
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Leo Quinlan | + 3079 word(s) | 3079 | 2021-06-25 09:44:58 | | | |
| 2 | Lily Guo | Meta information modification | 3079 | 2021-07-07 03:47:42 | | |
Video Upload Options
Targeted cellular ablation is being increasingly used in the treatment of arrhythmias and structural heart disease. Catheter-based ablation for atrial fibrillation (AF) is considered a safe and effective approach for patients who are medication refractory. Electroporation (EPo) employs electrical energy to disrupt cell membranes which has a minimally thermal effect. The nanopores that arise from EPo can be temporary or permanent. Reversible electroporation is transitory in nature and cell viability is maintained, whereas irreversible electroporation causes permanent pore formation, leading to loss of cellular homeostasis and cell death. Several studies report that EPo displays a degree of specificity in terms of the lethal threshold required to induce cell death in different tissues. However, significantly more research is required to scope the profile of EPo thresholds for specific cell types within complex tissues. Irreversible electroporation (IRE) as an ablative approach appears to overcome the significant negative effects associated with thermal based techniques, particularly collateral damage to surrounding structures. With further fine-tuning of parameters and longer and larger clinical trials, EPo
1. Introduction
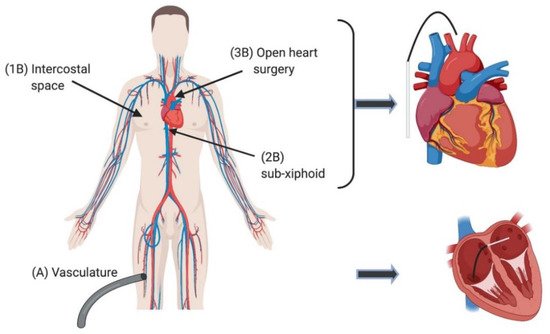
2. Current Ablation Approaches for Treating Arrhythmia
| Ref. | Subject | Follow-Up | Energy | Parameters | Monophasic/Biphasic Waveform | Monopolar/Bipolar Electrode Configuration | Reported Outcome |
|---|---|---|---|---|---|---|---|
| In Vitro | |||||||
| [3] | HL-1 cell line | N/A | 200 V; 1000 V/cm | PD- 50 µs, F- 10 Hz, PF- 10, 50, 99 pulses. | Not specified | Not specified | (1) IRE is effective for creating lesions on HL-1 cell line. |
| [11] | Cardiac strand-2D model | N/A | 0.4–0.5 V; 25 V/cm |
PD- 5 ms | Monophasic | Not specified | (1) Cardiac fibre exposed to a strong stimulus responds by developing pores in the first layer of cells immediately adjacent to the electrode. (2) IRE stops the growth of the macroscopic transmembrane potential, it does not affect intra- and extracellular potentials in the bulk of the tissue. |
| In Vivo Animal | |||||||
| [2] | Rat | 1 month | 50, 250, 500 V | PD- 70 vs. 100 μs, F- 1, 2, 3, 4 Hz, PF- 10 V’s 20. | Not specified | Not specified | (1) Longer pulse duration (100 μs vs. 70 μs) is associated with larger volume reduction. (2) More pulses (20 vs. 10) are associated with larger volume reduction. (3) Pulse voltage (500 V vs. 250 V, 50 V) has an important effect on tissue damage. (4) Lower pulse frequency (10 Hz vs 20 Hz) is correlated with harsher tissue damage. |
| [9] | Porcine | 24 h | 1500–2000 V | PD- 100 μs, PF- 8, 16, 32. | Not specified | Not specified | (1) Lesions were mean 0.9 cm in depth. (2) Complete transmural destruction of atrial tissue at the site of the electrode application. (3) No local temperature change and with demonstration of electrical isolation. |
| [12] | Porcine | 7 days | Not specified | F- 1 Hz, PF- 35 | Not specified | Bipolar | (1) Unlike RF lesions, SW lesions showed only mild denaturation and little disruption of endocardium. (2) Lesion depth from SW correlated to amount of energy used. (3) SWCA lesions showed transient inflammatory responses followed by accelerated healing process with preserved myocardial blood flow. |
| [13] | Porcine | 3 weeks | Not specified | Not specified | Monophasic | Not specified | (1) Mean depths ranged from 2.9 + 1.2 mm–6.5 + 2.7 mm. (2) 32% of lesions were transmural. (3) Coronary arteries do not develop significant stenosis within 3 weeks after epicardial IRE. |
| [14] | Porcine | 3 months | Not specified | PF- 3. | Monophasic | Not specified | (1) Mean value of the median lesion depths was 6.4 ± 2.6 mm. (2) 31% of lesions were transmural. (3) Apart from short-lasting (<30 min) coronary spasm, no long-term luminal narrowing was seen. |
| [15] | Porcine | 2 weeks | 500 V | PD- 90 µs, PF- 60. | Biphasic | Bipolar | (1) PFA lesions comparable to RFA lesions and had no collateral damage. |
| [16] | Canine | 29 days | 750 V | PD- 20 µs, F- 30–500 Hz, PF-10. | Not specified | Bipolar | (1) PEF can safely ablate Purkinje fibres. (2) Minimal collateral damage to myocardium. |
| [17] | Porcine | 3 weeks | Not specified | PF- 4. | Monophasic | Bipolar | (1) Low energy IRE is safe and efficient in creating lesions on the PV ostia. |
| [18] | Rat | N/A | 20 kV; 36 kV/cm | PD- 10 ns, F- 2 Hz, PF- 3. | Not specified | Not specified | (1) nsEP produces smaller pore size and reduced non-polar distribution of electro-pores over the cell body. (2) At near threshold intensities, both nsEPo and msEPo triggered Ca2+ transients. |
| [19] | Rabbit | N/A | 50–500 V | F- 1–2 kHz, PF- 6–10. | Monophasic | Bipolar | (1) IRE thresholds were 229 ± 81 and 318 ± 84 V for the endocardium and the epicardium, respectively. (2) Selective transient impairment of electrical activity in endocardial bundles is caused by IRE. (3) IRE might transiently reduce myocardial vulnerability to arrhythmias. |
| [20] | Ovine | N/A | Not specified | PD- 100–400 µs, F- 1–5 Hz, PF- 10–40 pulses. |
Not specified | Bipolar | (1) Lesions were well demarcated from the unaffected tissue. (2) The induced inflammatory reaction within these acute ablations was minimal. |
| [21] | Porcine | 3 weeks | 600 V | PD- 2 ms, F- 10 kHz, PF- 10. | Biphasic | Not specified | (1) Demonstrated the feasibility of a novel asymmetrical high frequency (aHF) waveform for IRE. (2) The aHF waveform led to significantly deeper lesions than the symmetrical HF waveform. (3) Both methods showed lesions of more than 4 mm deep. |
| [22] | Murine, rat, porcine | N/A | 100 V; 12.2 kV/cm | PD- 400 ns, PF- 20. | Not specified | Not specified | (1) Stimulation by 200 ns shocks can elicit Ca2+ transients. (2) Shortest shocks cause the least damage and their threshold energy is minimal. (3) Orientation of cardiomyocytes with respect for electric field does not affect threshold for ns shocks. |
| [23] | Murine | N/A | Not specified | PD- 200 µs | Not specified | Not specified | (1) 200 ns stimuli induced action potentials. (2) nsPEF caused Ca2+ entry, associated with a slow sustained depolarisation. |
| [24] | Rabbit | N/A | 200 V | PD- 350 ns, F- 1, 3 Hz, PF- 20, 6. | Not specified | Monopolar | (1) Nonconducting lesions created in less than 2 s with nsPEF application per site and minimal heating (<0.2 °C) of the tissue. (2) Lesion was smoother and more uniform throughout the wall in comparison to RF lesions. |
| [25] | Canine | 113 ± 7 days | 1000 V | PD- 100 µs, PF- 10 | Not specified | Bipolar | (1) Cardiac GP permanently damaged using DC for IRE. (2) Preservation of atrial myocardial architecture and absence of inflammatory reaction and fibrosis. |
| [26] | Porcine | 63 ± 3.3 days | 800–1800 V | Not specified | Monophasic | Bipolar | (1) Both waveforms created confluent myocardial lesions. (2) Biphasic PFA was more durable than monophasic PFA and radiofrequency ablation lesions. |
| [27] | Rabbit | 4 weeks | 300 V | Not specified | Monophasic | Bipolar | (1) Shock-induced IRE was spatially dependent on the location and dimension of the active region of the shock electrode. (2) The surviving anterior epicardial layers in the infarcted region were more susceptible to IRE. |
| [28] | Rabbit | Not specified | 200 V; 3 kV/cm | PD- 350 ns, F- 3 Hz, PF- 6. | Not specified | Not specified | (1) High anisotropy ratio substantially affects the ablation outcome, low anisotropy ratio does not. |
| [29] | Porcine | 3 months | Not specified | Not specified | Monophasic | Not specified | (1) Lesion size, depth and width corresponds to magnitude of energy used. (2) Initial spasm of coronary vasculature was noted, but this did not persist and was not recorded at follow-up. |
| [30] | Porcine | 3 months | Not specified | Not specified | Not specified | Not specified | (1) Mean depth of the 30 J, 100 J and 300 J lesions was 3.2 ± 0.7, 6.3 ± 1.8 and 8.0 ± 1.5 mm, respectively. (2) Mean width of the 30 J, 100 J, and 300 J lesions was 10.1 ± 0.8, 15.1 ± 1.5 and 17.1 ± 1.3 mm, respectively. (3) No luminal arterial narrowing was observed after 3 months. |
| [31] | Porcine | 3 weeks | 950–2150 V | PD- <10 ms, PF- 4. | Monophasic | Monopolar | (1) 200 J applications yielded median lesion depth of 5.2 ± 1.2 mm. (2) No signs of tissue heating. (3) Lesion would be sufficient for inducing PVI. |
| [32] | Canine | N/A | Not specified | PD- 60–300 s, F- 7 kHz. | Not specified | Not specified | (1) Device can successfully deliver both RF and IRE energy. (2) Addition of porous configuration on balloon can aid in enhancing drug delivery. |
| [33] | Porcine | 3 months | Not specified | Not specified | Monophasic | Not specified | (1) IRE ablation: PV ostial diameter decreased 11 ± 10% directly after ablation but had increased 19 ± 11% after 3 months. (2) RF ablation: PV ostial diameter decreased 23 ± 15% directly after ablation and remained 7 ± 17% smaller after 3 months than pre-ablation diameter, despite a 21 ± 7% increase in heart size during aging from 6 to 9 months. |
| [34] | Canine | N/A | Not specified | F- 1 Hz. | Not specified | Bipolar | (1) No evidence of collateral damage to surrounding structures. (2) Ventricular arrhythmias can occur during DC application and are more likely with use of higher energy. |
| [35] | Canine | 27 days | 2 kV/cm | PD- 100 µs, PF- 100. | Not specified | Bipolar | (1) No significant PV stenosis or oesophageal injury occurred. |
| [3] | Porcine | N/A | 500 V; 1200 V/cm | PD- 50 µs, F- 10 Hz, PF- 50. | Not specified | Not specified | (1) IREis effective for creating lesions on PV tissue. |
| [36] | Porcine | 35 days | 2200 V | PD- <60 s | Biphasic | Bipolar | (1) Fibrous tissue homogeneously replaced myocytes. (2) When present, nerve fascicles and vasculature were preserved within surrounding fibrosis. |
| [37] | Canine ex vivo | N/A | 750–2500 V; 250–833 V/cm | PD- 200 µs, F- 1 Hz, PF- 10 | Biphasic | Not specified | (1) Delivery of IRE energy significantly reduced the window of vulnerability to ventricular arrhythmia. (2) No evidence of myocardial damage. |
3. Electroporation as an Ablative Approach
| Ref. | Follow-Up | Energy | Parameters | Monophasic/Biphasic Waveform | Monopolar/Bipolar Electrode Configuration | Reported Outcome Reported Outcome |
|---|---|---|---|---|---|---|
| [56] | N/A | 900–2500 V | PF- 3. | Not specified | Bipolar | (1) PEF is a safe method for treating AF both endocardially and epicardially. (2) No incidences of atrial or ventricular arrythmia during procedure. (3) No collateral damage or PV stenosis recorded. |
| [58] | 4 months | 900–1000 V | Not specified | Monophasic | Bipolar | (1) Acute PVI achieved in 100% of patients using 6.4 ± 2.3 applications. (2) No injury to oesophagus or phrenic nerve. |
| [59] | 12 months | 0.011 ± 0.006 mV | PD- 3–5 s | Biphasic | Bipolar | (1) No adverse effects recorded related to PEF. (2) Freedom from AF was 94.4 ± 3.2%. |
| [60] | N/A | 2154 ± 59 V | Not specified | Monophasic | Monopolar | (1) Acute bidirectional electrical PVI achieved in all 40 PVs. (2) No PV reconnections occurred during waiting period (30 min). |
| [61] | 3 months | 900–1000 V | Not specified | Monophasic | Monopolar and Bipolar | (1) No change (0%) in PV diameter and no stenosis in PFA patients, but reduction in diameter in 32.5% of patients who received RFA. |
3. Conclusions
References
- Heart Disease Facts|cdc.gov. Available online: (accessed on 9 June 2020).
- Zager, Y.; Kain, D.; Landa, N.; Leor, J.; Maor, E. Optimization of irreversible electroporation protocols for in-vivo myocardial decellularization. PLoS ONE 2016, 11.
- Jiang, C.; Goff, R.; Patana-anake, P.; Iaizzo, P.A.; Bischof, J. Irreversible electroporation of cardiovascular cells and tissues. J. Med. Devices Trans. ASME 2013, 7.
- Avazzadeh, S.; McBride, S.; O’Brien, B.; Coffey, K.; Elahi, A.; O’Halloran, M.; Soo, A.; Quinlan, L.R. Ganglionated Plexi Ablation for the Treatment of Atrial Fibrillation. J. Clin. Med. 2020, 9, 3081.
- Holmes, D.R.; Valeti, U.S.; Nishimura, R.A. Alcohol septal ablation for hypertrophic cardiomyopathy: Indications and technique. Catheter. Cardiovasc. Interv. 2005, 66, 375–389.
- Nagueh, S.F.; Groves, B.M.; Schwartz, L.; Smith, K.M.; Wang, A.; Bach, R.G.; Nielsen, C.; Leya, F.; Buergler, J.M.; Rowe, S.K.; et al. Alcohol septal ablation for the treatment of hypertrophic obstructive cardiomyopathy: A multicenter north american registry. J. Am. Coll. Cardiol. 2011, 58, 2322–2328.
- Wei, C.; Qian, P.; Tedrow, U.; Mak, R.; Zei, P.C. Non-invasive stereotactic radioablation: A new option for the treatment of ventricular arrhythmias. Arrhythmia Electrophysiol. Rev. 2019, 8, 285–293.
- Chiu, M.H.; Mitchell, L.B.; Ploquin, N.; Faruqi, S.; Kuriachan, V.P.; Chiu, M. Review of Stereotactic Arrhythmia Radioablation Therapy for Cardiac Tachydysrhythmias. CJC Open 2020, 3, 236–247.
- Lavee, J.; Onik, G.; Rubinsky, B. A Novel Nonthermal Energy Source for Surgical Epicardial Atrial Ablation: Irreversible Electroporation Single cell manipulation and electroporation View project Isochoric Freezing: A new frontier for cryopreservation View project. Heart Surg. Forum 2007, 10, 96–101.
- Njeim, M.; Bogun, F. Selecting the appropriate ablation strategy: The role of endocardial and/or epicardial access. Arrhythmia Electrophysiol. Rev. 2015, 4, 184–188.
- Krassowska, W. Effects of Electroporation on Transmembrane Potential Induced by Defibrillation Shocks. Pacing Clin. Electrophysiol. 1995, 18, 1644–1660.
- Hirano, M.; Yamamoto, H.; Hasebe, Y.; Fukuda, K.; Morosawa, S.; Amamizu, H.; Ohyama, K.; Uzuka, H.; Takayama, K.; Shimokawa, H. Development of a Novel Shock Wave Catheter Ablation system—A Validation Study in Pigs in Vivo. EP Eur. 2018, 20, 1856–1865.
- Du Pre, B.C.; van Driel, V.J.; van Wessel, H.; Loh, P.; Doevendans, P.A.; Goldschmeding, R.; Wittkampf, F.H.; Vink, A. Minimal Coronary Artery Damage by Myocardial Electroporation Ablation. EP Eur. 2013, 15, 144–149.
- Neven, K.; van Driel, V.; van Wessel, H.; van Es, R.; du Pré, B.; Doevendans, P.A.; Wittkampf, F. Safety and feasibility of closed chest epicardial catheter ablation using electroporation. Circ. Arrhythm. Electrophysiol. 2014, 7, 913–919.
- Stewart, M.T.; Haines, D.E.; Verma, A.; Kirchhof, N.; Barka, N.; Grassl, E.; Howard, B. Intracardiac pulsed field ablation: Proof of feasibility in a chronic porcine model. Heart Rhythm 2019, 16, 754–764.
- Sugrue, A.; Vaidya, V.R.; Livia, C.; Padmanabhan, D.; Abudan, A.; Isath, A.; Witt, T.; DeSimone, C.V.; Stalboerger, P.; Kapa, S.; et al. Feasibility of selective cardiac ventricular electroporation. PLoS ONE 2020, 15.
- Wittkampf, F.H.; Van Driel, V.J.; Van Wessel, H.; Vink, A.; Hof, I.E.; GrÜndeman, P.F.; Hauer, R.N.; Loh, P. Feasibility of electroporation for the creation of pulmonary vein ostial lesions. J. Cardiovasc. Electrophysiol. 2011, 22, 302–309.
- Semenov, I.; Zemlin, C.; Pakhomova, O.N.; Xiao, S.; Pakhomov, A.G. Diffuse, non-polar electropermeabilization and reduced propidium uptake distinguish the effect of nanosecond electric pulses. Biochim. Biophys. Acta Biomembr. 2015, 1848, 2118–2125.
- Al-Khadra, A.; Nikolski, V.; Efimov, I.R. The role of electroporation in defibrillation. Circ. Res. 2000, 87, 797–804.
- Hong, J.; Stewart, M.T.; Cheek, D.S.; Francischelli, D.E.; Kirchhof, N. Cardiac ablation via electroporation. In Proceedings of the 31st Annual International Conference of the IEEE Engineering in Medicine and Biology Society: Engineering the Future of Biomedicine, EMBC 2009, Minneapollis, MN, USA, 3–6 September 2009; Volume 2009, pp. 3381–3384.
- Van Es, R.; Konings, M.K.; Du Pré, B.C.; Neven, K.; Van Wessel, H.; Van Driel, V.J.H.M.; Westra, A.H.; Doevendans, P.A.F.; Wittkampf, F.H.M. High-frequency irreversible electroporation for cardiac ablation using an asymmetrical waveform. Biomed. Eng. Online 2019, 18.
- Semenov, I.; Grigoryev, S.; Neuber, J.U.; Zemlin, C.W.; Pakhomova, O.N.; Casciola, M.; Pakhomov, A.G. Excitation and injury of adult ventricular cardiomyocytes by nano- to millisecond electric shocks. Sci. Rep. 2018, 8, 1–12.
- Azarov, J.E.; Semenov, I.; Casciola, M.; Pakhomov, A.G. Excitation of murine cardiac myocytes by nanosecond pulsed electric field. J. Cardiovasc. Electrophysiol. 2019, 30, 392–401.
- Xie, F.; Varghese, F.; Pakhomov, A.G.; Semenov, I.; Xiao, S.; Philpott, J.; Zemlin, C. Ablation of Myocardial Tissue With Nanosecond Pulsed Electric Fields. PLoS ONE 2015, 10, e0144833.
- Padmanabhan, D.; Naksuk, N.; Killu, A.K.; Kapa, S.; Witt, C.; Sugrue, A.; Desimon, C.V.; Madhavan, M.; de Groot, J.R.; O’Brien, B.; et al. Electroporation of epicardial autonomic ganglia: Safety and efficacy in medium-term canine models. J. Cardiovasc. Electrophysiol. 2019, 30, 607–615.
- Koruth, J.; Kuroki, K.; Iwasawa, J.; Enomoto, Y.; Viswanathan, R.; Brose, R.; Buck, E.D.; Speltz, M.; Dukkipati, S.R.; Reddy, V.Y. Preclinical Evaluation of Pulsed Field Ablation: Electrophysiological and Histological Assessment of Thoracic Vein Isolation. Circ. Arrhythmia Electrophysiol. 2019, 12.
- Kim, S.C.; Vasanji, A.; Efimov, I.R.; Cheng, Y. Spatial distribution and extent of electroporation by strong internal shock in intact structurally normal and chronically infarcted rabbit hearts. J. Cardiovasc. Electrophysiol. 2008, 19, 1080–1089.
- Xie, F.; Zemlin, C.W. Effect of Twisted Fiber Anisotropy in Cardiac Tissue on Ablation with Pulsed Electric Fields. PLoS ONE 2016, 11, e0152262.
- Neven, K.; Van Driel, V.; Van Wessel, H.; Van Es, R.; Doevendans, P.A.; Wittkampf, F. Myocardial Lesion Size after Epicardial Electroporation Catheter Ablation After Subxiphoid Puncture. Circ. Arrhythmia Electrophysiol. 2014, 7, 728–733.
- Neven, K.; Van Driel, V.; Van Wessel, H.; Van Es, R.; Doevendans, P.A.; Wittkampf, F. Epicardial linear electroporation ablation and lesion size. Hear. Rhythm 2014, 11, 1465–1470.
- Wittkampf, F.H.M.; Van Driel, V.J.; Van Wessel, H.; Neven, K.G.E.J.; Gründeman, P.F.; Vink, A.; Loh, P.; Doevendans, P.A. Myocardial lesion depth with circular electroporation ablation. Circ. Arrhythmia Electrophysiol. 2012, 5, 581–586.
- Desimone, C.V.; Ebrille, E.; Syed, F.F.; Mikell, S.B.; Suddendorf, S.H.; Wahnschaffe, D.; Ladewig, D.J.; Gilles, E.J.; Danielsen, A.J.; Holmes, D.R.; et al. Novel balloon catheter device with pacing, ablating, electroporation, and drug-eluting capabilities for atrial fibrillation treatment - Preliminary efficacy and safety studies in a canine model. Transl. Res. 2014, 164, 508–514.
- Van Driel, V.J.H.M.; Neven, K.G.E.J.; Van Wessel, H.; Du Pré, B.C.; Vink, A.; Doevendans, P.A.F.M.; Wittkampf, F.H.M. Pulmonary vein stenosis after catheter ablation electroporation versus radiofrequency. Circ. Arrhythmia Electrophysiol. 2014, 7, 734–738.
- Madhavan, M.; Venkatachalam, K.L.; Swale, M.J.; Desimone, C.V.; Gard, J.J.; Johnson, S.B.; Suddendorf, S.H.; Mikell, S.B.; Ladewig, D.J.; Nosbush, T.G.; et al. Novel Percutaneous Epicardial Autonomic Modulation in the Canine for Atrial Fibrillation: Results of an Efficacy and Safety Study. PACE - Pacing Clin. Electrophysiol. 2016, 39, 407–417.
- Witt, C.M.; Sugrue, A.; Padmanabhan, D.; Vaidya, V.; Gruba, S.; Rohl, J.; DeSimone, C.V.; Killu, A.M.; Naksuk, N.; Pederson, J.; et al. Intrapulmonary vein ablation without stenosis: A novel balloon-based direct current electroporation approach. J. Am. Heart Assoc. 2018, 7.
- Koruth, J.S.; Kuroki, K.; Iwasaw1, J.; Viswanathan, R.; Richard; Brose; Buck, E.D.; Donskoy, E.; Dukkipati, S.R.; Reddy, V.Y. Endocardial Ventricular Pulsed Field Ablation: A Proof-Of-Concept Preclinical Evaluation. EP Eur. 2020, 22, 434–439.
- Livia, C.; Sugrue, A.; Witt, T.; Polkinghorne, M.D.; Maor, E.; Kapa, S.; Lehmann, H.I.; DeSimone, C.V.; Behfar, A.; Asirvatham, S.J.; et al. Elimination of Purkinje Fibers by Electroporation Reduces Ventricular Fibrillation Vulnerability. J. Am. Heart Assoc. 2018, 7, e009070.
- Coltorti, F.; Bardy, G.H.; Reichenbach, D.; Greene, H.L.; Thomas, R.; Breazeale, D.G.; Alferness, C.; Ivey, T.D. Catheter-mediated electrical ablation of the posterior septum via the coronary sinus: Electrophysiologic and histologic observations in dogs. Circulation 1985, 72, 612–622.
- Nakagawa, H.; Jackman, M. Electroporation (revival of direct current ablation) new approach for increasing epicardial ablation safety in close proximity to a coronary artery. Circ. Arrhythmia Electrophysiol. 2014, 7, 779–780.
- Ahsan, A.J.; Cunningham, D.; Rowland, E.; Rickards, A.F. Catheter Ablation without Fulguration: Design and Performance of a New System. Pacing Clin. Electrophysiol. 1989, 12, 1557–1561.
- Wittkampf, F.H.M.; van Es, R.; Neven, K. Electroporation and its Relevance for Cardiac Catheter Ablation. JACC Clin. Electrophysiol. 2018, 4, 977–986.
- Tung, L.; Tovar, O.; Neunlist, M.; Jain, S.K.; O’neill, R.J. Effects of Strong Electrical Shock on Cardiac Muscle Tissue. Ann. N. Y. Acad. Sci. 1994, 720, 160–175.
- Davalos, R.V.; Mir, L.M.; Rubinsky, B. Tissue ablation with irreversible electroporation. Ann. Biomed. Eng. 2005, 33, 223–231.
- Maor, E.; Ivorra, A.; Mitchell, J.J.; Rubinsky, B. Vascular smooth muscle cells ablation with endovascular nonthermal irreversible electroporation. J. Vasc. Interv. Radiol. 2010, 21, 1708–1715.
- Wojtaszczyk, A.; Caluori, G.; Pešl, M.; Melajova, K.; Stárek, Z. Irreversible electroporation ablation for atrial fibrillation. J. Cardiovasc. Electrophysiol. 2018, 29, 643–651.
- Frandsen, S.K.; Gissel, H.; Hojman, P.; Tramm, T.; Eriksen, J.; Gehl, J. Direct therapeutic applications of calcium electroporation to effectively induce tumor necrosis. Cancer Res. 2012, 72, 1336–1341.
- Weaver, J.C. Electroporation of cells and tissues. IEEE Trans. Plasma Sci. 2000, 28, 24–33.
- Neumann, E.; Rosenheck, K. Permeability changes induced by electric impulses in vesicular membranes. J. Membr. Biol. 1972, 10, 279–290.
- Vižintin, A.; Vidmar, J.; Ščančar, J.; Miklavčič, D. Effect of interphase and interpulse delay in high-frequency irreversible electroporation pulses on cell survival, membrane permeabilization and electrode material release. Bioelectrochemistry 2020, 134, 107523.
- Andrei, G.; Pakhomov, D.; Miklavcic, M.S.M. Advanced Electroporation Techniques in Biology and Medicine. Available online: (accessed on 27 March 2020).
- Kotnik, T.; Pucihar, G.; Reberšek, M.; Miklavčič, D.; Mir, L.M. Role of pulse shape in cell membrane electropermeabilization. Biochim. Biophys. Acta Biomembr. 2003, 1614, 193–200.
- Stankevic, V.; Simonis, P.; Zurauskiene, N.; Stirke, A.; Dervinis, A.; Bleizgys, V.; Kersulis, S.; Balevicius, S. Compact square-wave pulse electroporator with controlled electroporation efficiency and cell viability. Symmetry 2020, 12, 412.
- Sano, M.B.; Fan, R.E.; Xing, L. Asymmetric Waveforms Decrease Lethal Thresholds in High Frequency Irreversible Electroporation Therapies. Sci. Rep. 2017, 7, 1–13.
- Polajžer, T.; Dermol-Cerne, J.; Erne, J.; Reberšek, M.; O’connor, R.; Miklavčič, D. Cancellation effect is present in high-frequency reversible and irreversible electroporation. Bioelectrochemistry 2019.
- Caluori, G.; Odehnalova, E.; Jadczyk, T.; Pesl, M.; Pavlova, I.; Valikova, L.; Holzinger, S.; Novotna, V.; Rotrekl, V.; Hampl, A.; et al. AC Pulsed Field Ablation Is Feasible and Safe in Atrial and Ventricular Settings: A Proof-of-Concept Chronic Animal Study. Front. Bioeng. Biotechnol. 2020, 8, 1374.
- Reddy, V.Y.; Koruth, J.; Jais, P.; Petru, J.; Timko, F.; Skalsky, I.; Hebeler, R.; Labrousse, L.; Barandon, L.; Kralovec, S.; et al. Ablation of Atrial Fibrillation With Pulsed Electric Fields: An Ultra-Rapid, Tissue-Selective Modality for Cardiac Ablation. JACC Clin. Electrophysiol. 2018, 4, 987–995.
- Cemazar, M.; Sersa, G.; Frey, W.; Miklavcic, D.; Teissié, J. Recommendations and requirements for reporting on applications of electric pulse delivery for electroporation of biological samples. Bioelectrochemistry 2018, 122, 69–76.
- Reddy, V.Y.; Neuzil, P.; Koruth, J.S.; Petru, J.; Funosako, M.; Cochet, H.; Sediva, L.; Chovanec, M.; Dukkipati, S.R.; Jais, P. Pulsed Field Ablation for Pulmonary Vein Isolation in Atrial Fibrillation. J. Am. Coll. Cardiol. 2019.
- Reddy, V.Y.; Anter, E.; Rackauskas, G.; Peichl, P.; Koruth, J.S.; Petru, J.; Funasako, M.; Minami, K.; Natale, A.; Jaïs, P.; et al. A Lattice-Tip Focal Ablation Catheter that Toggles Between Radiofrequency and Pulsed Field Energy to Treat Atrial Fibrillation: A First-in-Human Trial. Circ. Arrhythmia Electrophysiol. 2020, 13.
- Loh, P.; Van Es, R.; Groen, M.H.A.; Neven, K.; Kassenberg, W.; Wittkampf, F.H.M.; Doevendans, P.A. Pulmonary vein isolation with single pulse irreversible electroporation: A first in human study in 10 patients with atrial fibrillation. Circ. Arrhythmia Electrophysiol. 2020, 13, 1083–1091.
- Kuroki, K.; Whang, W.; Eggert, C.; Lam, J.; Leavitt, J.; Kawamura, I.; Reddy, A.; Morrow, B.; Schneider, C.; Petru, J.; et al. Ostial Dimensional Changes After Pulmonary Vein Isolation: Pulsed Field Ablation vs Radiofrequency Ablation. Hear. Rhythm 2020.




