| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Michelle Bagood | + 2595 word(s) | 2595 | 2021-06-18 09:51:36 |
Video Upload Options
TRPV1 is a nonspecific ion channel highly expressed by cutaneous sensory nerves and other skin cells, including circulating and skin resident immune cells. Understanding the role of TRPV1 in wound healing may inform future TRPV1-targeted therapies to improve healing in impaired and chronic wounds. Many factors contribute to the polymodal nature of TRPV1 channel activation including tetrameric composition, splice variant, accessory protein sensitization or desensitization, activator concentration/coupling, etc.
1. Introduction
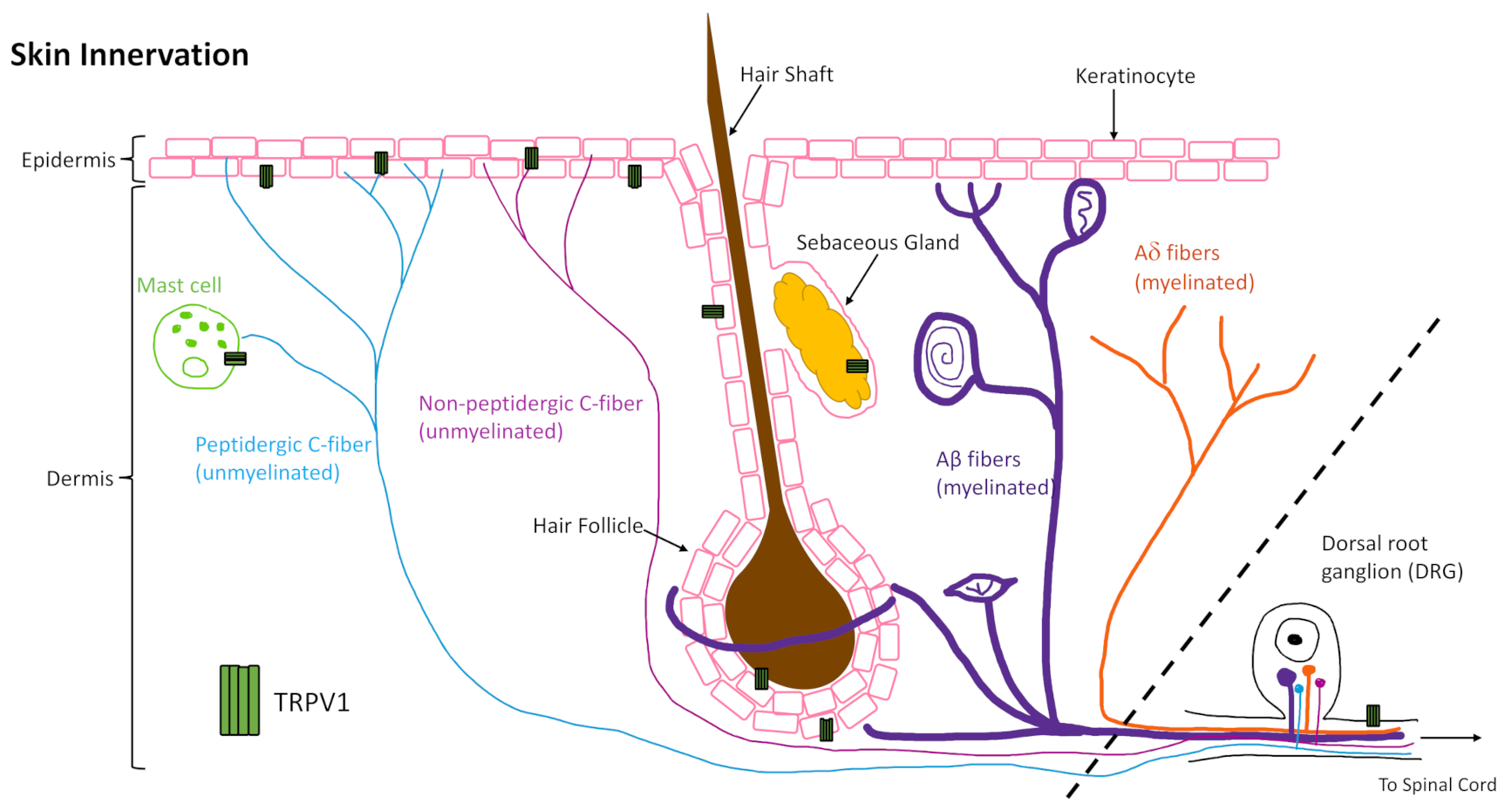
The skin is innervated by afferent sensory nerves, to detect and distinguish between innocuous and noxious stimuli, and efferent autonomic nerves, to maintain barrier tissue homeostasis [1]. About 70% of the unmyelinated C fibers are classified as C-polymodal nociceptors, which respond to various trophic stimuli determined by their membrane protein expression, such as calcium and sodium channels (e.g. TRPV1 and NaV1.8). Classification based on neurotransmitter expression does not align with classification based on membrane protein expression as there is overlap in some fibers but not in others. Moreover, C-fibers have been shown to interact with keratinocytes, mast cells (Figure 1), Langerhans cells, blood vessels, and other cells of the skin to perform efferent functions through release of neuropeptides, such as calcitonin gene related peptide (CGRP) [4,5,6,7,8,9,10,11,12].
One area where integration of neuronal signaling is critical is in tissue repair, where neuroimmune mechanisms signal for cellular responses that contribute to the repair mechanism. The global role of innervation and neuromediators in cutaneous wound healing has been reviewed previously [13,14,15]. Here we focus on a well-characterized channel, the transient receptor potential cation channel, subfamily V member 1 (TRPV1, also known as the vanilloid receptor 1 (VR1) and transient receptor potential vanilloid 1), that is highly expressed by cutaneous peripheral sensory nerves, as well as central nerve endings in the DRG, and in varying degrees on other skin and immune cells (Figure 1). Understanding what is already known about its role in wound healing, and how TRPV1-targeted therapies work in skin, as well as other tissues, may inform future therapies to improve healing in impaired and chronic wounds.
2. TRPV1 Structure and Function
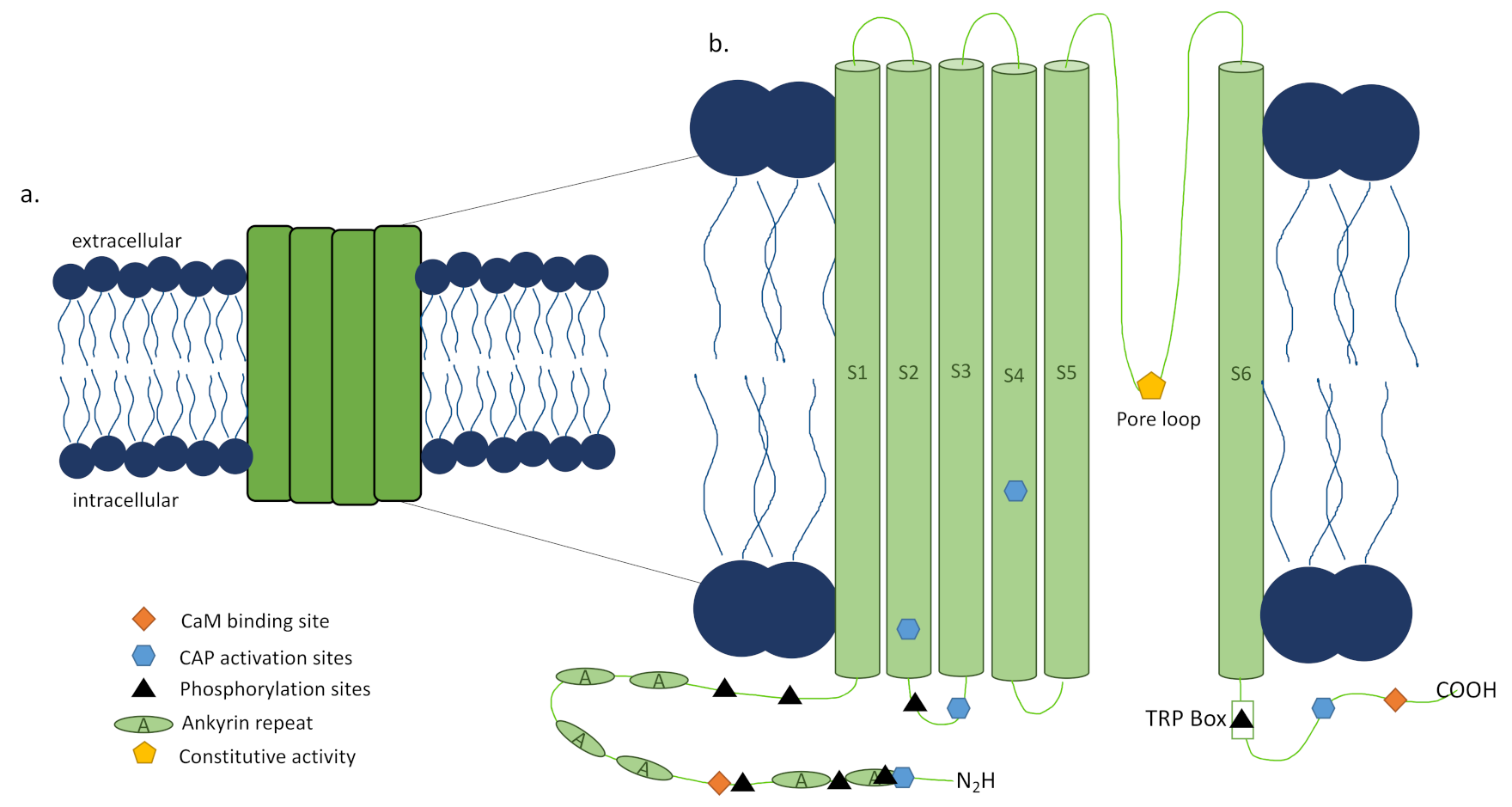
TRPV1 is a tetrameric ion channel [16], each subunit consisting of 838 amino acids (Figure 2a) [18], that has been found in homotetrameric and heterotetrameric forms [19,20], each with distinct functional properties [21]. The N and C termini of each subunit of the channel are located intracellularly [16], with the N terminus playing a role in the channel’s sensitivity to activators [22] and the TRP box containing C terminus impacting channel stability and function (Figure 2b). Upon activation by a ligand, channel opening is achieved through a dual gate mechanism involving conformational changes in a selectivity filter and a lower gate [35]. Opening the TRPV1 channel permeabilizes the cell membrane to ions in a nonselective manner, with very high relative permeability to calcium ions [16]. To further complicate matters, there have been reports of alternative splicing of TRPV1, which can lead to nonfunctional channels or splice variant co-expression with TRPV1 that inhibit function [36].
Prior to the discovery of the TRPV1 channel, capsaicin use to activate and desensitize sensory nerves became an active area of research in preclinical models in the 1970s [37], but the burning feeling and increased blood flow in skin after its application was observed by Hogyes a century before that in 1878 [38]. However, the reversibility and extent of desensitization reached using capsaicin or its ultrapotent analog, resiniferatoxin (RTX), remained elusive [34], as shown by RTX being sensory nerve specific and not affecting TRPV1 expression in skin [39]. In 2000, the Julius lab created and characterized a TRPV1 knockout mouse (Trpv1−/−) that lacks exon 13, encoding the pore loop and transmembrane domain 6 of the channel, and does not respond to capsaicin [40]. The development of this mouse and other tools have allowed preclinical studies and made TRPV1 an appealing pharmacological target, though the outcome of the therapy is as varied as the conditions it is utilized to treat and the precise mechanisms are still under investigation.
3. Using TRPV1-Targeted Therapy to Improve Wound Healing
While there is strong evidence of the presence of non-neuronal TRPV1 and its many roles in skin function, the evidence for targeting TRPV1 for skin wound healing has proven complicated. Despite keratinocytes, fibroblasts, lymphocytes, and vascular endothelial cells in the rat dorsal paw and plantar skin having TRPV1 immunopositivity, chemical treatment with RTX did not influence TRPV1 mRNA and protein expression levels in non-neural cells of either skin types as it does in sensory neurons [39]. The authors suggest that TRPV1B expression, a dominant negative splice variant, and the low level of TRPV1 expression contributed to human keratinocyte resistance to capsaicin and other vanilloids [129]. This may suggest the need for a more personal medicine approach where TRPV1-targeted therapies to improve wound healing are tailored to the channel splice variant expressed and dosage selected based on expression level to reach the desired outcome.
There is evidence of TRPV1-targeted therapy impacting the functionality of TRPV1+ peripheral sensory nerves in the skin, which may be utilized to improve wound healing. Systemic stimulation with RTX was shown to induce sensory neurons in the skin to release pituitary adenylate cyclase activating polypeptide (PACAP-38) [142], and later this same group showed that PACAP regulated TRPV1-mediated acute neurogenic edema in plantar skin after capsaicin injection, though this edema was not accompanied by immune cell infiltration as seen with CFA-induced edema [143]. In vivo, capsaicin treatment of sciatic nerve crush injury resulted in better thermal sensation recovery and improved epidermal axon reinnervation of the skin approaching contralateral skin levels [144]. This evidence demonstrates the role that TRPV1 activation plays in regeneration of adult sensory neurons and their axons, which may impact reinnervation of wounded skin by thermosensitive axons.
In the imiquimod (IMQ) induced psoriasis model, dermal dendritic cells (dDCs) were imaged in contact with cutaneous sensory neurons expressing TRPV1. Further, ablation of these nerves resulted in greatly reduced IMQ-associated inflammation including reduction in immune cell infiltration and cytokine production (IL-23, IL-17A/F, and IL-22) [91]. Using other mouse models, the authors confirmed that TRPV1+NaV1.8+nociceptors actively induced and controlled IL-23. In the squaric acid dibutylester (SADBE)-induced ACD model, SADBE not only directly activates the TRPV1 channel but also TRPV1 was expressed on MrgprA3 and MrgprD expressing DRG neurons, and the authors concluded that these TRPV1+ neurons were responsible for the persistent itch signal after SADBE exposure [92]. Additionally, TRPV1 deficiency resulted in increased macrophage infiltration and TNFα, IL-1β, and IL-6 expression in the SADBE-induced ACD model, providing further evidence of TRPV1+ sensory neuron involvement in cutaneous inflammation [92].
Further evidence for a role of TRPV1+ nociceptors in barrier tissues is found in the recent studies of infection in skin and lungs. Chiu et al. showed that TRPV1+ DRGs in vitro and NaV1.8+ nociceptors in vivo, which have 80% overlap with TRPV1+ nociceptors [91], are directly activated by pathogen associated molecular patterns (PAMPs), specifically formyl peptides and α-hemolysin [93]. Activated nociceptors released CGRP responsible for modulating the immune response to a subcutaneous injection of Staphylococcus aureus. The authors demonstrated this in vitro, where CGRP released by DRG nerves suppressed TNFα production by macrophages [93]. Chiu and colleagues went on to demonstrate the anti-inflammatory effects of TRPV1+ nociceptors in the lung in response to Staphylococcus aureus. Here, the CGRP released from TRPV1+ afferents suppressed recruitment and surveillance of neutrophils, leading to better outcomes for the infected animals due to reduced inflammation-associated damage [94].
Not only does TRPV1 play a role in modulating the immune response to bacterial pathogens, but work by the Kaplan group and others have extended this to fungal ones as well. In bone infection, Candida albicans stimulates NaV1.8+ nociceptors via Dectin-1 to release CGRP in a TRPV1/TRPA1 dependent manner [147]. CGRP reduces osteoinflammation in response to fungal infection by directly suppressing NFκB p65 via the transcriptional repressor Jdp2 [147]. Taken together, these studies show that skin nociceptors are directly activated by cutaneous invading microbes and/or microbial products and then communicate with tissue resident immune cells, however, the outcome of this communication, either stimulatory or regulatory, is tissue and context dependent.
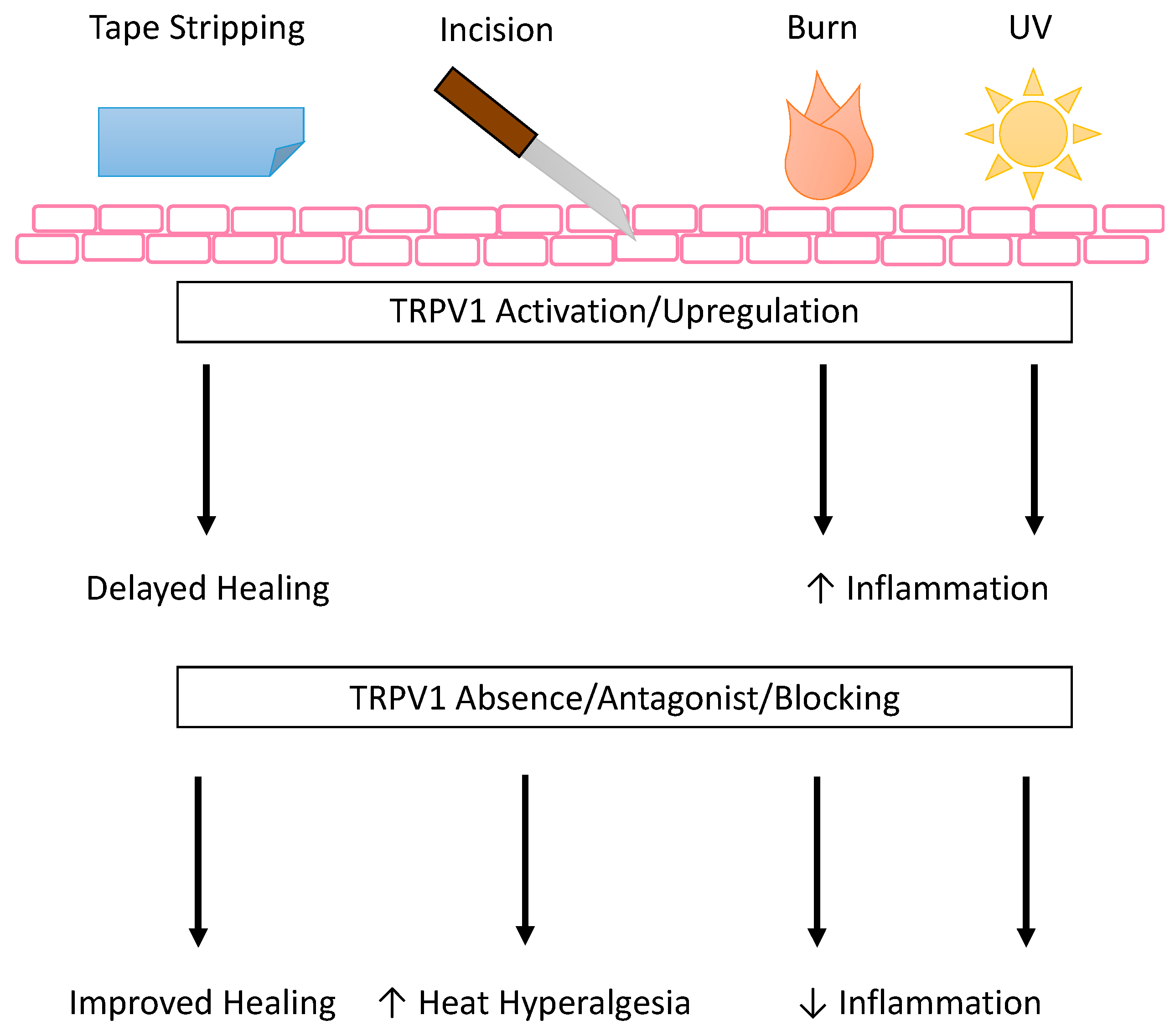
One of the earliest pieces of evidence that TRPV1+ nociceptors were involved in the wound environment was the TRPV1-dependent increase in heat hyperalgesia in a mouse hind paw incision model [149] (Figure 3). This was not mediated by a change in TRPV1 expression, as equivalent expression was shown on the ipsilateral peripheral TRPV1+ sensory nerves and the DRG and sciatic nerves as compared to the contralateral skin and nerves [149]. In a later study of the role of TRPV1 in tissue swelling after surgical incision, the loss of TRPV1 did not show a significant difference in hind paw swelling after incision, suggesting TRPV1 does not play a part in incision-associated edema [131]. Taken together, these studies show that the pain associated with incision wounds is dependent on TRPV1+ afferents, but the edema uses another pathway suggesting a combination therapy may be more beneficial when treating this type of wound.
TRPV1 expression and activation seem to be affected by burn injury, known to be a highly inflammatory process, and in turn affect recovery by contributing to the post injury inflammation in both central and peripheral mechanisms (Figure 3). Recent evidence suggests that, centrally, burn injury increased CGRP-associated TRPV1 channel expression on the DRG [132]. CGRP in the burn injury model induced inflammation in DRG nerves [132]. In the periphery, evidence suggests the role of oxidized linoleic acid metabolites in activating TRPV1+ sensory nerves [133] to increase leukocyte/macrophage infiltration after burn injury, a process that was dependent on cytochrome P450 metabolism of linoleic acid [133]. An example of a natural product modifying TRPV1 expression to treat third degree burns is honokiol, a poly-phenolic compound extracted from various magnolia species, though the exact mechanism by which it alters expression is not fully understood [134]. Additionally, keratinocytes from burn scars with pruritis have increased thymic stromal lymphopoietin protein levels when compared to keratinocytes from normal control skin, and this increase is enhanced by TRPV1 or TRPV3 activation [135]. The authors propose TRPV1 is important for the induction of growth factor and cytokine release in the recovery from chemical burn injury [83].
In another type of wound caused by UV damage, inhibition of TRPV1 on keratinocytes may help dampen UV-induced inflammation (Figure 3). Inflammatory mechanisms have been targeted in mouse keratinocytes using TRPV1-specific blocker 5′-iodoresiniferatoxin (I-RTX) to reduce UV-induced mRNA and protein expression of MMP-13, MMP-9, MMP-3, and MMP-2, mRNA expression of pro-inflammatory cytokines IL-1β, IL-2, IL-4, and TNFα, and protein expression of COX-2 and p53 [136]. More recently, Chung and colleagues have developed a TRPV1 inhibitory peptide701–709 and shown that it inhibited UV-induced MMP (MMP-13 and MMP-9 in mouse and MMP-1 and MMP-2 in human) and pro-inflammatory cytokine expression (IL-6 and TNFα in mouse and IL-6 and IL-8 in human), as well as calcium influx in HaCaT cells, mouse skin, and human skin [137]. Taken together, this evidence suggests that in wounds with hyperinflammation, inhibiting TRPV1 on keratinocytes may decrease the keratinocyte dependent inflammatory signals, decreasing feedback and reducing overall inflammation.
One recent study explored the role of CGRP in sterile wounds, although the work did not specifically address the TRPV1 channel. One high dose of UVB radiation induces inflammation, as evidenced by edema and sensitivity to mechanical von Frey stimuli. This inflammation is associated with increased infiltration of neutrophils and monocyte-derived macrophages and DCs on days two and three post irradiation followed by increased αβ T cells and dDCs on days 5 and 10 and Langerhans cells on day 10 [153]. The infiltration of T cells and dDCs is reduced when CGRP is knocked out of the mouse, suggesting that CGRP may play a role in the immune cell expansion in response to UVB induced inflammation [153]. This is a narrow example of UVB wounding and does not exclude the involvement of TRPV1 in low dose UVB wounds, as detailed in the studies noted above, or other TRP channels. However, it does shed light on the role of CGRP in immune cell infiltration after wounding, which may be induced in other models of wound healing via TRPV1 activation, as shown in infection.
4. Conclusions
TRPV1 is a nonspecific ion channel highly expressed by cutaneous sensory nerves and other skin cells, including circulating and skin resident immune cells. Many factors contribute to the polymodal nature of TRPV1 channel activation including tetrameric composition, splice variant, accessory protein sensitization or desensitization, activator concentration/coupling, etc. TRPV1 targeting is further complicated by the downstream pathways induced after its activation and the resulting functionality of the cell expressing it, which may lead to adverse or off-target effects, as well as potential shifts in functional outcomes with age. TRPV1-targeted therapies in use and in clinical trials, mainly composed of capsaicin-based treatments, are for pain relief, however, this work has been expanded into the fields of urology, oncology, and dermatology, including skin wound biology.
Perhaps in accord with this, skin diseases and wound types that are highly inflammatory benefit from the inhibition or absence of TRPV1. On the other hand, skin acid burn, cornea debridement, and cutaneous fungal infection showed improved outcomes when TRPV1 is activated on epithelium or nociceptors. These TRPV1+ nociceptors have been shown to promote Type 17 inflammation in some models and anti-inflammatory mechanisms in other models. Therefore, blocking TRPV1 may improve wound healing in inflammatory wounds, though the wound environment must be taken into account when deciding to employ this treatment.
It is clear TRPV1 is expressed in many cell populations in the skin that are involved in wound healing, and activation or inhibition of TRPV1 impacts the function of these cells. However, more work is needed to confirm the benefits of TRPV1-targeted therapies for healing various types of wounds, as well as resolve the dose and timing of these treatments.