Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Saeed Chehreh Chelgani | + 5427 word(s) | 5427 | 2021-06-30 08:20:54 | | | |
| 2 | Bruce Ren | -21 word(s) | 5406 | 2021-07-06 10:08:24 | | | | |
| 3 | Bruce Ren | + 1036 word(s) | 6442 | 2021-07-06 10:35:27 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Chehreh Chelgani, S. Talc Flotation. Encyclopedia. Available online: https://encyclopedia.pub/entry/11707 (accessed on 07 February 2026).
Chehreh Chelgani S. Talc Flotation. Encyclopedia. Available at: https://encyclopedia.pub/entry/11707. Accessed February 07, 2026.
Chehreh Chelgani, Saeed. "Talc Flotation" Encyclopedia, https://encyclopedia.pub/entry/11707 (accessed February 07, 2026).
Chehreh Chelgani, S. (2021, July 06). Talc Flotation. In Encyclopedia. https://encyclopedia.pub/entry/11707
Chehreh Chelgani, Saeed. "Talc Flotation." Encyclopedia. Web. 06 July, 2021.
Copy Citation
Talc is a naturally hydrophobic gangue mineral in most sulfide ores. However, talc has vast applications in the cosmetics, paper, and paint industries due to its high chemical stability, and its demand continues to grow. Since flotation is the most effective beneficiation technique for upgrading sulfides, the high hydrophobicity of talc has made its selective separation challenging.
surface properties
talc froth flotation
talc depression
talc hydrophobicity
1. Introduction
In nature, talc is commonly associated with different minerals such as carbonates (serpentine shear zones), silicates, and sulfides [1][2][3][4][5][6][7]. Talc has a different chemical composition and crystal morphology based on the origin, which may impact its mineral specifications and features (whiteness, particle shape, oil absorption, and amphiphilic specification) [2]. According to these precise features, talc has specific properties (platy, softness, hydrophobicity, organophilicity, and inertness), which can provide different functions in many varied applications (Table 1). Talc has high chemical stability, high surface affinity, and heat resistance compared to other silicate minerals [8]. Talc world production is documented and shown in the graph (Figure 1).
Table 1. Common talc applications.
| Industry | Applications and Utilization of Talc | Ref. |
|---|---|---|
| Ceramics |
|
[5,6,8,9,10,11,12,13] |
| Cosmetics |
|
[4,6,8,11,13,14] |
| Color and Paint |
|
[5,6,10,11,12,13] |
| Paper |
|
[5,6,8,9,10,11,13,14,15] |
| Plastic |
|
[5,6,8,9,12,13,15] |
| Roofing |
|
[5,12,15] |
| Rubbers |
|
[4,6,9] |
| Others |
|
[4,5,6,8,9,10,11,13,14,16,17,18] |
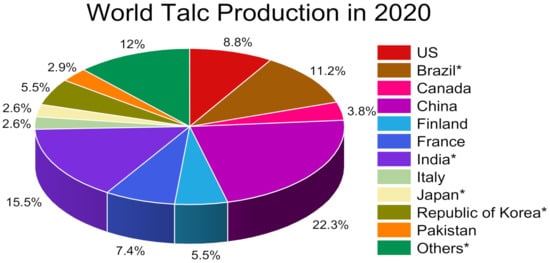
Figure 1. The estimated total talc mine production in 2020 was 5.82 million metric tons. Data were extracted from USGS Mineral Commodity Summaries 2020 [9]. * Includes pyrophyllite.
Talc with a theoretical chemical composition, Mg3Si4O10 (OH)2, is the softest mineral on Earth, mainly attributed to its chemical structure. The magnesium-based octahedral layer is in between silica rings through shared oxygen atoms. This exposes oxide surfaces that make talc only held together by weak van der Waals forces. Furthermore, these oxide surfaces make talc naturally hydrophobic. When hydrolyzed in water, the magnesia edges will show some hydrophilicity [10]. Talc has two faces: the hydrophobic basal surface and the hydrophilic edge surface [11]. The majority, around 90% of its total surface area, is a basal surface that accounts for the natural hydrophobicity or floatability of talc [5][6][7]. Ergo, preventing talc (as a typical gangue for sulfide ores) from being recovered to the flotation concentrate was the primary focus of the earlier studies.
As mentioned, valuable sulfide minerals such as chalcopyrite are commonly intergrown with gangue minerals such as talc [1][2]. This is generally the challenge in the selective flotation of such minerals. In other words, the talc separation from other valuable sulfide minerals can be difficult since talc has good natural floatability. The existence of talc in the sulfide flotation concentration as a MgO-containing gangue can cause problems in the downstream metallurgical processes [8][10][9][11]. In detail, talc has several negative effects on furnace operation in pyro-metallurgical processes to extract various metals, especially from sulfide minerals. In the smelters, MgO can change the properties of the primary slag and reduce the strengths of the sinter and pellets. The primary slag with high MgO being sticky, and the softening-melting properties of the burden in the blast furnace becoming deteriorated. In other words, increasing talc/MgO in the feed of smelters can increase liquidus temperature and slag viscosity, which requires operating the furnace at higher temperatures, thus shortening the campaign life of the furnace. To avoid these negative effects, talc content has to be significantly reduced in the ore concentrate from mineral processing plants as the feed to smelters [2][10][12][13].
These problems result in a cost increase and recovery reduction of subsequent smelting operations [10][14]. Thus, it is of great interest to remove talc beforehand from the processing plant concentrate. Different kinds of depressants have been reported to inhibit talc floatability, and polysaccharides are commonly used. However, these are expensive and can cost relatively more than other reagents used in the flotation. Although these depressants are able to depress talc, they have poor selectivity. For example, Beattie et al. reported that CMC, as a talc depressant, depressed not only talc but also chalcopyrite [15]. Such depressants also impair the flotation performance of other valuable minerals [16]. Some investigations have found that using ultrasonic treatment can further improve the depression of talc. It was shown that it had improved the flotation performance, specifically in terms of flotation rate, overall recovery, and selectivity of sulfides [16][17].
All these issues indicated that it would be worthwhile to conduct a comprehensive review that analyzes the various conditions of talc flotation. The purpose of this work is to highlight and classify the investigations that were conducted on talc flotation, defining sub-areas of studies, and highlighting the areas that require further exploration for better understanding and optimizing of talc flotation. To the best of our knowledge, this is the first review to offer an in-depth analysis and critique of talc flotation studies, including experimental works and detailed analysis procedures suggested in the literature. The literature analysis showed that while talc depression through flotation is extensively studied, insufficient attention is paid to its flotation kinetics and application of column flotation. It is also highlighted that the literature available on the talc column flotation is rare. The lack of fundamental understanding of pretreatment on the flotation efficiency is discussed, which is essential for the separation process.
2. Talc Surface Properties
2.1. Relative Humidity
Talc, the oldest known solid lubricant, has a low shear strength [6]. Different factors influencing the hydrophobicity of talc are elongation, flatness, roundness, and relative width. A talc particle with higher elongation and flatness and lower roundness and relative width will tend to be more hydrophobic since, in these cases, the adhesion force is stronger due to more areas and lines for contact [18]. These different surface properties of talc can be attributed to its unique molecular chain in the faces and edges (Table 2 and Table 3). Its crystal has three layers, with the two outer layers being composed of Si-O tetrahedra, while the layer in between is a brucite Mg(OH)2 layer or hydroxyl octahedral, bonded by van der Waals forces [4][5][6][7][8]. These two outer layers have zero residual charges, which means no interlayer cation is present [19], while the octahedral layer has charged ions Mg2+, OH- that draw many bonding possibilities with the water molecules[5][7][10]. Moreover, -OH groups of the octahedral layer are known to be influencing the surface behavior of talc [20]. In its natural state, talc adsorbs organic and inorganic species on its surface. The highly energetic sites on its surface are mostly blocked by nitrogen and water molecules [21].
Table 2. Effect of the molecular chain in the talc surface and surface properties.
| Surface Properties | The Molecular Chain in the Surface | Ref. |
|---|---|---|
| Hydrophobic | Neutral >Si-O-Si< groups | [29] |
| Has Si-O-Si siloxane linkages that are common for many silicates and can potentially serve as weak H-bond acceptors. | [30] | |
| Because of the lamellar geometry, the predominance of the hydrophobic basal surface explains the difficult dispersion of natural talc in an aqueous medium. | [5] | |
| Low energy silicate layers of talc plane | [3] | |
| No active sites (O-H groups) account for its natural hydrophobicity, but the negatively charged oxygen atoms can make a weak hydrogen bond with water molecules. | [31] | |
| Hydrophilic | Breaking ionic/covalent bonds | [32] |
| The broken >Si-O- and >Mg-O- bonds | [29] | |
| Weak H-bond donation from the internal Mg-OH sites is also at this surface. | [30] | |
| The formation of a hydrogen bond between the water molecule and the hydroxyl group | [33] | |
| Water is adsorbed on the octahedral layer where OH groups point directly to the surface face | [29] | |
| The more reactive lateral surface is containing -SiOH and -MgOH groups, presenting Bronsted acidity. | [5] | |
| pH-dependent edges with the hydroxyl groups (-SiOH) and (-MgOH) | [3] |
Table 3. Properties of surfaces formed after cleavage.
| Surface Type | Surface after Cleavage | Composition | Properties | Ref. |
|---|---|---|---|---|
| Face | Easy cleavage of the layers | Fully compensated oxygen atoms |
|
[3] |
| Edge | Rupture of ionic bonds within these layers | Composed of hydroxyl ions, silicon, oxygen, and magnesium ions |
|
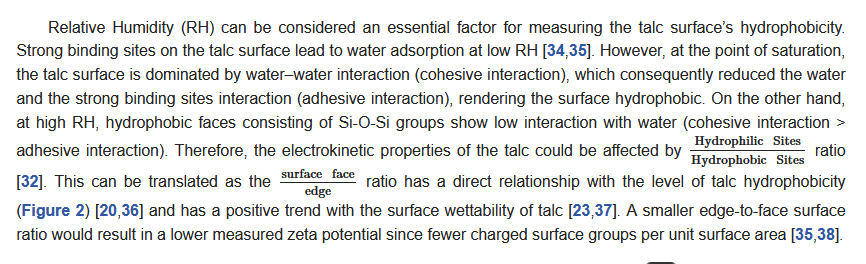
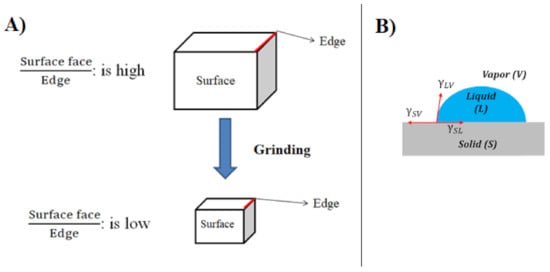
Figure 2. (A) The relation between the size of particles and surface/edge ratio (B) force of cohesion, work of cohesion, and hence surface tension.
Thus, due to talc’s delamination that produces more surface faces, the hydrophobicity increases by decreasing the particle size [28]. Beyond a certain particle size reduction limit (i.e. after subjecting a 10 microns-sized talc in an hour of high-intensity planetary ball milling), the hydrophobicity was seen to decrease (inferred to the loss of crystallinity in the talc and structural degradation) [29]. As examined, the hydrophobic face and hydrophilic edges constitute around 90% and 10% of the total surface area, respectively [11]. These properties also made a significant surface roughness effect on the talc hydrophobicity. A cleaved talc surface is considered to have a very low root mean square roughness, which indicates a very smooth surface. This cleaved surface becomes very hydrophobic, as manifested in the contact angle measurement [12][30][31][26][32][33].
2.2. Zeta Potential
Exploring electrical charge on the talc surface in various conditions indicated that its zeta potential (ZP) magnitude is negative at wide pH ranges (pH 2–12) [18][34][35][36][11][21][37]. This may be attributed to the Mg-OH and Si-OH groups ionization, passing of H+ into the water, and OH ions in water adsorbed on Si2+ and Mg+ [38]. Different studies reported the isoelectric point (IEP) of talc occurred at around pH 2–3 (Table 4). Furthermore, in phyllosilicate minerals such as talc, the IEP may depend on the mineral side where the measurement is made (the edge vs. the face) [14]. The hydrophobic faces consist of –Si–O–Si– groups, have a very slight electrical charge and are nonpolar in water [29][39]. On the other hand, the edges (constructed by breaking ionic/covalent bonds and composed of silicon, hydroxyl ions, oxygen, and magnesium ions [24]) are polar in water. They indicated a relatively high electrical charge, depending on pH [11][39].
Table 4. The talc IEP of the absence and presence of reagents.
Different reagents have various impacts on the talc electrical surface charge (Table 4). It was found that the addition of depressants such as tragacanth gum, guar gum, and carrageenan slightly decreases the absolute value of talc’s potential at a specific pH range [18][35][11][25][40]. Depressant adsorptions could consequently shift the slip surface electrical double layer towards the outside and cause this decrease. On the other hand, the talc IEP in the presence of depressants just increased negligibly. At a very low and very high pH, the addition of some depressants has an insignificant effect. This is attributed to the protonation of, for example, carboxyl groups (for gum Arabic (GA) at pH 2–4), which decreases surface charge; thus, talc’s ZP became less affected [41]. These carboxyl groups will mostly dissociate at a high pH (pH 10–12) and enhance the electrostatic repulsion between GA and talc. It was also found that the presence of chloride-based metal salts, such as NaCl, decreased the ZP of talc but did not have a significant change in IEP [42]. With the presence of an electrolyte such as Ca2+ in addition to depressant CMC, zeta potential is highly affected by pH changes since the electric potential is almost closer to zero in Ca2+ solution [43]. For the cases of leached talc, ZP remains the same, while calcinated talc shows a significant shift. The latter has become more negative, attributed to the change of brucite to MgO and loss of structural water from talc [44].
2.3. Contact Angle
Measuring the talc’s contact angle in air, water, and the number of organic solutions, indicated that its surface is not completely hydrophobic [45]. Various measurements and bulk/surface analysis had been applied to assess the talc contact angle and hydrophobicity for the gross content and cleavage specifications [46]. Assessment of these measurements demonstrated that the talc mono-crystal surface is hydrophobic; however, as mentioned, they indicated hydrophilic behavior at low relative humidity [44]. In other words, particle size could influence the contact angle. Since smaller particle sizes would have a larger edge-to-face ratio [32], the contact angle could also be smaller for these particles than larger ones. However, with the depressants’ presence, the contact angle might be lower for larger particles as the depressant adsorption density could be higher compared to smaller particles (Table 5) [47]. Thus, the talc contact angle is correlated to the surface coverage, and its surface wettability is influenced by the effect of polymer adsorptions [48]. It would be decreased (hydrophobicity increased) when cationic surfactants and different depressants (dextrin, starch, humic acid, and combination of calcium chloride and sodium lignosulfonate) were presented at their sufficient concentrations [4][12][30][32]. The extent of aluminum substitution or impurity in the tetrahedral silica layer could decrease the talc contact angle [19][42][49]. The talc contact angle would not vary significantly with pH variations and instead remained high, owing to the talc’s natural hydrophobicity [50]. It was documented that the static contact angle measurements might not be very reliable due to talc’s possible stick–slip behavior at the wetting front that could result in hysteresis in advancing and receding contact experiments [3][24].
Table 5. Contact angle measurement of talc in the different liquid mediums.
| Medium | Contact Angle (°) | Reference | |
|---|---|---|---|
| Advancing | Receding | ||
| Water | 60 | 50 | [57] |
| Formamide | 42 | 41 | |
| Diodomethane | 38 | 3 | |
| Glycerol | 52 | 42 | |
| Bromonaphtalene | <1 | - | |
| Water (nonwetting) Cyclohexane (wetting) |
80 | 60 | [55] |
| 10∙2 M KCl | 93 | 74 | [58] |
| CH2I2 | 44 * | [9] | |
3. Talc Flotation
3.1. Collectors
Talc shows excellent floatability in a wide pH range (3–11) [16]. Although talc can be considered a naturally hydrophobic mineral, it might need treatment to float. Investigation on pure talc flotation showed that it could float easily [18][33] in a collectorless flotation system by adding frothers (alcohols) [28][51][52][53]. However, various collectors could be considered for the talc selective flotation separation in complex ores [54]. When using xanthate as a collector, Atomic Force Microscope (AFM) and Fourier-transform infrared spectroscopy (FTIR) test results indicated that the collector was physically adsorbed on the talc surface, and adsorption would be negligible. For example, because the potassium amyl xanthate (PAX) did not adsorb onto talc, no competition was detected through the flotation in the adsorption of this collector depressant [39]. In addition, the absence and presence of potassium butyl xanthate (PBX) over the whole pH range did not affect the talc recovery [55]. Among anionic collectors, it was observed that increasing the oleic acid concentration created the multilayers of the collector on the talc surface and reduced its recovery [56]. Cationic collectors (Tallow amine acetate and Dodecylamine) could improve its floatability [5][57]. The adsorbed amount of collector affected the talc recovery, where a high concentration made the talc surface more hydrophobic [57]. It should be observed that adsorption capacity would be dependent on pH, particle size, temperature, adsorption time, and collector concentration [5]. It was reported that without a collector, the talc flotation does not follow the first-order kinetics model but follows the Kelsall model. With collector, it follows both models since the talc morphology had less effect on the flotation performance on minerals with naturally high floatability [58].
3.2. Depressants
The faces of talc crystals consist of quite compensated oxygen atoms, and the edges of them have consisted of hydroxyl ions, silicon, oxygen, and magnesium ions. Since the faces of talc crystals have a very slight electrical charge and are nonpolar in water, the depressant adsorption is expected to have occurred on a greater area of the faces. On the other hand, the edges of talc, which are simply hydrolyzable, have a high electrical charge and are polar in water. Thus, the large particles had higher depressant adsorption than the finer ones, resulting in a lower flotation recovery [32]. Therefore, particle size and the face-to-edge ratio was still considered the main factor for the depressant adsorption [44].
Some investigations documented that the adsorption of Dextrin WY and Polymer-H onto the silica models followed the Freundlich isotherm, which indicates a heterogeneous surface [59]. However, the later studies showed that the adsorption obeyed the Langmuir isotherm, indicating a homogeneous surface [33]. The adsorption isotherms of guar gum and dextrin onto talc followed the Langmuirian behavior and pH-independent. In general, it was also shown that the Langmuir adsorption isotherm fit the data better than the Freundlich model, except when the pH was under 2 (Table 6) [60]. These indicated that the adsorption mainly occurred in the dominant basal face of the talc [8][51]. However, talc edge could also play a role in the adsorption of polysaccharides [11][61]. In general, there is a significant relationship between adsorbed amount, the adsorbed morphology, layer thickness, depressant molecular weights, and the subsequent talc wettability. For some depressants, such as carboxymethyl cellulose (CMC), the enhancement of acidic pH and ionic strengths of the suspension medium increased the adsorption isotherms of the depressant on talc [62]. Adsorption isotherms of the polymers indicated that by increasing the concentration, the talc recovery decreased [23][63].
Table 6. Investigation of adsorption isotherms for different polymers on talc.
| Polymer | Adsorption Model | Maximum Adsorbed Amount (mg·m−2) | Results | Reference |
|---|---|---|---|---|
| MP Dextrin | Langmuir | 0.71 ± 0.04 |
|
[69] |
| CM Dextrin | 0.59 ± 0.02 | |||
| Dextrin TYM | 0.6 ± 0.1 | |||
| Polymer-H | Langmuir | 0.88 ± 0.01 | Adsorption isotherms of polymers indicated that the talc recovery reduced by increasing the depressant concentration | [35] |
| Polymer-N | 0.43 ± 0.01 | |||
| Dextrin WY | 0.92 ± 0.05 | |||
| HP-Dextrin | 0.82 ± 0.02 | |||
| HP-Starch | 4.82 ± 0.41 | |||
| HP Dextrin | Langmuir | 1.05 ± 0.05 | The isotherms indicated high-affinity behavior | [55] |
| CM Dextrin | 0.8 ± 0.02 | |||
| Dextrin TY | 0.62 ± 0.02 |
The polymers with greater layer thicknesses can efficiently depress talc particles [23]. Polysaccharides as dense polymers with high molecular weights (made up of monosaccharide (sugar) units) were between these depressant types [64]. Their adsorption on talc can occur by hydrogen bonding and chemical interaction [65], which strongly make talc faces hydrophilic [64]. The other depressants are CMC (a negatively charged polymer) [3][7][66][43][45][67], guar gum [68], and dextrin [3][7], which also had various degrees of depression on other minerals [68]. Polymer structure and molecular weights are effective factors on the talc depressant adsorption. For example, the high molecular weight of starch leads to a greater adsorbed amount and apparent layer thickness than its low molecular weighted polymers, which decreased the talc wettability [33]. Because of the more favorable cis-configuration of hydroxyl groups in guar gum and its higher molecular weight, it had higher adsorption densities onto talc in comparison with dextrin (Figure 3) [16][20][41][44][69][70][71]. Between CMC and guar gum, the molecular weight of the latter has a more significant effect on talc depression [25].
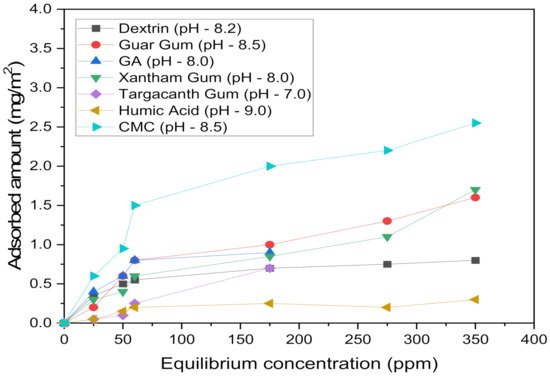
Figure 3. Adsorption of different depressants on the talc surface.
As mentioned, selective talc depression is complicated. For instance, flotation experiments have shown that the P(Am-co-VPD) copolymer system depressed talc and pentlandite in mixed mineral flotation. However, the copolymer system depressed the talc better than guar gum. From an absorption point of view, the low selectivity of depressants could be attributed to the little variation of the Langmuir affinity constant of these two minerals [72]. The effect of various types of depressants on the flotation of talc was summarized in Table 7. Other factors such as calcination also affected the adsorption density of guar gum onto talc. Calcination decreased the adsorption density for talc particles. This decrease was attributed to the desorption of nitrogen species found on the talc surface [20].
Table 7. Depressants and their effect on the talc flotation.
| Depressants | Effects and Results | Ref. |
|---|---|---|
| HP Dextrin, MP Dextrin, CM Dextrin, Dextrin TY, and Dextrin TYM |
|
[69,78] |
| Chitosan |
|
[71] |
|
[67,79] | |
| CMC |
|
[23] |
|
[51] | |
|
[70] | |
|
[80] | |
|
[34,81] | |
|
[82] | |
| Guar Gum |
|
[83] |
|
[27] | |
| Tragacanth Gum |
|
[24] |
| Synthetic polyacrylamides (PAM-A and PAM-N) |
|
[20] |
| Humic Acid (HA) |
|
[74] |
| Galactomannan (KGM) |
|
[73] |
| Sodium Silicate (SS) |
|
[46] |
| Sesbania Gum (SGM) |
|
|
| Lignosulfonates |
|
[47] |
| Galactomannan |
|
[54] |
| Zinc sulfate + sodium carbonate |
|
[50] |
3.3. Frothers
Talc can generally float by adding frothers. Frothers such as alcohols can adsorb on the mineral’s surface by using their hydroxyl group; thus, they are analogous to talc depressants such as starch, cellulose, and dextrin (their molecular structure includes alcohol groups) [73]. However, lower frother adsorption onto the talc surface would be desirable through separation since flotation water reduced the surface tension [31]. Methyl isobutyl carbinol (MIBC) as a typical frother could enhance its flotation recovery because of its collecting capability [74]. MIBC has the least frother adsorption capacity and is more efficient in terms of weight comparing pine oil and Aero 825 while obtaining the highest talc recovery. In collectorless flotation, the addition of MIBC alone could recover 90% of the talc in a micro-flotation test in a wide pH range (acidic to alkaline conditions) [55][75]. This increases relatively and depending on the molecular structure, which could be considered for different frother types (Table 8).
Table 8. Application of various frother types in the talc flotation.
| Frothers | Effects and Results | Ref. |
|---|---|---|
|
|
[12] |
|
|
[43,55] |
| MIBC | By using MIBC, the talc recovery reached more than 80% | [24] |
| Polypropylene glycol |
|
[86] |
| MIBC | In its presence, the talc recovery reached more than 90% | [73] |
| Polyfroth H57 (Huntsman) | By using the hypersaline water and this frother in the flotation system, the recovery was considerably enhanced | [77] |
| Flotanol F | It showed a higher flotation rate constant but lower overall recovery compared to MIBC. | [87] |
It is well understood that through flotation separation, the bubble size strongly depends on frother type and its concentration. Kho et al. (1989) investigated the effects of bubble size on the talc recovery in the column flotation system. The simulation results demonstrated that the air bubble diameter was the most significant parameter to increase talc recovery, where they had a negative correlation (Figure 4). Hence, the control of bubble size would be an essential factor in the talc flotation. Simulation through a column flotation indicated that bubble size significantly influences talc flotation recovery, although increasing the column height could also improve its recovery [76].
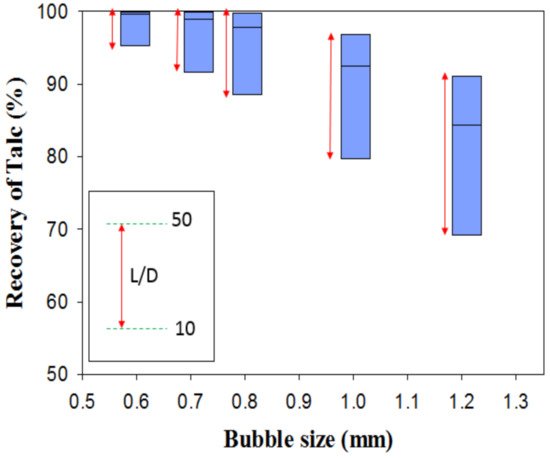
Figure 4. Effects of bubble size on the recovery of talc in the different column flotation (L/D: Length/Diameter) [76]).
3.4. pH Influence
Although the talc floatability is pH-independent, a negative trend between coagulation of talc suspension and pH could be observed [77]. It should be observed that the surfaces of talc particles are net-negatively charged [69][78], and when the pH is enhanced, the zeta-potentials of the talc surface decreased [79]. At pH 9.4, the solubility of talc is at its lowest level; by reducing pH, the negative charge of talc was reduced, and its solubility increased [42][24][80][81]. Thus, talc floatability could be altered by pH [68]. At neutral pH, talc has the highest natural floatability, and in both acidic and alkaline solutions, its floatability is reduced to various degrees [75]. The adsorption mechanisms of talc are different at various pH values [65]. The potential- specifying ions including H+ and OH− of talc mineral are enhancing pH of the solution and, as a result, reducing the adsorption capacity [5]. Ionic strength and pH of the solution indicated some effects on the depressant effectivity [7][8][14][70], although it is mainly suggested that depressant adsorption is independent of pH as the surface charge has a negligible effect [44]. It was shown that at neutral or moderately alkaline pH values (pH 7–9.3), the lignosulfonates would be weak talc depressants; however, their depressing ability significantly enhance under highly alkaline conditions when lime is applied as the pH regulator [78]. Adsorption studies indicated that at pH 3 and pH 9, the adsorption of chitosan is through physical interactions, hydrogen bonding. At pH 9, aside from adsorption due to hydrogen bonding, chitosan changed the potential of talc gradually to zero. In the mixed mineral flotation of chalcopyrite and talc, chitosan was seen as a selective depressant of talc at pH 3 due to the presence of more hydroxyl bonds on talc than chalcopyrite but unselective at pH 9 as chitosan gets deposited on both minerals [14]. At pH 9 with low ionic strength (0.001M KCl), the carboxyl groups of CMC dissociated; thus, electrostatic repulsion occurred between the negatively charged talc and CMC, which prevented the adsorption [11]. The pH also affected the adsorption, wherein CMC was protonated at acidic pH. At pH 3.5, the negative charge of talc was not significant; therefore, electrostatic repulsion is reduced, enhancing the talc depression with CMC [7][8][48][82].
3.5. Ions Influence
By dissolution of talc, the neutral or charged magnesium hydroxo complexes are liberated and, through hydrogen bonding or the chemical interaction, could react with depressants at the hydroxylated talc surface and in the bulk solution [5][17][32]. Hence, the presence of ions influences adsorption on talc. It was indicated that the addition of cations alone (without any depressant) at pH 9 could effectively depress talc floatability [7][66]. In other words, in the presence of metal cations, the importance of depressant molecular weight became less significant and the ionic strength became a more influential factor for the adsorption density [83]. A lower electrostatic repulsion between the talc surface and depressant in the presence of metal cations could be the main reason. The addition of monovalent potassium enhanced the range of depressant adsorption onto talc planes due to very few bonding sites’ availabilities and depressed the talc floatability [22]. However, divalent cations (e.g., Ca2+ and Mg2+) showed a higher talc depression level than monovalent cations (e.g., K+ and Na+). At pH 9, the addition of these ions could lead to a significant degree of talc depression. At this pH, the hydrophobicity reduction occurred through the ions’ adsorption via the addition of charges along the talc planes [66][61][64][71][84]. In the presence of Ca2+ ions, the CMC adsorption onto talc surfaces occurred from the hydrophobicity interactions between the hydrocarbon under the part of glucose rings and the hydrophobic face of talc [79]. The combination of talc, CaCl2, and sodium lignosulfonate, reduced the surface potential of talc due to the complex formation of lignosulfonate ions with Ca2+ ions on the talc surface [17].
In addition, trivalent metal ions had a deep influence on the talc hydrophobicity at different pH values. By strong adsorption of hydroxo complex species including Al3+, Cr3+, and Fe3+ onto the polar surfaces, the zeta potential of talc changed its sign from negative to positive, and its natural hydrophobicity markedly decreased [85]. The Si4+ in the tetrahedral silica layer of the talc structure could be replaced with Al3+. Because of this substitution and the interaction between the water molecules and the enhanced polarity of the talc surface state, the water film on the surface is stable. Thus, the contact angle of the sessile drop was reduced by enhancing the aluminum content from about 80 to 0 degrees [61].
3.6. Pre-Treatment
Ultrasonic pretreatment was seen to reduce the flotation recovery of talc at about 15% with lower depressant dosage and 8% with high depressant dosage. This could not be fully explained, but the possible reason could be the release of Mg ions after talc dissolution that might form Mg hydroxo complexes, which by H bonding and chemical interaction reacted with the polymer [20]. Ultrasound could create defects on the talc face that act as active sites and improved depressant adsorption. Furthermore, ultrasound could clean the edge surface and reveal the Mg sites where depressants could be adsorbed [32]. In industrial processing, the inherent hydrophobicity of talc is detrimental as it results in poor dispersion [86][87]. To address this, Yi et al. (2019) applied thermal treatment (calcination at 960 °C) instead of surfactants that break the siloxane structure to amorphous silica. By thermal treatment, the surface wettability changed from 68.87° to 55.34° attributed to the transformation from the siloxane structure (hydrophobic) to amorphous silica (hydrophilic) [88][89].
4. Summary
Talc is a common gangue phase in sulfide ores and remains a challenge in the beneficiation of target commodities from concentration techniques such as froth flotation and pyrometallurgical processes due to the presence of MgO in the latter. Besides being a gangue phase, there is an increasing demand for talc due to its specific properties such as thermal and chemical stability, which find extensive application in cosmetics, papermaking, paint, etc. Based on its natural hydrophobicity, talc remains a challenge in froth flotation, besides its proven versatility in mineral separation.
Due to its chemical composition and structure, talc is a naturally hydrophobic mineral. However, talc is composed of two different surfaces, the basal surface, and the edge, with different surface properties, the former being the majority (approx. 90%) hydrophobic surface, and the latter being the minority (approx 10%) hydrophilic surface. In determining the level of talc hydrophobicity, the surface faceedge
ratio is often the basis. Zeta potential measurements of talc showed that it is charged negatively at pH 2–12 and its IEP is at pH 2–3. The talc flotation showed that the IEP had little to no change when reagents such as depressants were added to the pulp, although the talc’s potential decreased at certain pH due to shifting the slips surface double layer towards the outside. Talc’s contact angle also showed that its floatability did not vary and remained high even though pH was changed until depressants (e.g., dextrin, guar gum, CMC) were added to the process. Impurities in talc can also decrease the talc contact angle. Although both inorganic and organic depressants have been reported in the literature, the latter seems to be gaining popularity. Given the possible influence of cations and the relatively hazardous nature of inorganic surfactants, using organic depressants is expected to grow. In some cases, using mixtures (inorganic and organic) is reported to improve selectivity, thus offering another potential solution. Considering nonionic polymers (such as starch, dextrin, guar gums) will be commonly opposed to ionic ones (such as CMC) due to their sensitivity to ions present in processed water due to the increased recycling of water.
Due to its natural hydrophobicity, the rejection of talc in the flotation process is challenging. In the collectorless flotation of talc, MIBC has shown to be the most suitable frother that can be used since it had the least frother adsorption capacity and could obtain high recovery. Typical talc depressants are CMC, guar gum, dextrin, etc. The ionic strength and pH of the solution also have some effects on the depressant effectivity. Molecular weight, degree of substitution, and concentration of depressants are some of the factors that affect the depressant performance in the talc flotation. In addition, different ions, including Ca2+, Mg2+, K+, Al3+, CO32-, H+, OH−, Cr3+ and Fe3+ influence talc flotation. The ions’ presence has a desirable influence on talc depression. Di and trivalent cations, in comparison with monovalent cations, have higher talc depression effects. It is undeniable however that all these depressants, even with the presence of different ions, do not seem to depress talc in mixed mineral flotation selectively, and so investigating for new and selective talc depressant is still of great interest. Pre-treatment of talc using ultrasound has also increased the talc recovery (~15%) and could be a promising method on an industrial scale. The poor selectivity and efficiency of depressants point to the lack of full understanding of the flotation and depression behaviors of talc and associated sulfide minerals. As such, there is a need for fundamental studies on the froth stability, surface properties, and their alteration by using more advanced and sensitive techniques such as TOF-SIMS.
Due to the existing limitation in surface modification methods, such as using surfactants (depressants), which have poor selectivity and non-uniformity, other techniques were suggested in the literature. Thermal treatment is shown to decrease the wettability of talc from 68.87° to 55.34°, which should also decrease the degree of hydrophobicity. Considering the siloxane structure is mainly composed of talc (001), and pyrophyllite (001) surfaces (basal surface) are mainly hydrophobic. The edge surface due to the broken bonds is hydrophilic on a microscopic scale. Thus any process that can alter the siloxane structure should reduce the hydrophobicity of talc. Pre-treatment of talc using ultrasound has been reported and decreased the talc recovery by 15 %. The well-reported hydrophobicity of talc is also further enhanced by the associated, very poor dispersion. This poor dispersion is detrimental to flotation due to the formation of flocs which may entrain and/or entrap valuable mineral phases, ultimately leading to poor separation performance. For improved dispersion, the application of hydrodynamic cavitation (nanobubbles) can be explored since the depressants-particle contact is very poor due to the tendency of talc to form flocs. These pretreatment techniques may have great potential in surface modification for talc and even other clay minerals.
Although much of the literature on the flotation of talc reports on the treatment of talc as a gangue phase and is set to report to tailings. With the growing number of waste valorization projects, the recovery of talc should also be considered in initial flowsheet development, considering the growing need for talc and also the challenges associated with talc in waste streams, such as poor water recovery in high talc-containing effluent. The discussion should also focus on the recovery of talc instead of its rejection for better resource utilization and reduced environmental impacts.
References
- Claverie, M.; Dumas, A.; Careme, C.; Poirier, M.; Le Roux, C.; Micoud, P.; Martin, F.; Aymonier, C. Synthetic Talc and Talc-Like Structures: Preparation, Features and Applications. Chem. A Eur. J. 2017, 24, 519–542.
- Castillo, L.A.; Barbosa, S.E.; Capiati, N. Influence of talc genesis and particle surface on the crystallization kinetics of polypropylene/talc composites. J. Appl. Polym. Sci. 2012, 126, 1763–1772.
- Kalantari, K.; Ahmad, M.B.; Masoumi, H.R.F.; Shameli, K.; Basri, M.; Khandanlou, R. Rapid adsorption of heavy metals by Fe3O4/talc nanocomposite and optimization study using response surface methodology. Int. J. Mol. Sci. 2014, 15, 12913–12927.
- Gibson, B.; Wonyen, D.G.; Chelgani, S.C. A review of pretreatment of diasporic bauxite ores by flotation separation. Miner. Eng. 2017, 114, 64–73.
- Kursun, H.; Ates, A. Adsorption and column flotation studies on talc using anionic and cationic collectors. Korean J. Chem. Eng. 2010, 27, 1922–1927.
- Kumar, S.H.; Singh, K.J.; Somani, A.K. Estimatation of talc properties after milling. AIP Conf. Proc. 2016, 1728, 020139.
- Hildick-Smith, G.Y. The biology of talc. Br. J. Ind. Med. 1976, 33, 217–229.
- Qin, W.L.; Xia, T.; Ye, Y.; Zhang, P.P. Fabrication and electromagnetic performance of talc/NiTiO3 composite. R. Soc. Open Sci. 2018, 5, 171083.
- U.S. Geological Survey. Mineral Commodity Summaries 2021; U.S. Geological Survey: Reston, VA, USA, 2021.
- Wallqvist, V.; Claesson, P.M.; Swerin, A.; Schoelkopf, J.; Gane, P.A.C. Influence of Wetting and Dispersing Agents on the Interaction between Talc and Hydrophobic Particles. Langmuir 2009, 25, 6909–6915.
- Morris, G.; Fornasiero, D.; Ralston, J. Polymer depressants at the talc-water interface: Adsorption isotherm, microflotation and electrokinetic studies. Int. J. Miner. Process. 2002, 67, 211–227.
- Kwon, S.; Cho, M.; Lee, S.G. Intrinsic Kinetics of Platy Hydrated Magnesium Silicate (Talc) for Geological CO2 Sequestration: Determination of Activation Barrier. Ind. Eng. Chem. Res. 2014, 53, 16523–16528.
- Farrokhpay, S.; Ndlovu, B.; Bradshaw, D. Behavior of talc and mica in copper ore flotation. Appl. Clay Sci. 2018, 160, 270–275.
- Feng, B.; Peng, J.; Guo, W.; Zhang, W.; Ai, G.; Wang, H. The effect of changes in pH on the depression of talc by chitosan and the associated mechanisms. Powder Technol. 2018, 325, 58–63.
- Mierczynska-Vasilev, A.; Beattie, D.A. Adsorption of tailored carboxymethyl cellulose polymers on talc and chalcopyrite: Correlation between coverage, wettability, and flotation. Miner. Eng. 2010, 23, 985–993.
- Wei, G.; Bo, F.; Jinxiu, P.; Wenpu, Z.; Xianwen, Z. Depressant behavior of tragacanth gum and its role in the flotation separation of chalcopyrite from talc. J. Mater. Res. Technol. 2018, 8, 697–702.
- Fu, Y.; Zhu, Z.; Yao, J.; Han, H.; Yin, W.; Yang, B. Improved depression of talc in chalcopyrite flotation using a novel depressant combination of calcium ions and sodium lignosulfonate. Colloids Surf. A 2018, 558, 88–94.
- Yekeler, M.; Ulusoy, U.; Hiçyılmaz, C. Effect of particle shape and roughness of talc mineral ground by different mills on the wettability and floatability. Powder Technol. 2004, 140, 68–78.
- Lainé, M.; Allard, T.; Balan, E.; Martin, F.O.; Von Bardeleben, H.J.; Robert, J.-L.; Caè;r, S.L. Reaction mechanisms in talc under ionizing radiation: Evidence of a high stability of H• atoms. J. Phys. Chem. C 2016, 120, 2087–2095.
- Rath, R.K.; Subramanian, S.; Laskowski, J.S. Adsorption of Dextrin and Guar Gum onto Talc. A Comparative Study. Langmuir 1997, 13, 6260–6266.
- Michot, L.J.; Villieras, F.; Francois, M.; Yvon, J.; Le Dred, R.; Cases, J.M. The Structural Microscopic Hydrophilicity of Talc. Langmuir 1994, 10, 3765–3773.
- Parolis, L.A.; van der Merwe, R.; Groenmeyer, G.V.; Harris, P.J. The influence of metal cations on the behaviour of carboxymethyl celluloses as talc depressants. Colloids Surf. A 2008, 317, 109–115.
- Beattie, D.A.; Huynh, L.; Kaggwa, G.B.; Ralston, J. Influence of adsorbed polysaccharides and polyacrylamides on talc flotation. Int. J. Miner. Process. 2006, 78, 238–249.
- Ozkan, A.; Dudnik, V.; Esmeli, K. Hydrophobic flocculation of talc with kerosene and effects of anionic surfactants. Part. Sci. Technol. 2016, 34, 235–240.
- Shortridge, P.; Harris, P.; Bradshaw, D.; Koopal, L. The effect of chemical composition and molecular weight of polysaccharide depressants on the flotation of talc. Int. J. Miner. Process. 2000, 59, 215–224.
- Pan, G.; Zhang, G.; Shi, Q.; Chen, W. The effect of sodium alginate on chlorite and serpentine in chalcopyrite flotation. Minerals 2019, 9, 196.
- Wonyen, D.G.; Kromah, V.; Gibson, B.; Nah, S.; Chelgani, S.C. A Review of Flotation Separation of Mg Carbonates (Dolomite and Magnesite). Minerals 2018, 8, 354.
- Leterme, P.; Gayot, A.; Finet, G.; Bizi, M.; Flament, M. Influence of the morphogranulometry and hydrophobicity of talc on its antisticking power in the production of tablets. Int. J. Pharm. 2005, 289, 109–115.
- Walsh, B.J.M. The use of talc as a tlc adsorbent. J. Chem. Educ. 1967, 44, 294.
- Andrade Jr, M.A.; Pastore, H.O. Toward a Delaminated Organotalc: The Use of Polyamidoamine Dendrons. ACS Appl. Mater. Interfaces 2016, 8, 1884–1892.
- Kursun, H. Adsorption and Flotation Characteristics of the Different Types of Frothers. Part. Sci. Technol. 2014, 32, 632–636.
- Feng, D.; Aldrich, C. Effect of Ultrasonication on the Flotation of Talc. Ind. Eng. Chem. Res. 2004, 43, 4422–4427.
- Kaggwa, G.B.; Huynh, L.; Ralston, J.; Bremmell, K. The influence of polymer structure and morphology on talc wettability. Langmuir 2006, 22, 3221–3227.
- Blount, A.M.; Vassiliou, A.H. Identification of Chlorite and Serpentine in Cosmetic or Pharmaceutical Talc. Environ. Health Perspect. 1983, 51, 379–385.
- Kelebek, S.; Yoruk, S.; Smith, G. Wetting behavior of molybdenite and talc in lignosulphonate/MIBC solutions and their separation by flotation. Sep. Sci. Technol. 2001, 36, 145–157.
- Wallqvist, V.; Claesson, P.M.; Swerin, A.; Schoelkopf, J.; Gane, P.A.C. Interaction Forces between Talc and Pitch Probed by Atomic Force Microscopy. Langmuir 2007, 23, 4248–4256.
- Caban-Nevarez, R.; Perales Perez, O.J. Size-dependence hydrophobicity in nanocrystalline talc produced by high-intensity planetary ball milling. MRS Proc. 2015, 1805, mrss15-2138017.
- Alvarez-Silva, M.; Mirnezami, M.; Uribe-Salas, A.; Finch, J. Point of zero charge, isoelectric point and aggregation of phyllosilicate minerals. Can. Metall. Q. 2010, 49, 405–410.
- Beattie, D.A.; Huynh, L.; Kaggwa, G.B.N.; Ralston, J. The effect of polysaccharides and polyacrylamides on the depression of talc and the flotation of sulphide minerals. Miner. Eng. 2006, 19, 598–608.
- Feng, D.; Aldrich, C. Effect of particle size on flotation performance of complex sulphide ores. Miner. Eng. 1999, 12, 721–731.
- Liu, D.; Zhang, G.; Huang, G.; Gao, Y.; Wang, M. Investigations on the selective flotation of chalcopyrite from talc using gum Arabic as depressant. Sep. Sci. Technol. 2020, 55, 3438–3446.
- Ersoy, B. Influence of pH and Chloride-Based Metal Salts on Coagulation/Dispersion Behavior of Talc Suspension. Sep. Sci. Technol. 2011, 46, 1519–1527.
- Wang, X.; Liu, R.; Ma, L.; Qin, W.; Jiao, F. Depression mechanism of the zinc sulfate and sodium carbonate combined inhibitor on talc. Colloids Surf. A 2016, 501, 92–97.
- Liu, G.; Feng, Q.; Ou, L.; Lu, Y.; Zhang, G. Adsorption of polysaccharide onto talc. Miner. Eng. 2006, 19, 147–153.
- Bartell, F.E.; Zuidema, H.H. Wetting Characteristics of Solids of Low Surface Tension such as Talc, Waxes and Resins. J. Am. Chem. Soc. 1936, 58, 1449–1454.
- Mierczynska-Vasilev, A.; Beattie, D.A. The effect of impurities and cleavage characteristics on talc hydrophobicity and polymer adsorption. Int. J. Miner. Process. 2013, 118, 34–42.
- Chen, Z.; Gu, G.; Li, S.; Song, S.; Wang, C. Influence of Particle Size in Talc Suppression by a Galactomannan Depressant. Minerals 2018, 8, 122.
- Mierczynska-Vasilev, A.; Ralston, J.; Beattie, D.A. Adsorption of Modified Dextrins on Talc: Effect of Surface Coverage and Hydration Water on Hydrophobicity Reduction. Langmuir 2008, 24, 6121–6127.
- Wang, J.; Kalinichev, A.; James Kirkpatrick, R. Asymmetric Hydrogen Bonding and Orientational Ordering of Water at Hydrophobic and Hydrophilic Surfaces: A Comparison of Water/Vapor, Water/Talc, and Water/Mica Interfaces. J. Phys. Chem. C 2009, 113.
- Long, T.; Xiao, W.; Yang, W. The effect of molecular assembly between collectors and inhibitors on the flotation of pyrite and talc. R. Soc. Open Sci. 2019, 6, 191133.
- Beaussart, A.; Mierczynska-Vasilev, A.; A Beattie, D. Adsorption of Dextrin on Hydrophobic Minerals. Langmuir 2009, 25, 9913–9921.
- Chelgani, S.C.; Makaremi, S. Explaining the relationship between common coal analyses and Afghan coal parameters using statistical modeling methods. Fuel Process. Technol. 2013, 110, 79–85.
- Jin, S.; Zhang, P.; Ou, L. Study on the depression mechanism of zinc sulfate on talc in chalcopyrite flotation. Colloids Surf. A Physicochem. Eng. Asp. 2021, 619, 126474.
- Zhao, K.; Gu, G.; Wang, X.; Yan, W.; Hu, Y. The effect of depressant sesbania gum on the flotation of a talc-containing scheelite ore. J. Mater. Res. Technol. 2019, 8, 14–21.
- Deng, W.; Xu, L.; Tian, J.; Hu, Y.; Han, Y. Flotation and Adsorption of a New Polysaccharide Depressant on Pyrite and Talc in the Presence of a Pre-Adsorbed Xanthate Collector. Minerals 2017, 7, 40.
- Ahmed, M.M.; Ibrahim, G.A.; Hassan, M.M.A. Improvement of Egyptian talc quality for industrial uses by flotation process and leaching. Int. J. Miner. Process. 2007, 83, 132–145.
- Jiang, H.; Gao, Y.; Khoso, S.A.; Ji, W.; Hu, Y. Interpretation of Hydrophobization Behavior of Dodecylamine on Muscovite and Talc Surface through Dynamic Wettability and AFM Analysis. Minerals 2018, 8, 391.
- Bai, L.; Liu, J.; Han, Y.; Jiang, K.; Zhao, W. Effects of Xanthate on Flotation Kinetics of Chalcopyrite and Talc. Minerals 2018, 8, 369.
- Kaggwa, G.B.; Froebe, S.; Huynh, L.; Ralston, J.; Bremmell, K. Morphology of adsorbed polymers and solid surface wettability. Langmuir 2005, 21, 4695–4704.
- Lee, Y.-C.; Kim, J.-Y.; Shin, H.-J. Removal of Malachite Green (MG) From Aqueous Solutions by Adsorption, Precipitation, and Alkaline Fading Using Talc. Sep. Sci. Technol. 2013, 48, 1093–1101.
- Atluri, V.; Jin, J.; Shrimali, K.; Dang, L.; Wang, X.; Miller, J.D. The hydrophobic surface state of talc as influenced by aluminum substitution in the tetrahedral layer. J. Colloid Interface Sci. 2019, 536, 737–748.
- Burdukova, E.; Bradshaw, D.; Laskowski, J. Effect of CMC and pH on the rheology of suspensions of isotropic and anisotropic minerals. Can. Metall. Q. 2007, 46, 273–278.
- Beattie, D.A.; Huynh, L.; Mierczynska-Vasilev, A.; Myllynen, M.; Flatt, J. Effect of Modified Dextrins on the Depression of Talc and Their Selectivity in Sulphide Mineral Flotation: Adsorption Isotherms, AFM Imaging and Flotation Studies. Can. Metall. Q. 2007, 46, 349–358.
- Khraisheh, M.; Holland, C.; Creany, C.; Harris, P.; Parolis, L. Effect of molecular weight and concentration on the adsorption of CMC onto talc at different ionic strengths. Int. J. Miner. Process. 2005, 75, 197–206.
- Feng, B.; Peng, J.; Guo, W.; Zhu, X.; Huang, W. The stimulus response of chitosan and its depression effect on talc flotation. Miner. Process. Extr. Metall. 2018, 127, 56–61.
- Ma, X.; Pawlik, M. The effect of lignosulfonates on the floatability of talc. Int. J. Miner. Process. 2007, 83, 19–27.
- Zhong, C.; Wang, H.; Zhang, L.; Guo, M.; Bo, F. Flotation separation of molybdenite and talc by xanthan gum. Powder Technol. 2021, 388, 158–165.
- Zhao, K.; Gu, G.; Wang, C.; Rao, X.; Wang, X.; Xiong, X. The effect of a new polysaccharide on the depression of talc and the flotation of a nickel–copper sulfide ore. Miner. Eng. 2015, 77, 99–106.
- Yuan, D.; Xie, L.; Shi, X.; Yi, L.; Zhang, G.; Zhang, H.; Liu, Q.; Zeng, H. Selective flotation separation of molybdenite and talc by humic substances. Miner. Eng. 2018, 117, 34–41.
- Pan, G.; Shi, Q.; Zhang, G.; Huang, G. Selective depression of talc in chalcopyrite flotation by xanthan gum: Flotation response and adsorption mechanism. Colloids Surf. A 2020, 600, 124902.
- Cawood, S.; Harris, P.; Bradshaw, D. A simple method for establishing whether the adsorption of polysaccharides on talc is a reversible process. Miner. Eng. 2005, 18, 1060–1063.
- Leung, A.; Wiltshire, J.; Blencowe, A.; Fu, Q.; Solomon, D.; Qiao, G.G. The effect of acrylamide- co-vinylpyrrolidinone copolymer on the depression of talc in mixed nickel mineral flotation. Miner. Eng. 2011, 24, 449–454.
- Marabini, A.; Belardi, G.; Spaziani, E. Beneficiation of talc from the Valmalenco mine, Italy. Min. Metall. Explor. 1995, 12, 143–148.
- Bulatovic, S.M. Handbook of Flotation Reagents: Chemistry, Theory and Practice: Volume 1: Flotation of Sulfide Ores; Elsevier: Amsterdam, The Netherlands, 2007.
- AL-WAKEEL, A.Y.A.M.I. Talc separation from talc-carbonate ore to be suitable for different Industrial applications. Miner. Eng. 2000, 13, 111–116.
- Kho, C.-J.; Sohn, H.-J. Column flotation of talc. Int. J. Miner. Process. 1989, 27, 157–167.
- Ozkan, A. Coagulation and flocculation characteristics of talc by different flocculants in the presence of cations. Miner. Eng. 2003, 16, 59–61.
- Blaha, J.; Rosasco, G. Raman microprobe spectra of individual microcrystals and fibers of talc, tremolite, and related silicate minerals. Anal. Chem. 1978, 50, 892–896.
- Jin, S.; Shi, Q.; Li, Q.; Ou, L.; Ouyang, K. Effect of calcium ionic concentrations on the adsorption of carboxymethyl cellulose onto talc surface: Flotation, adsorption and AFM imaging study. Powder Technol. 2018, 331, 155–161.
- Feng, B.; Peng, J.; Zhang, W.; Ning, X.; Guo, Y.; Zhang, W. Use of locust bean gum in flotation separation of chalcopyrite and talc. Miner. Eng. 2018, 122, 79–83.
- Cuba-Chiem, L.T.; Huynh, L.; Ralston, J.; Beattie, D.A. In situ particle film ATR FTIR spectroscopy of carboxymethyl cellulose adsorption on talc: Binding mechanism, pH effects, and adsorption kinetics. Langmuir 2008, 24, 8036–8044.
- Douillard, J.; Zajac, J.; Malandrini, H.; Clauss, F. Contact angle and film pressure: Study of a talc surface. J. Colloid Interface Sci. 2002, 255, 341–351.
- Parolis, L.; Groenmeyer, G.; Harris, P. Equilibrium adsorption studies of polysaccharides on talc: The effects of molecular weight and charge and the influence of metal cations. Min. Metall. Explor. 2005, 22, 12–16.
- Jin, S.; Shi, Q.; Feng, Q.; Zhang, G.; Chang, Z. The role of calcium and carbonate ions in the separation of pyrite and talc. Miner. Eng. 2018, 119, 205–211.
- Fuerstenau, M.C.; Lopez-Valdivieso, A.; Fuerstenau, D.W. Role of hydrolyzed cations in the natural hydrophobicity of talc. Int. J. Miner. Process. 1988, 23, 161–170.
- Liu, S.; Liu, X.; Guo, Z.; Liu, Y.; Guo, J.; Zhang, S. Wettability modification and restraint of moisture re-adsorption of lignite using cationic gemini surfactant. Colloids Surf. A Physicochem. Eng. Asp. 2016, 508, 286–293.
- Yi, H.; Zhao, Y.; Rao, F.; Song, S. Hydrophobic agglomeration of talc fines in aqueous suspensions. Colloids Surf. A Physicochem. Eng. Asp. 2018, 538, 327–332.
- Yi, H.; Zhao, Y.; Liu, Y.; Wang, W.; Song, S.; Liu, C.; Li, H.; Zhan, W.; Liu, X. A novel method for surface wettability modification of talc through thermal treatment. Appl. Clay Sci. 2019, 176, 21–28.
- Liu, X.; Liu, X.; Hu, Y. Investigation of the thermal decomposition of talc. Clays Clay Miner. 2014, 62, 137–144.
More
Information
Subjects:
Mining & Mineral Processing
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.6K
Revisions:
3 times
(View History)
Update Date:
29 Jul 2021
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




