Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Nicola Tirelli | + 1798 word(s) | 1798 | 2021-05-31 11:59:09 | | | |
| 2 | Rita Xu | Meta information modification | 1798 | 2021-07-02 06:02:54 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Tirelli, N. Yeast Cells in Microencapsulation. Encyclopedia. Available online: https://encyclopedia.pub/entry/11602 (accessed on 07 February 2026).
Tirelli N. Yeast Cells in Microencapsulation. Encyclopedia. Available at: https://encyclopedia.pub/entry/11602. Accessed February 07, 2026.
Tirelli, Nicola. "Yeast Cells in Microencapsulation" Encyclopedia, https://encyclopedia.pub/entry/11602 (accessed February 07, 2026).
Tirelli, N. (2021, July 02). Yeast Cells in Microencapsulation. In Encyclopedia. https://encyclopedia.pub/entry/11602
Tirelli, Nicola. "Yeast Cells in Microencapsulation." Encyclopedia. Web. 02 July, 2021.
Copy Citation
Yeasts are uni/multicellular eukaryotic organisms, originally thought to be ascomycetous fungi, but later recognized to also comprise basidiomycetous organisms; more typically, yeasts reproduce asexually (rapid duplication) but can also adopt sexual reproduction.
drug delivery
food technology
diffusion phenomena
1. Introduction
Short recap about yeast. Yeasts are uni/multicellular eukaryotic organisms, originally thought to be ascomycetous fungi, but later recognized to also comprise basidiomycetous organisms; more typically, yeasts reproduce asexually (rapid duplication) but can also adopt sexual reproduction. A consensus definition has been proposed, and identifies yeasts “as those fungi whose asexual growth predominantly results from budding or fission, and which do not form their sexual states within or upon a fruiting body” [1]. Yeasts are best known as fermentative fungi, but it is worth noting that they are also very adaptive organisms, and have evolved the capacity to thrive (and ferment) both under aerobic and anaerobic conditions [2]. As a further testament of their capacity of adaptation, they have shown the capacity to acquire resistance to bisulfite [3] or synthetic antifungals [4][5] to the point that some yeasts can even survive nearly saturated brine solutions (e.g., Debaryomyces hansenii, also known as Candida farmata [6]). The industrial importance of these microorganisms resides in their rich metabolic activity (the very word ‘enzyme’ derives from the Greek word for leaven, ζύμη (zýmē)), which since the dawn of time has been heavily employed by humans, predominantly in food processing. Among the most popular yeasts in this business area, one could mention Saccharomyces cerevisiae and Schizosaccharomyces pombe (baking, beer making [7]), Saccharomyces bayanus (previously Saccharomyces uvarum, wine production [8]), Candida kefyr (formerly Candida pseudotropicalis, of which Kluyveromyces fragilis is the sexual stage used in dairy industry, flavors and enzymes synthesis, [9] conversion of lactose to ethanol for biofuel production [10]), and Cyberlindnera jadinii (commonly known as Candida utilis, for food flavoring [11]).
It is worth mentioning, however, that not all yeasts are beneficial; aside from the possibility for humans to develop allergic reactions [12], some yeasts are directly pathogenic such as Candida albicans (previously known as Monilia albicans; candidiasis in the gastrointestinal, reproductive and respiratory systems [13]) or Cryptococcus neoformans (a major source of lymphocytic meningitis [14][15]).
Of specific interest for this review are yeast strains capable of intracellular accumulation of large amounts of lipids, which are known as oleaginous yeasts. They can accommodate both endogenous and exogenous lipids routinely up to 20% (in some cases reportedly much more) of their weight. Examples in the literature go back decades; for example, in the 50s Cryptococcus lipofer (also known as Torulopsis lipofera) was one of the first yeasts to be shown to accumulate large amounts of sterols, [16] while in the 70s, Cutaneotrichosporon curvatum (formerly known as Candida curvata) and Cutaneotrichosporon cutaneum (also known as Basidiotrichosporon cutaneum) were first used to biotechnologically produce food-grade oil from cheese whey [17]. We refer the reader to specialized reviews for both the biochemical mechanisms presiding over lipid accumulation [18] and for the specific applications of these oleaginous yeasts [19], which range from the production of bio-diesel to that of food oils (e.g., cocoa butter substitutes). In terms of the identity of these oleaginous yeasts, possibly the most commonly employed are Cutaneotrichosporon curvatus (until recently known as Cryptococcus curvatus) [20] and Yarrowia lipolytica (formerly known as Saccharomycopsis lipolytica) [21]; a list of yeast strains with large lipid content, and therefore potentially apt for these applications, can be found in a review by Boundy-Mills [22], but it is worth noting that metabolic engineering of selected yeast types (above all of Y. lipolytic) is increasingly employed [23], as opposed to the use of a wider variety of microorganisms.
Yeast cells as microcapsules—technological advantages. Encapsulation allows for compounds of interest to be protected from a potentially aggressive environment, and released in an active form; in principle, the release occurs at specific locations and with a desired time law. A microcapsule does so at 1–1000 μm scale.
Dimensionally, yeast cells suit this definition well. For example, S. cerevisiae (baker’s yeast) cells most commonly range around 5–10 μm, always with a rather narrow size distribution; the upper end of dimensions in the yeast world is likely the Blastomyces dermatitidis, which can be as large as 40 μm [24]. Yeasts fit the definition also from a functional point of view; for a long time (1976 patent) [25], yeast cells have been known for their capacity to absorb large amounts of hydrophobes, and the process can be tuned to produce hydrophobe-loaded cells, i.e. yeast-based microcapsules (YBMCs). YBMCs have been applied to encapsulate poorly soluble actives such as flavoring agents [26], antioxidants [27], biocides (acaricides) [28], increasing their water dispersibility [29], but also providing mechanical protection thanks to the robustness of the yeast cell walls [30]. Actually, cell walls do not fulfill only a mechanical role; they are also the main barrier to both loading [31] and release. It is noteworthy that the latter (the release of actives) typically requires the presence of water, which means that in a dry state (during storage), encapsulated actives are retained with a higher efficiency [32], and release is better obtained upon wet heating [33]. Whether due to solely to these barrier properties, or maybe also to the inherent antioxidant properties of their constituents [34][35], encapsulation in yeast increases the thermo/oxidative stability of actives [36][37], considerably more than e.g. then using ‘standard’ encapsulating agents such as oligo/polysaccharides, e.g. maltodextrins [33], β-glucans [38] or modified starch, or cyclodextrins [39].
Some YBMC features can be perceived as societal advantages. Firstly, they have a sustainable and low-cost origin. Since viability of yeast is not crucial for encapsulation [31], under-utilized by-products such as spent yeast from beer making [40] can be employed. Secondly, they can be seen as safe and consumer-friendly products. Aside from the presence of yeast in virtually all bakery products, whole yeast cells, their lysates or selected components are commonly sold as super-food or flavoring agents.
The relevant literature on YBMCs is, however, poorly coherent to the point that a common parameter such as the encapsulation yield (EY%: the weight ratio between loaded compound and yeast mass) is randomly reported in relation to dry [41] or variably hydrated yeast [31][42]. A more fundamental source of variability is due to the yeast species, since different strains of the same organism might behave differently, and particular culture conditions/various levels of biological stress are likely to also have an effect.
YBMCs—commercial and medical interest. After the first seminal patent in 1976, the encapsulation of lipophilic materials in yeast cells has been protected by patents in applications related to carbonless paper, [43], fabric softeners [44], or fragrances [45] in the textile industry, nicotine for smoking cessation [46], drug delivery targeted to mucosal membranes [47], plant extracts [48], and food flavors [49]. For a more extensive review of these patents, please refer to Paramera et al. [50]. YBMCs may also have effects directly relevant to healthcare, although it is often difficult to pinpoint the precise biomolecular mechanisms and differentiate them from inflammatory (foreign-body) responses. For example, yeast cells have been linked to anti-mutagenic activity, potentially synergic with chemotherapy [51]; this may be related to the activation of inflammatory cells, which recognize yeast cells [52], phagocyte them [53] (also when in the form of yeast particles [54]), and end up exerting protective effects as a result [55]. Yeasts have also been reported to be able to trigger the opening of tight junctions in epithelial models [56], suggesting their use to control permeation through gut barriers, but this effect may also be caused by yeast triggering an inflammatory reaction: for example, C. albicans is well known to damage epithelia upon entry [57][58]. The kind of inflammatory responses elicited by YBMCs is still unclear; for example, when loaded with antigens for vaccines [59][60][61], siRNA [62], or even nanoparticles [63], YBMCs have produced impressive immunosuppressive effects in vivo [64], even without the use of immunosuppressive drugs [65], reportedly through combined pro-apoptotic and immunomodulatory effects [66].
2. Yeast Cells and Their Barrier Structures
Yeast cells feature an external cell wall and an inner membrane surrounding its intracellular environment, as shown in Figure 1.
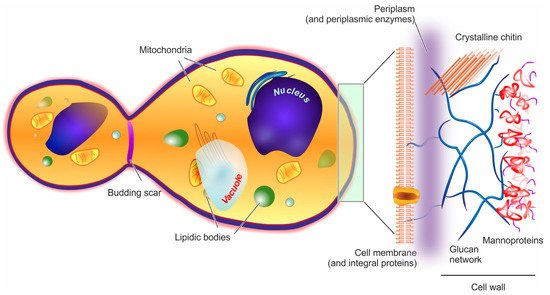
Figure 1. In the structure of a yeast cell (here represented in a post-budding state), the elements potentially acting as barriers to encapsulation (cell wall and cell membrane) are depicted in a magnified fashion on the right hand side of the figure.
Cell wall. In yeast, the wall accounts for approx. 15–20% of the cell dry mass. Its thickness is very variable (70–200 nm), since it increases in response to compression or osmotic forces [67]; structurally, however, it is always a highly polar double-layered matrix (a kind of hydrogel). Its inner part is mainly composed of branched β-(1,3) and β-(1,6) glucans (about 50% of the overall wall) [68] hydrogen-bonded to 3–4% of mostly crystalline chitin [69]. This inner layer is likely to be the main contributor to the overall mechanical resistance of the whole cell wall [70]. The outer layer consists chiefly of mannoproteins, which are negatively charged proteins highly N- or O-glycosylated with mannose or mannosyl phosphate residues linked by 1,2-, 1,3-, 1,4- and 1,6-α-linkages (the latter mostly composed of short chains typically referred to as mannan) [71]. Mannoproteins are the main contributors to the cell wall surface properties, for example, the anionic mannosyl phosphate residues determine the yeast cell surface charge [72] and their reduction in number determines an increase in its hydrophobicity [73]. Furthermore, through their covalent linkage to the β-glucan layer, mannoproteins contribute to the wall outer porosity [74], and above all to yeast adhesion, for example, the C. albicans adhesins that allow its binding to oral epithelial cells are all mannoproteins [75].
Of note, the overall wall composition is rather constant throughout the different yeast species [76], and that of S. cerevisiae can be considered as representative for almost all Ascomycetes [77]; it must be pointed out, however, that although the main components are preserved, the actual detailed composition is not at all simple, with about 1200 genes having being linked to a role in the cell wall [78].
Plasma membrane. It is composed in equal parts of lipids (mainly glycerophospholipids and fatty acids, with a smaller quantity of sterols such as ergosterol and sphingolipids) and proteins, and is connected by glycoproteins and glycolipids to the cell wall, from which it is separated by a non-continuous, enzyme-rich region known as the periplasm. The membrane main functional role is the regulation of the transport from/to the cell. [79] In the outer part of the membrane, phosphatidylcholine, phosphatidylethanolamine, phosphatidic acid, and sphingolipids are mostly found, while the more negatively charged phosphatidylserine and phosphatidylinositol are mostly present only in the inner membrane [70]; as in mammalian cells, lipid rafts are commonly observed, and have important roles in protein segregation and localization [80], which in turn have crucial effects in cell growth and death [81], or mating and division [82].
Intracellular ultrastructure. The yeast interior is compartmented into a nucleus, one or, more commonly, several vacuoles, mitochondria, the endoplasmic reticulum, and a number of vesicular bodies that include peroxisomes and oxisomes. It is worth noting that vacuoles are large (sometimes similar in size to nuclei), mildly acidic (pH 6–6.5) vesicles, whose functions in both storage and stress response/digestion bear some functional analogies to mammalian lysosomes/autophagosomes [83][84].
References
- Kurtzman, C.P.; Fel, J.W.; Boekhout, T. (Eds.) Definition, classification and nomenclature of the yeasts. In The Yeasts, a Taxonomic Study; Elsevier Science: Amsterdam, The Netherlands, 2011.
- Dashko, S.; Zhou, N.; Compagno, C.; Piskur, J. Why, when, and how did yeast evolve alcoholic fermentation? FEMS Yeast Res. 2014, 14, 826–832.
- Nadai, C.; Treu, L.; Campanaro, S.; Giacomini, A.; Corich, V. Different mechanisms of resistance modulate sulfite tolerance in wine yeasts. Appl. Microbiol. Biotechnol. 2016, 100, 797–813.
- Prasad, R.; Lata Panwar, S.; Smriti, K. Drug resistance in yeasts—An emerging scenario. In Advances in Microbial Physiology; Academic Press: Cambridge, MA, USA, 2002; Volume 46, pp. 155–201.
- Whaley, S.G.; Berkow, E.L.; Rybak, J.M.; Nishimoto, A.T.; Barker, K.S.; Rogers, P.D. Azole Antifungal Resistance in Candida albicans and Emerging Non-albicans Candida Species. Front. Microbiol. 2017, 7, 2173.
- Breuer, U.; Harms, H. Debaryomyces hansenii—An extremophilic yeast with biotechnological potential. Yeast 2006, 23, 415–437.
- Benito, Á.; Calderón, F.; Benito, S. Mixed alcoholic fermentation of Schizosaccharomyces pombe and Lachancea thermotolerans and its influence on mannose-containing polysaccharides wine composition. AMB Express 2019, 9, 17.
- González, S.S.; Barrio, E.; Gafner, J.; Querol, A. Natural hybrids from Saccharomyces cerevisiae, Saccharomyces bayanus and Saccharomyces kudriavzevii in wine fermentations. FEMS Yeast Res. 2006, 6, 1221–1234.
- Güneşer, O.; Demirkol, A.; Karagül Yüceer, Y.; Özmen Toğay, S.; İşleten Hoşoğlu, M.; Elibol, M. Bioflavour production from tomato and pepper pomaces by Kluyveromyces marxianus and Debaryomyces hansenii. Bioprocess Biosyst. Eng. 2015, 38, 1143–1155.
- Löser, C.; Urit, T.; Gruner, E.; Bley, T. Efficient growth of Kluyveromyces marxianus biomass used as a biocatalyst in the sustainable production of ethyl acetate. Energy Sustain. Soc. 2015, 5, 2.
- Kieliszek, M.; Kot, A.; Bzducha-Wróbel, A.; Błażejak, S.; Gientka, I.; Kurcz, A. Biotechnological use of Candida yeasts in the food industry: A review. Fungal Biol. Rev. 2017, 31, 185–198.
- Bansal, R.A.; Tadros, S.; Bansal, A.S. Beer, Cider, and Wine Allergy. Case Rep. Immunol. 2017, 2017, 7958924.
- Dermawan, J.K.T.; Ghosh, S.; Keating, M.K.; Gopalakrishna, K.V.; Mukhopadhyay, S. Candida pneumonia with severe clinical course, recovery with antifungal therapy and unusual pathologic findings: A case report. Medicine 2018, 97, e9650.
- Shribman, S.; Noyce, A.; Gnanapavan, S.; Lambourne, J.; Harrison, T.; Schon, F. Cryptococcal meningitis in apparently immunocompetent patients: Association with idiopathic CD4+ lymphopenia. Pract. Neurol. 2018, 18, 166–169.
- Poley, M.; Koubek, R.; Walsh, L.; McGillen, B. Cryptococcal Meningitis in an Apparent Immunocompetent Patient. J. Investig. Med. High Impact Case Rep. 2019, 7, 2324709619834578.
- Schuytema, C.G.; Lata, G.F. Ergosterol synthesis and storage in the yeast Torulopsis lipofera. Arch. Biochem. Biophys. 1958, 75, 40–45.
- Moon, N.J.; Hammond, E.G.; Glatz, B.A. Conversion of Cheese Whey and Whey Permeate to Oil and Single-Cell Protein. J. Dairy Sci. 1978, 61, 1537–1547.
- Beopoulos, A.; Nicaud, J.M.; Gaillardin, C. An overview of lipid metabolism in yeasts and its impact on biotechnological processes. Appl. Microbiol. Biotechnol. 2011, 90, 1193–1206.
- Papanikolaou, S.; Aggelis, G. Lipids of oleaginous yeasts. Part II: Technology and potential applications. Eur. J. Lipid Sci. Technol. 2011, 113, 1052–1073.
- Liang, Y.N.; Cui, Y.; Trushenski, J.; Blackburn, J.W. Converting crude glycerol derived from yellow grease to lipids through yeast fermentation. Bioresour. Technol. 2010, 101, 7581–7586.
- Beopoulos, A.; Cescut, J.; Haddouche, R.; Uribelarrea, J.-L.; Molina-Jouve, C.; Nicaud, J.-M. Yarrowia lipolytica as a model for bio-oil production. Prog. Lipid Res. 2009, 48, 375–387.
- Sitepu, I.R.; Garay, L.A.; Sestric, R.; Levin, D.; Block, D.E.; German, J.B.; Boundy-Mills, K.L. Oleaginous yeasts for biodiesel: Current and future trends in biology and production. Biotechnol. Adv. 2014, 32, 1336–1360.
- Shi, S.; Zhao, H. Metabolic Engineering of Oleaginous Yeasts for Production of Fuels and Chemicals. Front. Microbiol. 2017, 8, 2185.
- Walker, K.; Skelton, H.; Smith, K. Cutaneous lesions showing giant yeast forms of Blastomyces dermatitidis. J. Cutan. Pathol. 2002, 29, 616–618.
- Shank, J.L. Encapsulation Process Utilizing Microorganisms and Products Produced Thereby. U.S. Patent US4001480A, 16 August 1976.
- Sultana, A.; Miyamoto, A.; Hy, Q.L.; Tanaka, Y.; Fushimi, Y.; Yoshii, H. Microencapsulation of flavors by spray drying using Saccharomyces cerevisiae. J. Food Eng. 2017, 199, 36–41.
- Shi, G.; Rao, L.; Yu, H.; Xiang, H.; Pen, G.; Long, S.; Yang, C. Yeast-cell-based microencapsulation of chlorogenic acid as a water-soluble antioxidant. J. Food Eng. 2007, 80, 1060–1067.
- da Silva Lima, A.; Maciel, A.P.; Mendonça, C.d.J.S.; Costa Junior, L.M. Use of encapsulated carvacrol with yeast cell walls to control resistant strains of Rhipicephalus microplus (Acari: Ixodidae). Ind. Crops Prod. 2017, 108, 190–194.
- Shi, G.; Rao, L.; Yu, H.; Xiang, H.; Yang, H.; Ji, R. Stabilization and encapsulation of photosensitive resveratrol within yeast cell. Int. J. Pharm. 2008, 349, 83–93.
- Stenson, J.; Hartley, P.; Wang, C.; Thomas, C. Determining the Mechanical Properties of Yeast Cell Walls. Biotechnol. Prog. 2011, 27, 505–512.
- Ciamponi, F.; Duckham, C.; Tirelli, N. Yeast cells as microcapsules. Analytical tools and process variables in the encapsulation of hydrophobes in S. cerevisiae. Appl. Microbiol. Biotechnol. 2012, 95, 1445–1456.
- Dardelle, G.; Normand, V.; Steenhoudt, M.; Bouquerand, P.-E.; Chevalier, M.; Baumgartner, P. Flavour-Encapsulation and flavour-release performances of a commercial yeast-based delivery system. Food Hydrocoll. 2007, 21, 953–960.
- Sultana, A.; Tanaka, Y.; Fushimi, Y.; Yoshii, H. Stability and release behavior of encapsulated flavor from spray-dried Saccharomyces cerevisiae and maltodextrin powder. Food Res. Int. 2018, 106, 809–816.
- Kogan, G.; Pajtinka, M.; Babincova, M.; Miadokova, E.; Rauko, P.; Slamenova, D.; Korolenko, T.A. Yeast cell wall polysaccharides as antioxidants and antimutagens: Can they fight cancer? Neoplasma 2008, 55, 387–393.
- Kogan, G.; Staško, A.; Bauerová, K.; Polovka, M.; Šoltés, L.; Brezová, V.; Navarová, J.; Mihalová, D. Antioxidant properties of yeast (1→3)-β-d-glucan studied by electron paramagnetic resonance spectroscopy and its activity in the adjuvant arthritis. Carbohydr. Polym. 2005, 61, 18–28.
- Iassonova, D.R.; Hammond, E.G.; Beattie, S.E. Oxidative stability of polyunsaturated triacylglycerols encapsulated in oleaginous yeast. J. Am. Oil Chem. Soc. 2008, 85, 711–716.
- Wu, J.; Guan, Y.; Zhong, Q. Yeast mannoproteins improve thermal stability of anthocyanins at pH 7.0. Food Chem. 2015, 172, 121–128.
- Beikzadeh, S.; Shojaee-Aliabadi, S.; Dadkhodazade, E.; Sheidaei, Z.; Abedi, A.-S.; Mirmoghtadaie, L.; Hosseini, S.M. Comparison of Properties of Breads Enriched with Omega-3 Oil Encapsulated in β-Glucan and Saccharomyces cerevisiae Yeast Cells. Appl. Food Biotechnol. 2019, 7, 11–20.
- Paramera, E.I.; Konteles, S.J.; Karathanos, V.T. Stability and release properties of curcumin encapsulated in Saccharomyces cerevisiae, β-cyclodextrin and modified starch. Food Chem. 2011, 125, 913–922.
- Jaeger, A.; Arendt, E.K.; Zannini, E.; Sahin, A.W. Brewer’s Spent Yeast (BSY), an Underutilized Brewing By-Product. Fermentation 2020, 6, 123.
- Czerniak, A.; Kubiak, P.; Białas, W.; Jankowski, T. Improvement of oxidative stability of menhaden fish oil by microencapsulation within biocapsules formed of yeast cells. J. Food Eng. 2015, 167, 2–11.
- Bishop, J.R.P.; Nelson, G.; Lamb, J. Microencapsulation in yeast cells. J. Microencapsul. 1998, 15, 761–773.
- Pannell, N.A. Microbial Encapsulation. International Patent Application No. EP0242135B1, 12 April 1986.
- Behan, J.M.; Perring, K.D. Fabric Softening Compositions Containing Microorganism-Encapsulated Perfume. U.S. Patent US5078904A, 7 January 1992.
- Nelson, G. Application of microencapsulation in textiles. Int. J. Pharm. 2002, 242, 55–62.
- McNeight, D.L. Nicotine Delivery Systems. International Patent Application No. EP1176961B1, 5 November 2003.
- Gordon, N.; Duckham, S.C.; Round, A.E. Targeted Delivery of Microbially Encapsulated Drugs. International Patent Application No. GB2394416A, 11 May 2000.
- Siegel, S.; Mavric, E.; Krammer, G. Encapsulated Vaccinium Extracts with Balanced Gastrointestinal Release. U.S. Patent US20090041872A1, 7 August 2008.
- Barra, J.; Dardelle, G.; Marty, M.; Castioni, N.V.; Wick, M.; Zampieri, D. Process for Encapsulating an Active Ingredient. International Patent Application No. WO2012084467A1, 28 June 2012.
- Paramera, E.I.; Karathanos, V.T.; Konteles, S.J. Yeast cells and yeast-based materials for microencapsulation. In Microencapsulation in the Food Industry; Gaonkar, A.G., Vasisht, N., Khare, A.R., Sobel, R., Eds.; Academic Press: San Diego, CA, USA, 2014; Chapter 23; pp. 267–281.
- Chorvatovičová, D.; Machová, E.; Šandula, J.; Kogan, G. Protective effect of the yeast glucomannan against cyclophosphamide-induced mutagenicity. Mutat. Res. Genet. Toxicol. Environ. 1999, 444, 117–122.
- Fuller, E.; Duckham, C.; Wood, E. Disruption of Epithelial Tight Junctions by Yeast Enhances the Paracellular Delivery of a Model Protein. Pharm. Res. 2007, 24, 37–47.
- Yang, W.; Yan, L.; Wu, C.; Zhao, X.; Tang, J. Fungal invasion of epithelial cells. Microbiol. Res. 2014, 169, 803–810.
- Wächtler, B.; Citiulo, F.; Jablonowski, N.; Förster, S.; Dalle, F.; Schaller, M.; Wilson, D.; Hube, B. Candida albicans-Epithelial Interactions: Dissecting the Roles of Active Penetration, Induced Endocytosis and Host Factors on the Infection Process. PLoS ONE 2012, 7, e36952.
- Nicola, A.M.; Casadevall, A.; Goldman, D.L. Fungal killing by mammalian phagocytic cells. Curr. Opin. Microbiol. 2008, 11, 313–317.
- Erwig, L.P.; Gow, N.A.R. Interactions of fungal pathogens with phagocytes. Nat. Rev. Microbiol. 2016, 14, 163–176.
- Upadhyay, T.K.; Fatima, N.; Sharma, D.; Saravanakumar, V.; Sharma, R. Preparation and characterization of beta-glucan particles containing a payload of nanoembedded rifabutin for enhanced targeted delivery to macrophages. EXCLI J. 2017, 16, 210–228.
- Stubbs, A.C.; Martin, K.S.; Coeshott, C.; Skaates, S.V.; Kuritzkes, D.R.; Bellgrau, D.; Franzusoff, A.; Duke, R.C.; Wilson, C.C. Whole recombinant yeast vaccine activates dendritic cells and elicits protective cell-mediated immunity. Nat. Med. 2001, 7, 625–629.
- Huang, H.; Ostroff, G.R.; Lee, C.K.; Specht, C.A.; Levitz, S.M. Robust Stimulation of Humoral and Cellular Immune Responses following Vaccination with Antigen-Loaded β-Glucan Particles. mBio 2010, 1, e00164-10.
- Pan, Y.; Li, X.; Kang, T.; Meng, H.; Chen, Z.; Yang, L.; Wu, Y.; Wei, Y.; Gou, M. Efficient delivery of antigen to DCs using yeast-derived microparticles. Sci. Rep. 2015, 5, 10687.
- De Smet, R.; Demoor, T.; Verschuere, S.; Dullaers, M.; Ostroff, G.; Leclercq, G.; Allais, L.; Pilette, C.; Dierendonck, M.; De Geest, B.; et al. Beta-Glucan microparticles are good candidates for mucosal antigen delivery in oral vaccination. J. Control. Release 2013, 172, 671–678.
- Aouadi, M.; Tesz, G.J.; Nicoloro, S.M.; Wang, M.; Chouinard, M.; Soto, E.; Ostroff, G.R.; Czech, M.P. Orally delivered siRNA targeting macrophage Map4k4 suppresses systemic inflammation. Nature 2009, 458, 1180–1184.
- Soto, E.; Caras, A.; Kut, L.; Castle, M.; Ostroff, G. Glucan Particles for Macrophage Targeted Delivery of Nanoparticles. J. Drug Deliv. 2012, 2012, 143524.
- Gao, X.; Gao, C.; Liu, G.; Hu, J. MAP4K4: An emerging therapeutic target in cancer. Cell Biosci. 2016, 6, 56.
- Zhang, X.; Xu, X.; Chen, Y.; Dou, Y.; Zhou, X.; Li, L.; Li, C.; An, H.; Tao, H.; Hu, H.-Y.; et al. Bioinspired yeast microcapsules loaded with self-assembled nanotherapies for targeted treatment of cardiovascular disease. Mater. Today 2017, 20, 301–313.
- Ghoneum, M.; Badr El-Din, N.K.; Noaman, E.; Tolentino, L. Saccharomyces cerevisiae, the Baker’s Yeast, suppresses the growth of Ehrlich carcinoma-bearing mice. Cancer Immunol. Immunother. 2008, 57, 581–592.
- Nelson, G.; Duckham, S.C.; Crothers, M.E.D. Microencapsulation in Yeast Cells and Applications in Drug Delivery. In Polymeric Drug Delivery I; American Chemical Society: Washington, DC, USA, 2006; Volume 923, pp. 268–281.
- Klis, F.; Mol, P.; Hellingwerf, K.; Brul, S. Dynamics of cell wall structure in Saccharomyces cerevisiae. FEMS Microbiol. Rev. 2002, 26, 239–256.
- McLellan, W.L., Jr.; McDaniel, L.E.; Lampen, J.O. Purification of phosphomannanase and its action on the yeast cell wall. J. Bacteriol. 1970, 102, 261–270.
- Aguilar-Uscanga, B.; François, J.M. A study of the yeast cell wall composition and structure in response to growth conditions and mode of cultivation. Lett. Appl. Microbiol. 2003, 37, 268–274.
- Lesage, G.; Bussey, H. Cell Wall Assembly in Saccharomyces cerevisiae. Microbiol. Mol. Biol. Rev. 2006, 70, 317–343.
- Friis, J.; Ottolenghi, P. The genetically determined binding of alcian blue by a minor fraction of yeast cell walls. C. R. Trav. Lab. Carlsberg 1970, 37, 327–341.
- Singleton, D.R.; Masuoka, J.; Hazen, K.C. Surface hydrophobicity changes of two Candida albicans serotype B mnn4 delta mutants. Eukaryot. Cell 2005, 4, 639–648.
- Zlotnik, H.; Fernandez, M.P.; Bowers, B.; Cabib, E. Saccharomyces cerevisiae mannoproteins form an external cell wall layer that determines wall porosity. J. Bacteriol. 1984, 159, 1018–1026.
- Cannon, R.D.; Chaffin, W.L. Oral colonization by Candida albicans. Crit. Rev. Oral Biol. Med. 1999, 10, 359–383.
- Gow, N.; Munro, C.; Latge, J.-P. The Fungal Cell Wall: Structure, Biosynthesis, and Function. Microbiol. Spectr. 2017, 5, 1–25.
- Kapteyn, J.C.; Van Den Ende, H.; Klis, F.M. The contribution of cell wall proteins to the organization of the yeast cell wall. Biochim. Biophys. Acta 1999, 1426, 373–383.
- de Groot, P.W.; Ruiz, C.; Vázquez de Aldana, C.R.; Duenas, E.; Cid, V.J.; Del Rey, F.; Rodríquez-Peña, J.M.; Pérez, P.; Andel, A.; Caubín, J.; et al. A genomic approach for the identification and classification of genes involved in cell wall formation and its regulation in Saccharomyces cerevisiae. Comp. Funct. Genom. 2001, 2, 124–142.
- Grillitsch, K.; Tarazona, P.; Klug, L.; Wriessnegger, T.; Zellnig, G.; Leitner, E.; Feussner, I.; Daum, G. Isolation and characterization of the plasma membrane from the yeast Pichia pastoris. BBA Biomembr. 2014, 1838, 1889–1897.
- Bagnat, M.; Keranen, S.; Shevchenko, A.; Shevchenko, A.; Simons, K. Lipid rafts function in biosynthetic delivery of proteins to the cell surface in yeast. Proc. Natl. Acad. Sci. USA 2000, 97, 3254–3259.
- Mollinedo, F. Lipid raft involvement in yeast cell growth and death. Front. Oncol. 2012, 2, 140.
- Bagnat, M.; Simons, K. Cell surface polarization during yeast mating. Proc. Natl. Acad. Sci. USA 2002, 99, 14183–14188.
- Thumm, M. Structure and function of the yeast vacuole and its role in autophagy. Microsc. Res. Tech. 2000, 51, 563–572.
- Li, S.C.; Kane, P.M. The yeast lysosome-like vacuole: Endpoint and crossroads. BBA Mol. Cell Res. 2009, 1793, 650–663.
More
Information
Subjects:
Biochemistry & Molecular Biology
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.7K
Revisions:
2 times
(View History)
Update Date:
02 Jul 2021
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




