2013, 46, 208–213.
- Marchese, V.; Beltrame, A.; Angheben, A.; Monteiro, G.B.; Giorli, G.; Perandin, F.; Buonfrate, D.; Bisoffi, Z. Schistosomiasis in immigrants, refugees and travellers in an Italian referral centre for tropical diseases. Infect. Dis. Poverty 2018, 7, 55.
- Beltrame, A.; Zammarchi, L.; Zuglian, G.; Gobbi, F.; Angheben, A.; Marchese, V.; Degani, M.; Mantella, A.; Bianchi, L.; Montagnani, C.; et al. Schistosomiasis screening of travelers from Italy with possible exposure in Corsica, France. Emerg. Infect. Dis. 2015, 21, 1887–1889.
- Tosswill, J.H.; Ridley, D.S. An evaluation of the ELISA for schistosomiasis in a hospital population. Trans. R. Soc. Trop. Med. Hyg. 1986, 80, 435–438.
- Mott, K.E.; Dixon, H.; Osei-Tutu, E.; England, E.C. Relation between intensity of Schistosoma haematobium infection and clinical haematuria and proteinuria. Lancet 1983, 321, 1005–1008.
- Ochodo, E.A.; Gopalakrishna, G.; Spek, B.; Reitsma, J.B.; van Lieshout, L.; Polman, K.; Lamberton, P.; Bossuyt, P.M.; Leeflang, M.M. Circulating antigen tests and urine reagent strips for diagnosis of active schistosomiasis in endemic areas. Cochrane Database Syst. Rev. 2015, 2015, Cd009579.
- Adekiya, T.A.; Aruleba, R.T.; Oyinloye, B.E.; Okosun, K.O.; Kappo, A.P. The effect of climate change and the snail-schistosome cycle in transmission and bio-control of schistosomiasis in sub-Saharan Africa. Int. J. Environ. Research Public Health 2019, 17, 181.
- Rollinson, D.; Knopp, S.; Levitz, S.; Stothard, J.R.; Tchuem Tchuenté, L.-A.; Garba, A.; Mohammed, K.A.; Schur, N.; Person, B.; Colley, D.G.; et al. Time to set the agenda for schistosomiasis elimination. Acta Trop. 2013, 128, 423–440.
- McManus, D.P.; Gordon, C.; Weerakoon, K.G.A.D. Testing of water samples for environmental DNA as a surveillance tool to assess the risk of schistosome infection in a locality. Int. J. Infect. Dis. 2018, 76, 128–129.
- Allan, F.; Ame, S.M.; Tian-Bi, Y.-N.T.; Hofkin, B.V.; Webster, B.L.; Diakité, N.R.; N’Goran, E.K.; Kabole, F.; Khamis, I.S.; Gouvras, A.N.; et al. Snail-related contributions from the schistosomiasis consortium for operational research and evaluation program including xenomonitoring, focal mollusciciding, biological control, and modeling. Am. Soc. Trop. Med. Hyg. 2020, 103 (Suppl. S1), 66–79.
- Sokolow, S.H.; Huttinger, E.; Jouanard, N.; Hsieh, M.H.; Lafferty, K.D.; Kuris, A.M.; Riveau, G.; Senghor, S.; Thiam, C.; N’Diaye, A.; et al. Reduced transmission of human schistosomiasis after restoration of a native river prawn that preys on the snail intermediate host. Proc. Natl. Acad. Sci. USA 2015, 112, 9650–9655.
- Yang, K.; Sun, L.P.; Liang, Y.S.; Wu, F.; Li, W.; Zhang, J.F.; Huang, Y.X.; Hang, D.R.; Liang, S.; Bergquist, R.; et al. Schistosoma japonicum risk in Jiangsu province, People’s Republic of China: Identification of a spatio-temporal risk pattern along the Yangtze River. Geospat. Health 2013, 8, 133–142.
- Hamburger, J.; Xu, Y.; Ramzy, R.M.; Jourdane, J.; Ruppel, A. Development and laboratory evaluation of a polymerase chain reaction for monitoring Schistosoma mansoni infestation of water. Am. J. Trop. Med. Hyg. 1998, 59, 468–473.
- Hertel, J.; Kedves, K.; Hassan, A.H.M.; Haberl, B.; Haas, W. Detection of Schistosoma mansoni cercariae in plankton samples by PCR. Acta Trop. 2004, 91, 43–46.
- Amarir, F.; Sebti, F.; Abbasi, I.; Sadak, A.; Fellah, H.; Nhammi, H.; Ameur, B.; El Idrissi, A.L.; Rhajaoui, M. Schistosoma haematobium detection in snails by DraI PCR and Sh110/Sm-Sl PCR: Further evidence of the interruption of schistosomiasis transmission in Morocco. Parasites Vectors 2014, 7, 288.
- Abbasi, I.; King, C.H.; Sturrock, R.F.; Kariuki, C.; Muchiri, E.; Hamburger, J. Differentiation of Schistosoma haematobium from related schistosomes by PCR amplifying an inter-repeat sequence. Am. J. Trop. Med. Hyg. 2007, 76, 950–955.
- Hamburger, J.; Hoffman, O.; Kariuki, H.C.; Muchiri, E.M.; Ouma, J.H.; Koech, D.K.; Sturrock, R.F.; King, C.H. Large-scale, polymerase chain reaction-based surveillance of Schistosoma haematobium DNA in snails from transmission sites in coastal Kenya: A new tool for studying the dynamics of snail infection. Am. J. Trop. Med. Hyg. 2004, 71, 765–773.
- Melo, F.L.; Gomes, A.L.; Barbosa, C.S.; Werkhauser, R.P.; Abath, F.G. Development of molecular approaches for the identification of transmission sites of schistosomiasis. Trans. R. Soc. Trop. Med. Hyg. 2006, 100, 1049–1055.
- Sato, M.O.; Rafalimanantsoa, A.; Ramarokoto, C.; Rahetilahy, A.M.; Ravoniarimbinina, P.; Kawai, S.; Minamoto, T.; Sato, M.; Kirinoki, M.; Rasolofo, V.; et al. Usefulness of environmental DNA for detecting Schistosoma mansoni occurrence sites in Madagascar. Int. J. Infect. Dis. 2018, 76, 130–136.
- Schols, R.; Carolus, H.; Hammoud, C.; Mulero, S.; Mudavanhu, A.; Huyse, T. A rapid diagnostic multiplex PCR approach for xenomonitoring of human and animal schistosomiasis in a ‘One Health’ context. Trans. R. Soc. Trop. Med. Hyg. 2019, 113, 722–729.
- Pennance, T.; Archer, J.; Lugli, E.B.; Rostron, P.; Llanwarne, F.; Ali, S.M.; Amour, A.K.; Suleiman, K.R.; Li, S.; Rollinson, D.; et al. Development of a Molecular Snail Xenomonitoring Assay to Detect Schistosoma haematobium and Schistosoma bovis Infections in their Bulinus Snail Hosts. Molecules 2020, 25, 4011.
More
Information
Subjects:
Infectious Diseases
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
2.5K
Revisions:
2 times
(View History)
Update Date:
02 Nov 2021
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




