
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Jeremy Yuvaraj | + 1362 word(s) | 1362 | 2021-05-24 09:00:37 | | | |
| 2 | Lindsay Dong | Meta information modification | 1362 | 2021-06-29 10:21:02 | | | | |
| 3 | Conner Chen | Meta information modification | 1362 | 2021-09-22 03:28:56 | | |
Video Upload Options
Coronary computed tomography angiography (CTA) provides a means of mapping inflammatory changes to both epicardial adipose tissue (EAT) and pericoronary adipose tissue (PCAT) as independent markers of coronary risk.
1. Introduction
In recent decades, atherosclerosis has become well recognised as a disease of chronic vascular inflammation. Where the excess accumulation of lipid in the arterial wall was thought to be the most prominent driver of plaque formation, a strong body of evidence highlights the critical effect of vascular inflammatory mechanisms in plaque formation and morphology, as well as their contribution to the onset of major coronary events. The poor localisation of traditional systemic biomarkers to the coronary vasculature has led to exploration and discovery of suitable alternative methods of quantifying inflammatory risk using non-invasive coronary computed tomography angiography (CTA) imaging.
2. Coronary Computed Tomography Angiography (CTA) Assessment of Cardiac Adipose Tissue as a Marker of Coronary Inflammation
2.1. EAT Quantification
CT attenuation of adipose tissue reflects morphological derangements of adipocytes exposed to the effects of local vascular inflammation. Coronary CTA readily quantifies EAT volume and density as independent markers of adverse cardiometabolic risk each bearing associations with CAD (Figure 1) [1][2]. Increased EAT volume is a predictor of the presence of CAD, acute MI and ‘high-risk’ CAD phenotypes [2][3][4]. Likewise, EAT attenuation has been associated with CAD, although the nature of this association demonstrates significant heterogeneity. Factors such as coronary calcification [5][6][7][8][9], coronary events [1][5] and statin therapy [10][11] all producing seemingly disparate effects on EAT attenuation. This may be owed to the anatomy of EAT as a depot, which inherently encompasses a wide range of adipocytes varying in proximity to the vessel wall and, accordingly, exposure to coronary inflammation.
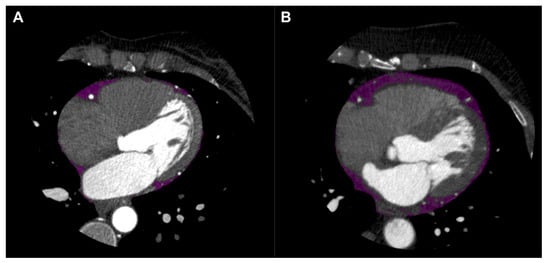
Figure 1. Epicardial adipose tissue (EAT) shown in purple on axial view of coronary computed tomography angiography (CTA). EAT in patient without coronary artery disease (CAD) shown in left panel (A), and EAT in patient with CAD shown in right panel (B).
2.2. Pericoronary Adipose Tissue and Fat Attenuation Index
Pericoronary adipose tissue (PCAT) resides directly adjacent to the coronary adventitia, and harbours the most profound exposure to inflammatory mediators that may arise from the vasculature. Accordingly, it is emerging as a prominent non-invasive metric of coronary inflammation. The current primary definition of PCAT on coronary CTA is adipose tissue residing within a volume that extends to an orthogonal distance equivalent to the diameter of the target vessel. PCAT is typically measured around select lesions, or in the proximal segments of the major coronary arteries, particularly the right coronary artery (RCA) due to the low number of side branches, abundance of adipose tissue and uniformity of luminal diameter from its ostial to distal segments [12][13] (Figure 2).
The pericoronary fat attenuation index (FAI) is an AI-driven quantitation of adipose tissue attenuation computationally adjusted for a range of additional factors, such as CT technical parameters and adipocyte morphology [12][13]. Increased pericoronary FAI has been associated with inflammatory mediators [12], high-risk coronary plaque [14] and coronary event endpoints [13][15]. It also reflects the effects of anti-inflammatory therapy [16][17].
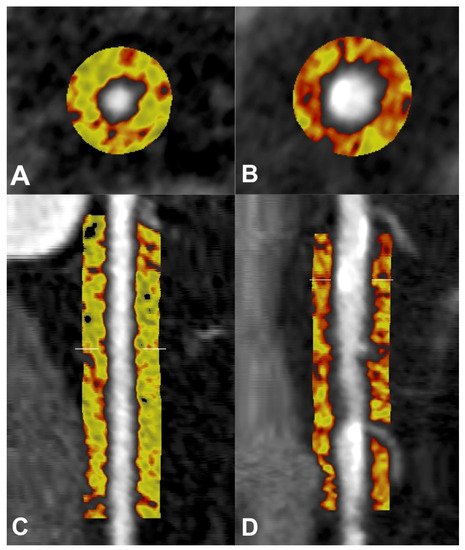
Figure 2. Pericoronary adipose tissue (PCAT) shown in cross-sectional (A,B) and longitudinal (C,D) views of the right coronary artery (RCA) on coronary computed tomography angiography (CTA). PCAT in RCA without plaque represented in the left panels (A,C), and PCAT in RCA with calcified and non-calcified plaque represented in the right panels (B,D). Colour map describes spectrum of adipose tissue attenuation values in Hounsfield units (HU), ranging from −190 HU (yellow) to −30 HU (red), with higher attenuation values indicating inflammatory changes.
2.3. ‘Crude’ PCAT Attenuation and CAD
Numerous observational studies aside from those listed above have together cultivated a mounting level of evidence into the nature of coronary inflammation shown through PCAT attenuation, albeit without the adjustments afforded by the algorithm that characterises FAI. A range of methodologies have been previously employed to study and classify PCAT on CT. While these studies are informative, a key limitation is the absence of a clear demarcation between pericoronary and ‘non-pericoronary’ fat, and thus the influence of ‘non-perivascular’ fractions of EAT in analysis cannot be dismissed. Many recent studies, however, have adopted the standardised approach described previously [12][13] and while these studies may assess the ‘unadjusted’ or ‘crude’ form of PCAT attenuation, the employment of a consistent methodology has been conducive to the generation of results that are more readily reproducible and cross-verifiable [18].
PCAT attenuation studies have shown that coronary inflammatory changes may occur incrementally with the burden of CAD and coronary events [19]. PCAT attenuation is also increased around high-risk coronary plaque [20][21] and in male patients [22][23][24][25].
2.4. PCAT Assessment of Haemodynamically-Significant Lesions
It is important to note that high-risk coronary plaque phenotypes are often non-obstructive in nature, and thus differential pathophysiological mechanisms may be pertinent to plaque morphology compared to plaque-related stenosis. Nevertheless, several studies comparing PCAT attenuation with measures of coronary flow and myocardial ischaemia denote the potential role of vascular inflammation in coronary stenosis [26][27][28].
2.5. PCAT Assessment in Non-Atherosclerotic Disease States
Therefore, both adjusted and ‘crude’ PCAT assessment have demonstrated distinctly increased coronary inflammation in patients with developing high-risk lesions or major coronary events, collectively providing further validation for the potential clinical utility of this technique. In addition to these studies on orthodox coronary atherosclerosis, PCAT attenuation has been explored in a number other coronary and inflammatory disease states, including vasospastic angina [29][30], atherosclerotic intraplaque cholesterol crystals [31], and spontaneous coronary artery dissection [32].
2.6. Technical Parameter Influence in PCAT Assessment
A number of observational studies have evaluated the impact of scan parameters, particularly contrast enhancement, on PCAT attenuation. Given the nature of coronary CTA as one of the first-line imaging modalities with wide usage in assessment of suspected CAD, PCAT attenuation studies have thus far analysed primarily contrast-enhanced CT acquisitions in both retrospective and prospective cohorts. The current literature demonstrates PCAT attenuation is increased in contrast-enhanced scans compared to non-contrast scans [33]. This is an important consideration as the LAD has reportedly higher PCAT attenuation at the ostium than the distal vessel, which corresponds to decreasing luminal diameter and contrast volume [34][35].
3. Current Limitations and Future Directions
Current knowledge on PCAT attenuation as an imaging biomarker of inflammation is, therefore, ever-expanding, but is not without some limitations. Differences in PCAT attenuation owed to epicardial and overall adiposity [33], as well as due to sex, call for future studies to adjust for these potential patient-specific confounders. While the impact of specific scan parameters on PCAT attenuation has been increasingly evaluated, validation of ‘crude’ PCAT assessment across different scanners has yet to be explored. As discussed, PCAT attenuation has been assessed previously using a range of methodologies, and this has been to a large extent accounted for via the use of validated per-patient and per-lesion forms of assessment. However, there is some heterogeneity as to the degree to which plaque within the RCA may affect the per-patient assessment of PCAT in this vessel [19][24]. Moreover, the present definition of PCAT (adipose tissue within a distance equivalent to the vessel diameter) includes an inherently larger volume of adipose tissue in the RCA than in its counterpart vessels. The consistent luminal diameter of the RCA ensures that adipocytes within up to approximately 2–3 mm from the vessel wall are considered PCAT throughout the vessel’s passage; conversely, the LAD and LCx are encased in less adipose tissue overall, and decreases in luminal diameter mean a progressively decreasing volume of adipose tissue volume in the mid-to-distal segments of these vessels falls within the classification of PCAT. In theory, the broader inclusion of adipose tissue around the RCA indicates PCAT assessment in this vessel may include adipocytes that are distinctly less ‘inflamed’, while PCAT in the LAD or LCx would consist only of adipocytes that are most proximal to the vessel wall. In this regard, alternative methodologies consistent with the theoretical framework outlined previously would be worthy of consideration. For example, a recent study [25] evaluated per-patient PCAT attenuation as the mean attenuation of adipose tissue within a region of 1 mm thickness around all three coronary vessels, and thus a consistent volume of PCAT being assessed for each vessel was ensured. Nevertheless, extensive validation and a wealth of literature highlights the utility of the current methods of PCAT assessment at the per-patient and per-lesion level [12][13].
References
- Amir Abbas Mahabadi; Bastian Balcer; Iryna Dykun; Michael Forsting; Thomas Schlosser; Gerd Heusch; Tienush Rassaf; Cardiac computed tomography-derived epicardial fat volume and attenuation independently distinguish patients with and without myocardial infarction. PLOS ONE 2017, 12, e0183514, 10.1371/journal.pone.0183514.
- Yan Xu; Xiaoshu Cheng; Kui Hong; Chahua Huang; Li Wan; How to interpret epicardial adipose tissue as a cause of coronary artery disease. Coronary Artery Disease 2012, 23, 227-233, 10.1097/mca.0b013e328351ab2c.
- Amir A. Mahabadi; Marie H. Berg; Nils Lehmann; Hagen Kälsch; Marcus Bauer; Kaffer Kara; Nico Dragano; Susanne Moebus; Karl-Heinz Jöckel; Raimund Erbel; et al.Stefan Möhlenkamp Association of Epicardial Fat With Cardiovascular Risk Factors and Incident Myocardial Infarction in the General Population. Journal of the American College of Cardiology 2013, 61, 1388-1395, 10.1016/j.jacc.2012.11.062.
- Nitesh Nerlekar; Adam J. Brown; Rahul G. Muthalaly; Andrew Talman; Thushan Hettige; James Cameron; Dennis T. L. Wong; Association of Epicardial Adipose Tissue and High‐Risk Plaque Characteristics: A Systematic Review and Meta‐Analysis. Journal of the American Heart Association 2017, 6, e006379, 10.1161/jaha.117.006379.
- Markus Goeller; Stephan Achenbach; Mohamed Marwan; Mhairi K. Doris; Sebastien Cadet; Frederic Commandeur; Xi Chen; Piotr J. Slomka; Heidi Gransar; J. Jane Cao; et al.Nathan D. WongMoritz H. AlbrechtAlan RozanskiBalaji K. TamarappooDaniel S. BermanDamini Dey Epicardial adipose tissue density and volume are related to subclinical atherosclerosis, inflammation and major adverse cardiac events in asymptomatic subjects. Journal of Cardiovascular Computed Tomography 2018, 12, 67-73, 10.1016/j.jcct.2017.11.007.
- Rami M. Abazid; Osama A. Smettei; Mohammad Obadah Kattea; Sawsan Sayed; Hanaa Saqqah; Adel M. Widyan; Maksymilian P. Opolski; Relation Between Epicardial Fat and Subclinical Atherosclerosis in Asymptomatic Individuals. Journal of Thoracic Imaging 2017, 32, 378-382, 10.1097/rti.0000000000000296.
- Bas T. Franssens; Hendrik M. Nathoe; Frank L.J. Visseren; Yolanda van der Graaf; Tim Leiner; Ale Algra; Diederick E. Grobbee; Guy E.H.M. Rutten; Gert Jan de Borst; L.J. (Jaap) Kappelle; et al. Relation of Epicardial Adipose Tissue Radiodensity to Coronary Artery Calcium on Cardiac Computed Tomography in Patients at High Risk for Cardiovascular Disease. The American Journal of Cardiology 2017, 119, 1359-1365, 10.1016/j.amjcard.2017.01.031.
- Radoslaw Pracon; Mariusz Kruk; Cezary Kepka; Jerzy Pregowski; Maksymilian Opolski; Zofia Dzielinska; Ilona Michalowska; Zbigniew Chmielak; Marcin Demkow; Witold Ruzyllo; et al. Epicardial Adipose Tissue Radiodensity Is Independently Related to Coronary Atherosclerosis - A Multidetector Computed Tomography Study . Circulation Journal 2011, 75, 391-397, 10.1253/circj.cj-10-0441.
- Zihou Liu; Shunjun Wang; Yongqiang Wang; Ningbo Zhou; Jie Shu; Christof Stamm; Meng Jiang; Fanyan Luo; Association of epicardial adipose tissue attenuation with coronary atherosclerosis in patients with a high risk of coronary artery disease. Atherosclerosis 2019, 284, 230-236, 10.1016/j.atherosclerosis.2019.01.033.
- Paolo Raggi; Varuna Gadiyaram; Chao Zhang; Zhengjia Chen; Gary Lopaschuk; Arthur E. Stillman; Statins Reduce Epicardial Adipose Tissue Attenuation Independent of Lipid Lowering: A Potential Pleiotropic Effect. Journal of the American Heart Association 2019, 8, e013104, 10.1161/jaha.119.013104.
- Nitesh Nerlekar; Udit Thakur; Andrew Lin; Ji Quan Samuel Koh; Elizabeth Potter; David Liu; Rahul G. Muthalaly; Hashrul N. Rashid; James D. Cameron; Damini Dey; et al.Dennis T. L. Wong The Natural history of Epicardial Adipose Tissue Volume and Attenuation: A long-term prospective cohort follow-up study. Scientific Reports 2020, 10, 1-7, 10.1038/s41598-020-63135-z.
- Alexios Antonopoulos; Fabio Sanna; Nikant Sabharwal; Sheena Thomas; Evangelos K. Oikonomou; Laura Herdman; Marios Margaritis; Cheerag Shirodaria; Anna-Maria Kampoli; Ioannis Akoumianakis; et al.Mario PetrouRana SayeedGeorge KrasopoulosConstantinos PsarrosPatricia CicconeCarl M. BrophyJanet DigbyAndrew KelionRaman UberoiSuzan AnthonyNikolaos AlexopoulosDimitris TousoulisStephan AchenbachStefan NeubauerKeith ChannonCharalambos Antoniades Detecting human coronary inflammation by imaging perivascular fat. Science Translational Medicine 2017, 9, eaal2658, 10.1126/scitranslmed.aal2658.
- Evangelos K Oikonomou; Mohamed Marwan; Milind Y Desai; Jennifer Mancio; Alaa Alashi; Erika Hutt Centeno; Sheena Thomas; Laura Herdman; Christos Kotanidis; Katharine E Thomas; et al.Brian P GriffinScott D FlammAlexios AntonopoulosCheerag ShirodariaNikant SabharwalJohn DeanfieldStefan NeubauerJemma C HopewellKeith ChannonStephan AchenbachCharalambos Antoniades Non-invasive detection of coronary inflammation using computed tomography and prediction of residual cardiovascular risk (the CRISP CT study): a post-hoc analysis of prospective outcome data. The Lancet 2018, 392, 929-939, 10.1016/s0140-6736(18)31114-0.
- Xu Dai; Jianhong Deng; Mengmeng Yu; Zhigang Lu; Chengxing Shen; Jiayin Zhang; Perivascular fat attenuation index and high-risk plaque features evaluated by coronary CT angiography: relationship with serum inflammatory marker level. The International Journal of Cardiovascular Imaging 2020, 36, 723-730, 10.1007/s10554-019-01758-8.
- Nicola Gaibazzi; Chiara Martini; Andrea Botti; Antonio Pinazzi; Barbara Bottazzi; Anselmo A. Palumbo; Coronary Inflammation by Computed Tomography Pericoronary Fat Attenuation in MINOCA and Tako‐Tsubo Syndrome. Journal of the American Heart Association 2019, 8, e013235, 10.1161/jaha.119.013235.
- Youssef A. Elnabawi; Evangelos K. Oikonomou; Amit K. Dey; Jennifer Mancio; Justin A. Rodante; Milena Aksentijevich; Harry Choi; Andrew Keel; Julie Erb-Alvarez; Heather L. Teague; et al.Aditya A. JoshiMartin P. PlayfordBenjamin LockshinAndrew D. ChoiJoel M. GelfandMarcus Y. ChenDavid BluemkeCheerag ShirodariaCharalambos AntoniadesNehal N. Mehta Association of Biologic Therapy With Coronary Inflammation in Patients With Psoriasis as Assessed by Perivascular Fat Attenuation Index. JAMA Cardiology 2019, 4, 885-891, 10.1001/jamacardio.2019.2589.
- Xu Dai; Lihua Yu; Zhigang Lu; Chengxing Shen; Xinwei Tao; Jiayin Zhang; Serial change of perivascular fat attenuation index after statin treatment: Insights from a coronary CT angiography follow-up study. International Journal of Cardiology 2020, 319, 144-149, 10.1016/j.ijcard.2020.06.008.
- Evangelos Tzolos; Priscilla McElhinney; Michelle C. Williams; Sebastien Cadet; Mark R. Dweck; Daniel S. Berman; Piotr J. Slomka; David E. Newby; Damini Dey; Repeatability of quantitative pericoronary adipose tissue attenuation and coronary plaque burden from coronary CT angiography. Journal of Cardiovascular Computed Tomography 2021, 15, 81-84, 10.1016/j.jcct.2020.03.007.
- Andrew Lin; Nitesh Nerlekar; Jeremy Yuvaraj; Katrina Fernandes; Cathy Jiang; Damini Dey; Stephen J. Nicholls; Dennis Tl Wong; PERICORONARY ADIPOSE TISSUE COMPUTED TOMOGRAPHY ATTENUATION IN DIFFERENT STAGES OF CORONARY ARTERY DISEASE: A CROSS-SECTIONAL STUDY. Journal of the American College of Cardiology 2020, 75, 1718, 10.1016/s0735-1097(20)32345-7.
- Markus Goeller; Stephan Achenbach; Sebastien Cadet; Alan C. Kwan; Frederic Commandeur; Piotr J. Slomka; Heidi Gransar; Moritz H. Albrecht; Balaji K. Tamarappoo; Daniel S. Berman; et al.Mohamed MarwanDamini Dey Pericoronary Adipose Tissue Computed Tomography Attenuation and High-Risk Plaque Characteristics in Acute Coronary Syndrome Compared With Stable Coronary Artery Disease. JAMA Cardiology 2018, 3, 858-863, 10.1001/jamacardio.2018.1997.
- Markus Goeller; Balaji K Tamarappoo; Alan C Kwan; Sebastien Cadet; Frederic Commandeur; Aryabod Razipour; Piotr J Slomka; Heidi Gransar; Xi Chen; Yuka Otaki; et al.John D FriedmanJ Jane CaoMoritz H AlbrechtDaniel O BittnerMohamed MarwanStephan AchenbachDaniel S BermanDamini Dey Relationship between changes in pericoronary adipose tissue attenuation and coronary plaque burden quantified from coronary computed tomography angiography. European Heart Journal - Cardiovascular Imaging 2019, 20, 636-643, 10.1093/ehjci/jez013.
- Jeremy Yuvaraj; Andrew Lin; Nitesh Nerlekar; Ravi Munnur; James Cameron; Damini Dey; Stephen Nicholls; Dennis Wong; Pericoronary Adipose Tissue Attenuation Is Associated with High-Risk Plaque and Subsequent Acute Coronary Syndrome in Patients with Stable Coronary Artery Disease. Cells 2021, 10, 1143, 10.3390/cells10051143.
- Markus Goeller; Abdul Rahman Ihdayhid; Sebastien Cadet; Andrew Lin; Daniel Adams; Udit Thakur; Grace Yap; Mohamed Marwan; Stephan Achenbach; Damini Dey; et al.Brian Ko Pericoronary adipose tissue and quantitative global non-calcified plaque characteristics from CT angiography do not differ in matched South Asian, East Asian and European-origin Caucasian patients with stable chest pain. European Journal of Radiology 2020, 125, 108874, 10.1016/j.ejrad.2020.108874.
- Tomoyo Sugiyama; Yoshihisa Kanaji; Masahiro Hoshino; Masao Yamaguchi; Masahiro Hada; Hiroaki Ohya; Yohei Sumino; Hidenori Hirano; Yoshinori Kanno; Tomoki Horie; et al.Toru MisawaKai NogamiHiroki UenoRikuta HamayaEisuke UsuiTadashi MuraiTetsumin LeeTaishi YonetsuTetsuo SasanoTsunekazu Kakuta Determinants of Pericoronary Adipose Tissue Attenuation on Computed Tomography Angiography in Coronary Artery Disease. Journal of the American Heart Association 2020, 9, -, 10.1161/jaha.120.016202.
- Runlei Ma; Daan Ties; Marly Van Assen; Gert Jan Pelgrim; Grigory Sidorenkov; Peter van Ooijen; Pim Van Der Harst; Randy Van Dijk; Rozemarijn Vliegenthart; Towards reference values of pericoronary adipose tissue attenuation: impact of coronary artery and tube voltage in coronary computed tomography angiography. European Radiology 2020, 30, 6838-6846, 10.1007/s00330-020-07069-0.
- Mengmeng Yu; Xu Dai; Jianhong Deng; Zhigang Lu; Chengxing Shen; Jiayin Zhang; Diagnostic performance of perivascular fat attenuation index to predict hemodynamic significance of coronary stenosis: a preliminary coronary computed tomography angiography study. European Radiology 2019, 30, 673-681, 10.1007/s00330-019-06400-8.
- Masahiro Hoshino; Seokhun Yang; Tomoyo Sugiyama; Jinlong Zhang; Yoshihisa Kanaji; Masao Yamaguchi; Masahiro Hada; Yohei Sumino; Tomoki Horie; Kai Nogami; et al.Hiroki UenoToru MisawaEisuke UsuiTadashi MuraiTetsumin LeeTaishi YonetsuTsunekazu Kakuta Peri-coronary inflammation is associated with findings on coronary computed tomography angiography and fractional flow reserve. Journal of Cardiovascular Computed Tomography 2020, 14, 483-489, 10.1016/j.jcct.2020.02.002.
- Cesar H Nomura; Antonildes N Assuncao-Jr; Patricia O Guimarães; Gabriela Liberato; Thamara C Morais; Mateus G Fahel; Maria C P Giorgi; José C Meneghetti; Jose R Parga; Roberto Dantas Jr; et al.Giovanni G Cerri Association between perivascular inflammation and downstream myocardial perfusion in patients with suspected coronary artery disease. European Heart Journal - Cardiovascular Imaging 2020, 21, 599-605, 10.1093/ehjci/jeaa023.
- Hiroki Ueno; Masahiro Hoshino; Tomoyo Sugiyama; Yoshihisa Kanaji; Kai Nogami; Tomoki Horie; Masao Yamaguchi; Masahiro Hada; Yohei Sumino; Toru Misawa; et al.Hidenori HiranoTaishi YonetsuTetsuo SasanoTsunekazu Kakuta Pericoronary Adipose Tissue Inflammation on Coronary Computed Tomography in Patients With Vasospastic Angina. JACC: Cardiovascular Imaging 2021, 14, 511-512, 10.1016/j.jcmg.2020.08.002.
- Tomoki Horie; Tomoyo Sugiyama; Yoshihisa Kanaji; Masahiro Hoshino; Tsunekazu Kakuta; Serial Assessment of Pericoronary Adipose Tissue Inflammation in a Patient With MINOCA Potentially Complicated With Vasospasm. CJC Open 2021, 3, 204-206, 10.1016/j.cjco.2020.10.001.
- Andrew Lin; Nitesh Nerlekar; Ravi Kiran Munnur; Yu Kataoka; Jordan Andrews; Damini Dey; Stephen J. Nicholls; Dennis Tl. Wong; Cholesterol crystal-induced coronary inflammation: Insights from optical coherence tomography and pericoronary adipose tissue computed tomography attenuation. Journal of Cardiovascular Computed Tomography 2020, 14, 277-278, 10.1016/j.jcct.2019.11.011.
- Jeremy Yuvaraj; Andrew Lin; Nitesh Nerlekar; Hashrul Rashid; James D. Cameron; Sujith Seneviratne; Stephen Nicholls; Peter J. Psaltis; Dennis T. L. Wong; Is spontaneous coronary artery dissection (SCAD) related to vascular inflammation and epicardial fat? —insights from computed tomography coronary angiography. Cardiovascular Diagnosis and Therapy 2020, 10, 239-241, 10.21037/cdt.2020.01.09.
- Michaela M. Hell; Stephan Achenbach; Annika Schuhbaeck; Lutz Klinghammer; Matthias S. May; Mohamed Marwan; CT-based analysis of pericoronary adipose tissue density: Relation to cardiovascular risk factors and epicardial adipose tissue volume. Journal of Cardiovascular Computed Tomography 2016, 10, 52-60, 10.1016/j.jcct.2015.07.011.
- Shone Almeida; Megan Pelter; Kashif Shaikh; Lavanya Cherukuri; Divya Birudaraju; Kyle Kim; Jenil Modi; Chandana Shekar; Mohammad Sheikh; April Kinninger; et al.Elizabeth HillChristy MutchlerLaura TabbRobert FalkDamini DeyJorge GonzalezRonald KarlsbergGeorge WesbeyMatthew Budoff Feasibility of measuring pericoronary fat from precontrast scans: Effect of iodinated contrast on pericoronary fat attenuation. Journal of Cardiovascular Computed Tomography 2020, 14, 490-494, 10.1016/j.jcct.2020.04.004.
- Bastian Balcer; Iryna Dykun; Thomas Schlosser; Michael Forsting; Tienush Rassaf; Amir A. Mahabadi; Pericoronary fat volume but not attenuation differentiates culprit lesions in patients with myocardial infarction. Atherosclerosis 2018, 276, 182-188, 10.1016/j.atherosclerosis.2018.05.035.




