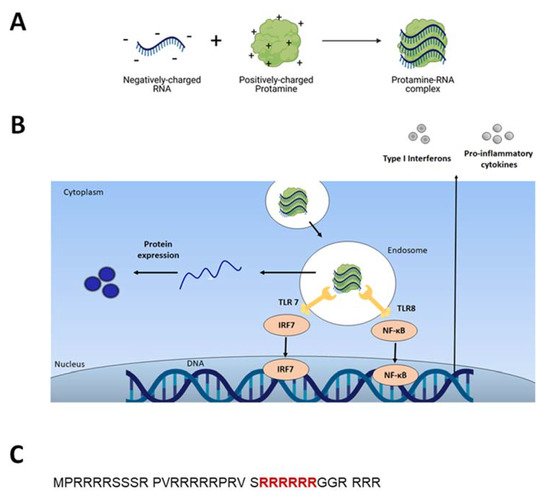
2.1. Adjuvant and Immunostimulatory Properties of Protamine-RNA Formulations
The first reports of using Protamine as an mRNA condensation and protection agent for vaccination was published in 2000 by Hoerr et al.
[3]. The authors proved that mice injected with Protamine-protected mRNA coding for the model antigen of beta-galactosidase (βgalZβgα
n RNA) were able to produce antigen-specific cytotoxic T lymphocytes (CTLs) and IgG antibodies against this antigen. Interestingly, the specific immune response was detectable only after injection in ear pinnae and not after intravenous injections. Only 1 μg of Protamine-condensed βgalZβgα
n RNA was sufficient for in vivo CTL priming. It was then reported for the first time that RNA can be qualified as a danger signal since when stabilized (modified or mixed with Protamine) it triggers innate immunity
[15]. Indeed, it was thereafter found that RNA stimulates endosomal-resident Toll-like receptors 7 and 8 (TLR 7 and 8)
[8][16]. When triggered, TLRs induce specific intracellular activation pathways that can result in the expression of different types of innate immune response molecules, such as type I interferons and TNF-alpha (Figure 1B)
[11]. Unmodified single-stranded RNA (ssRNA) is recognized by human TLR7 (expressed in plasmacytoid dendritic cells) and human TLR8 (expressed in monocytes). A TLR-induced cellular response consists of the activation of different signal transduction cascades and ultimately leads to induction of secretion of cytokines (e.g., IL-12, IFNα, TNFα)
[15][17].
This feature suggested the possibility of using RNA as an anti-tumor treatment
[8]. Glioblastoma-challenged mice were treated with series of intra-tumoral injections consisting of naked mRNA, CpG DNA, mRNA condensed with Protamine or Protamine alone. Injections of mRNA alone or Protamine-protected mRNA as well as injections of CpG DNA into tumors led to a significant delay in tumor growth and in the long term, circa 20% of mice remained tumor-free in all nucleic acid-injected groups. The tumor-free mice were subsequently re-challenged with glioblastoma cells. None of the mice that had recovered from the primary tumor graft as a consequence of nucleic acid treatment showed any palpable tumors, which indicated that immunotherapy of solid tumors using RNA as a danger signal led to long-term anti-tumor immunity. It was postulated that Protamine-stabilized RNA could represent a safer alternative replacement of CpG DNA-based adjuvants to be applied in the context of many immunotherapeutic or prophylactic treatments.
Fotin-Mleczek and colleagues explored another aspect of immunostimulatory RNA formulations for cancer immunotherapy
[18]. Since mRNA complexation with Protamine can inhibit translation of mRNA, the authors investigated a new formulation consisting of two components: mRNA complexed with Protamine for providing good innate immune stimulation, and free mRNA for antigen expression. This was named RNActive vaccine (Figure 3, RNActive formulation)
[19]. Animal studies showed a delay in tumor growth (melanoma cell line B16 expressing ovalbumin) of about 10 days in groups vaccinated with a two-component formulation containing OVA-coding mRNA. The authors observed significant superiority of the two-component vaccine compared with a single component (naked mRNA). This two-component vaccine induced complete adaptive immune responses, including activation of antigen-specific B and T cells. The study was repeated with mRNA coding a weaker antigen, PSMA (Prostate carcinoma-associated antigen) and gave lower, but detectable levels of innate and adaptive immune responses. The two-component RNActive formulation was also effective as a therapeutic vaccine: mice receiving the two-component OVA vaccine after tumor transplantation displayed inhibited tumor growth rates in comparison with non-vaccinated mice.
2.2. Clinical Trials with Protamine as an mRNA Carrier
Protamine has been widely used in clinics as a heparin antagonist and in slow-release insulin formulations for many years. When it comes to its application as an RNA carrier, there have been several clinical trials performed in the past 20 years aiming at testing Protamine-RNA complexes’ performance in cancer immunotherapy in patients. All of the below mentioned trials aimed at assessment of safety and efficacy. In all described studies, vaccines were well tolerated, with most common side effects being skin irritation at injection sites and flu-like symptoms. Every investigated Protamine-mRNA based vaccine induced detectable levels of appropriate immune responses, however, the results suggested the necessity for further optimization and the potential need, in the context of cancer, to combine the system with checkpoint inhibitors or other anti-cancer therapies, such as local radiotherapy. Published studies are described in detail in the below section and summarized in Table 1.
Table 1. Published clinical trials with Protamine-mRNA.
| Condition |
Protamine Formulation |
Number |
Reference |
| Metastatic Melanoma |
Protamine ICM |
NCT00204607 |
[20] |
| Prostate Cancer |
RNactive CV9103 |
EudraCT 2008-003967-37 |
[21] |
| Prostate Cancer |
RNactive CV9104 |
NCT01817738 |
[22] |
| Rabies |
RNactive CV7201 |
NCT02241135 |
[23] |
| Non-small Cell Lung Cancer |
RNactive CV9201 |
NCT00923312 |
[24] |
| Non-small Cell Lung Cancer |
RNactive CV9202 |
NCT01915524 |
[25] |
Just after the evaluation of naked mRNA vaccine in melanoma patients
[26], the Tuebingen-based research group explored Protamine-mRNA complexes in a Phase I/II vaccination trial in metastatic melanoma patients (NCT00204607)
[20]. In this study, 21 patients with metastatic melanoma were injected with Protamine-condensed mRNAs coding for melanoma antigens. The most frequently occurring side effect was an inflammatory skin reaction at the injection site. Fatigue was reported in 86% of the patients. No adverse effects exceeding grade 2 were observed. The addition of Protamine caused more intensive injection site reactions compared with naked mRNA
[20]. A reproducible increase of vaccine-induced T cells was observed in two out of four immunologically evaluable patients. One of seven patients with measurable disease showed a response of lung metastases at the end of the treatment. Upon ongoing vaccinations these lesions regressed completely 13 months after starting the therapy. The authors concluded that although Protamine-protected mRNA is feasible and safe as a vaccination method, the clinical or immunological responses were low, probably due to cellular immunosuppression (significantly decreased levels of Foxp3+/CD4+ regulatory T cells in treated patients). Indeed, in some murine models, Protamine-RNA based immunotherapies combined with low doses of anti-CTLA-4 or anti-PD-1 showed synergistic effects, resulting in complete tumor rejection
[27].
The RNActive technology was tested in healthy volunteers using mRNA coding for a rabies virus glycoprotein (NCT02241135)
[23]. Healthy adults received three doses of mRNA and Protamine containing vaccines (CV7201) intradermally or intramuscularly, with a booster after one year. The goals were to assess safety and tolerability as well as to determine the lowest dose of the vaccine needed to elicit rabies virus neutralizing titers. Rabies virus was selected as a model antigen to explore mRNA technology in humans, as the population is naïve to the virus unless previously vaccinated. This vaccination was also proven safe and well tolerated. All described adverse reactions were transient and mild to moderate in severity. There were four serious adverse events: one due to human error and a case of Bell’s palsy, nasal septum deviation and campylobacter infection.
Analysis of functional antibody titers against the rabies virus revealed clear differences between administration with needle-syringe or needle-free injector devices. In needle-syringe cohorts there were no detectable levels of antibody responses, while 77% of the group vaccinated with the injector device developed detectable virus neutralizing titers. This pattern was observed in both intramuscular and intradermal vaccine administration. In most patients, RABV-G-specific IgM titers peaked at day 21, IgG peaked at day 42. One year after the boost there was no change in RABV-G specific IgM antibody levels. This predominantly IgG response is indicative of an established immune memory response during the initial vaccination schedule. RAVB-G-specific CD4+ T cells were increased at day 42 compared to baseline, they declined to baseline at day 91.
RNActive vaccines were also evaluated in cancer patients
[21][24]. Vaccine against prostate cancer, CV9103, that contained four different mRNAs and Protamine, was administered intradermal to 44 patients at up to 1280 micrograms RNA per injection. Side effects included local reactogenicity and fatigue, pyrexia, chills and influenza-like illness. Immune responses were detected in the majority of the patients (and those survived also longer than immunological non-responders). One patient demonstrated a PSA response
[21]. Follow-up studies with a vaccine (CV9104) including two more mRNA species (coding for two additional antigens) have been performed. However in a placebo control study with 197 patients, there was no impact of the CV9104 vaccine on overall survival or progression free survival
[22].
The CV9201 vaccine encoding five non-small lung cancer antigens was tested in 46 patients in a I/IIa dose-escalation trial. The objectives of the study were safety assessment and evaluation of T cell responses against the five antigens. Different doses were investigated, ranging from 400 to 1600 μg of RNA per intradermal injection. Most of the adverse effects were mild-to-moderate injection site reactions and flu-like symptoms, whereas three patients had grade 3 related adverse events. In the phase IIa trial, antigen-specific immune responses against more than one antigen were detected in 63% of patients. No clear dose–response relationship was observed, but higher frequencies of immune responses in patients treated with lower mRNA doses were noticed. Nine patients had stable disease as best overall response in 29 evaluated patients. Median overall survival was 11.5 months in the total population. No cases of clinically apparent autoimmune disease were observed. In part IIa of the trial, patients were injected with 1600 μg of CV9201. Both cellular and humoral immune responses were detected against all antigens, however the responses were modest and revealed high inter-patient variability. No objective tumor responses were observed with the vaccine and, again, they were associated with tumor-induced inhibition of the immune system. According to the authors, the vaccine showed an acceptable tolerability profile and evidence of immune activation. In a follow-up Phase Ib study, CureVac evaluated combined therapy consisting of RNActive CV9202 encoding six non-small cell lung cancer-associated antigens and local radiotherapy for the treatment of stage IV non-small cell lunch cancer, NSCLC (vaccine called BI1361849). Again, the most common side effect was an injection site reaction and flu-like symptoms. Three patients had grade 3 adverse events like fatigue and pyrexia. In comparison with baseline, immunomonitoring studies revealed vaccine-induced antigen-specific immune responses in 84% of patients. Antigen-specific antibody levels were increased in 80%, and functional T cells in 40% of the patients. Frequencies of functional CD4+ and CD8+ T cells following BI1361849 combined with radiotherapy increased over time. One patient achieved a partial response with decreasing measurable tumor size. Twelve out of 26 patients demonstrated stable disease as best overall response. The immunomonitoring results were comparable to those observed with CV9201 vaccine alone without radiation
[24]. An increase in tumor antigen-specific T cells and antibodies were detected in all experimental groups. Again, the observed responses were at low frequencies of CD4 and CD8 cells. The group suggested for both vaccine studies to be tried in combination with immune checkpoint inhibitors to help break the tolerance against endogenous antigens, e.g., by enhancing effector T-cell function and inhibition of Tregs
[28].