Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Sara Hooshmand | + 1364 word(s) | 1364 | 2021-06-21 10:40:27 | | | |
| 2 | Vicky Zhou | -2 word(s) | 1362 | 2021-06-29 03:08:59 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Hooshmand, S. Skin Wound Management. Encyclopedia. Available online: https://encyclopedia.pub/entry/11394 (accessed on 12 January 2026).
Hooshmand S. Skin Wound Management. Encyclopedia. Available at: https://encyclopedia.pub/entry/11394. Accessed January 12, 2026.
Hooshmand, Sara. "Skin Wound Management" Encyclopedia, https://encyclopedia.pub/entry/11394 (accessed January 12, 2026).
Hooshmand, S. (2021, June 28). Skin Wound Management. In Encyclopedia. https://encyclopedia.pub/entry/11394
Hooshmand, Sara. "Skin Wound Management." Encyclopedia. Web. 28 June, 2021.
Copy Citation
Etiologically, the skin wounds could be classified as acute, chronic, or malignant wounds. In general, an ordered and normal wound healing process happens after acute wounds. On the contrary, chronic wounds are characterized by prolonged inflammatory phase, sustained infection, and the generation of drug-resistance microbial biofilms, leading to an impaired wound healing cascade.
mesoporous silica nanoparticles
mesoporous bioactive glass
angiogenesis
skin regeneration
cancer therapy
drug release
tissue engineering
1. Introduction
Generally, skin wound healing comprises four overlapping phases with a well-orchestrated interaction of specialized cells, bioactive molecules, and extracellular matrix (ECM) components. In the first phase, the so-called hemostasis, the formed fibrin clot provides a temporary scaffold for cellular attachment and migration [1]. In addition, platelets produce pro-inflammatory cytokines (e.g., PDGF and TGF-β) that contribute to the inflammatory phase. The inflammatory phase is the second phase in which neutrophils are infiltrated and support reinforcing immune response through releasing TNF-α, IL-1β, and IL-6. Furthermore, monocytes migrate to the wound bed and differentiate into macrophages that serve as phagocytes and release bioactive molecules including IL-1, PDGF, TGF-α, TGF-β, FGF, IGF-1, and VEGF [2]. The third phase, i.e., proliferation, is remarked by re-epithelialization, angiogenesis, and granulation tissue formation. In the last phase, i.e., remodeling, the residing collagen type Ⅲ in granulation tissue is replaced by bundles of collagen type Ⅰ that result in scar tissue formation and subsequently enhance the tensile strength of ECM [3].
Etiologically, the skin wounds could be classified as acute, chronic, or malignant wounds. In general, an ordered and normal wound healing process happens after acute wounds [4]. On the contrary, chronic wounds are characterized by prolonged inflammatory phase [5], sustained infection [6], and the generation of drug-resistance microbial biofilms [7], leading to an impaired wound healing cascade. In the case of malignant lesions, the edges of wounds are constantly expanded because of the cell migration inward and outward of the wounds, which results in tumor proliferation as well as its invasion into the adjacent tissues [8].
It is well-known that successful and efficient wound healing requires improved angiogenesis, re-epithelialization, and less fibrous and scar tissue formation. Hence, numerous studies have been focused on developing innovative wound substitutes and dressings.
2. MBGs for Wound Healing and Skin Regeneration
The therapeutic multifunctionality of MBGs makes them highly interesting biomaterials to be applied in wound healing and skin tissue regeneration (Table 1). Acute wounds normally heal in a very orderly and efficient manner characterized by four distinct but overlapping phases: hemostasis, inflammation, proliferation, and remodeling. MBGs can be engineered to improve wound healing and skin regeneration through stimulating angiogenesis and inhibiting bacterial formation on the wound site. For example, Cu-containing MBGs were utilized in nanofibrillated cellulose matrix (NFC) composite aerogels to release Cu2+ ions for its angiogenic effect on promoting wound healing and its antibacterial effect as well [9]. A 3D fibrin spheroid assay showed that NFC: MBGSi75Cu5 (10:1) aerogel significantly induced sprouting of human umbilical vein endothelial cells (HUVECs) and enhanced the expressions of vimentin and fibronectin in the HUVEC spheroid compared to control groups (Figure 1). This suggested that NFC: MBGSi75Cu5 (10:1) aerogel can promote fibroblast–endothelial cell interaction and ECM production, similar to the in vivo fibrin clot during the wound healing process.
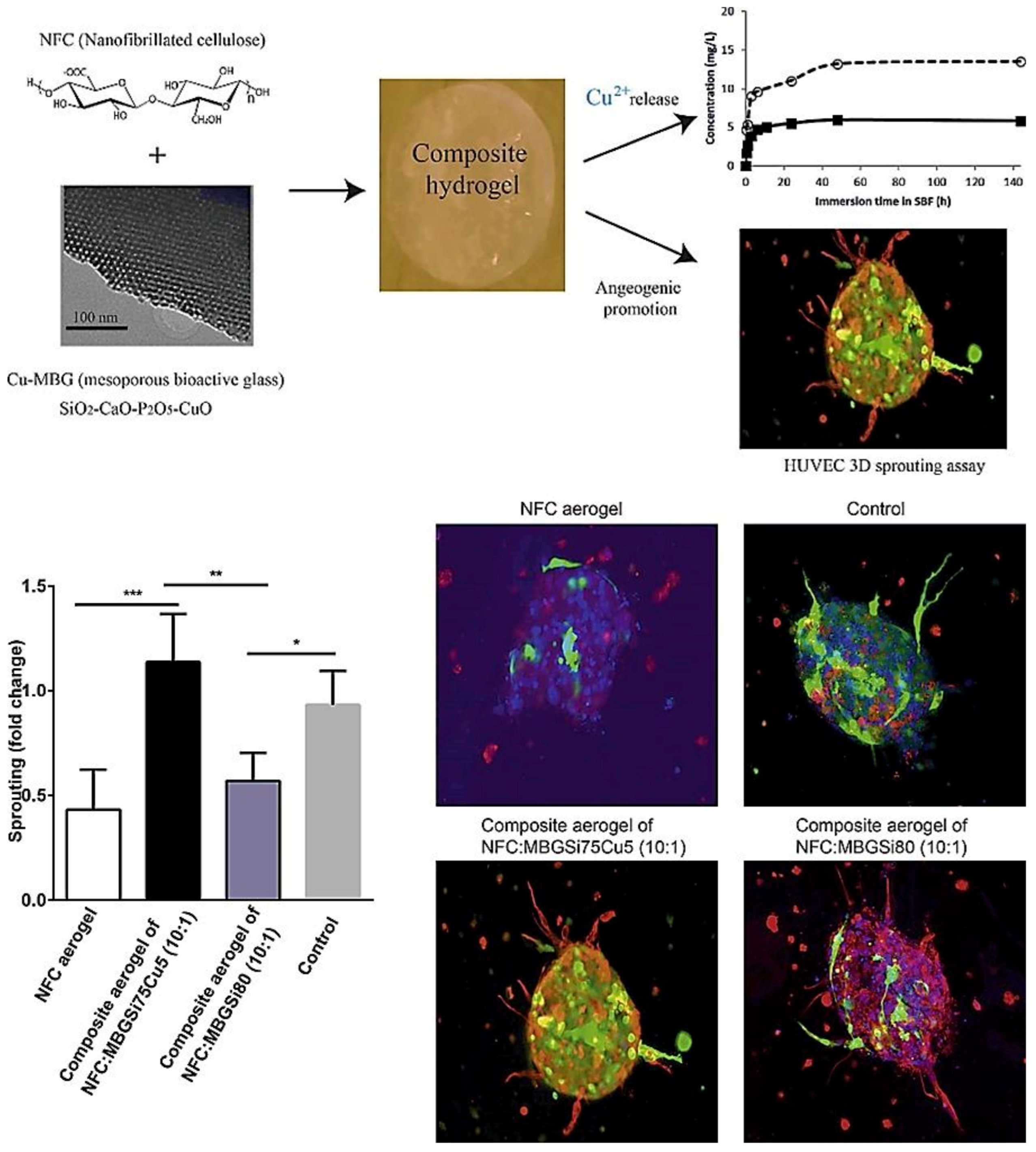 Figure 1. Sprouting of HUVEC spheroids with NFC, NFC: MBGSi80 (10:1) and NFC: MBGSi75Cu5 (10:1) composite aerogels: representative confocal images of expression of vimentin (in red) and fibronectin (in green) in HUVEC spheroids. The cell nuclei are counterstained with DAPI (blue). Bar = mean of sprout numbers ± s.e.m., n = 4. *—p < 0.1; **—p < 0.01; ***—p < 0.001. With permission from [9]. Copyright 2016 Elsevier.
Figure 1. Sprouting of HUVEC spheroids with NFC, NFC: MBGSi80 (10:1) and NFC: MBGSi75Cu5 (10:1) composite aerogels: representative confocal images of expression of vimentin (in red) and fibronectin (in green) in HUVEC spheroids. The cell nuclei are counterstained with DAPI (blue). Bar = mean of sprout numbers ± s.e.m., n = 4. *—p < 0.1; **—p < 0.01; ***—p < 0.001. With permission from [9]. Copyright 2016 Elsevier.Table 1. A short list of experimental studies performed on ion-doped MBGs for potential use in wound healing.
| Composition | Synthesis Method | Dopant | Application | Remarks | Ref |
|---|---|---|---|---|---|
| 1%Ga-MBG (79SiO2–15CaO–5P2O5–1Ga2O3) (10, 30, and 50 wt%) with CHT | Sol–gel using EISA with freeze-drying | Ga | Hemostatic and antibacterial |
|
[10] |
| (80 − x) SiO2 − 15CaO − 5P2O5 − xTa2O5, where x = 0, 0.5, 1, 5, 10 | Sol–gel using EISA | Ta | Hemostatic |
|
[11] |
| ([1 − (x + y)] (58SiO2 − 33P2O5 − 9CaO) − xCaF2 − yAg2O), where 0 ≤ x ≤ 20, 0 ≤ y ≤ 2 | Sol–gel | Ag | Antibacterial |
|
[12] |
| MBGN (96.60SiO2-3.40CaO) and MBGN with Ag | Sol–gel using EA-CTAB-water micro-emulsion droplets | Ag | Antibacterial |
|
[13] |
| MBGSi80 (molar ratio Si/Ca/P = 80/15/5)&&&MBGSi78Cu2 (molar ratio Si/Cu/Ca/P = 78/2/15/5) and MBGSi75Cu5 (molar ratio Si/Cu/Ca/P = 75/5/15/5) | Sol–gel using EISA | Cu | Angiogenic and antibacterial |
|
[9] |
| 85SiO2–13CaO–2CuO | Ultra-sound-assisted base catalyzed sol–gel method | Cu | Angiogenic and antibacterial |
|
[14] |
Abbreviations: CHT, chitosan; CXR, CeloxTM Rapid gauze; EISA, evaporation induced self-assembly; HDF, human dermal fibroblast; MBGs, mesoporous bioactive glasses; NFC, nanofibrillated cellulose.
3. Conclusions and Future Challenges
A lot of experimental studies carried out over the last decade have convincingly proved that mesoporous silicate biomaterials—MSNs and MBGs—are suitable for applications not only in contact with bone but also in the fields of wound management and skin tissue engineering. Of course, there is an obvious mismatch between the physico-mechanical properties of these hard and rigid inorganic materials and those of soft tissues; hence, in most cases, they need to be embedded in polymeric matrices to obtain softer composites. This approach also carries the advantage of making the topical application of these materials particularly easy, which is so common for treating skin or superficial injuries.
MSNs MBGs exhibit an exceptional conformational versatility as they can be produced according to a variety of structures and morphologies (e.g., external geometry, mesopore size, and, in general, textural properties): therefore, a “universal” criterion of choice cannot be defined, but the type, shape, size, and dosage of these materials should be carefully selected depending on the specific application.
MSNs and MBGs have in common the property of acting as drug delivery vehicles: textural characteristics (primarily mesopore size) can be properly tailored depending on the synthesis process, the parameters of which can be selected on the basis of the specific biomolecule to be hosted. As compared to MSNs, MBGs have the additional extra-functionality of acting as vehicles for the release of therapeutic ions as well. In principle, the synergy between the release of drugs/growth factors from the mesopores and ions upon material dissolution can lead to potentiated effects and better therapy; however, the interactions between these two agents, including mutual inhibitory and side effects, should be carefully evaluated. This is a highly fascinating field of research, but, at present, there is a lack of specific regulations and protocols, also in terms of ad hoc in vitro and in vivo models, to study such synergistic interactions.
The release of ions and biomolecules can be finely modulated if the walls of mesopores are properly functionalized and/or the mesoporous materials are embedded in stimuli-responsive polymeric matrices: in both cases, mesopores behave as “intelligent gates” that can be selectively opened or closed depending on the conditions of the environment (e.g., bloodstream, intact surface of the skin, injured skin/wound, etc.) These smart biomaterials also carry the added value of minimizing toxicity in non-target tissues, allowing localization of the release and associated therapeutic actions only when the carrier reaches the wound region, which may exhibit peculiar biochemical conditions, e.g., pH, that are different as compared to those of intact skin. Indeed, a wise, safe, and reproducible exploitation of this therapeutic approach requires the understanding and selection of the most suitable biochemical stimuli that can activate such intelligent MSN-/MBG-based systems.
References
- Gurtner, G.C.; Werner, S.; Barrandon, Y.; Longaker, M.T. Wound repair and regeneration. Nature 2008, 453, 314–321.
- Schultz, G.S.; Wysocki, A. Interactions between extracellular matrix and growth factors in wound healing. Wound Repair Regen. 2009, 17, 153–162.
- Madden, J.W.; Peacock, E.E., Jr. Studies on the biology of collagen during wound healing. 3. Dynamic metabolism of scar collagen and remodeling of dermal wounds. Ann. Surg. 1971, 174, 511.
- Singer, A.J.; Clark, R.A. Cutaneous wound healing. N. Engl. J. Med. 1999, 341, 738–746.
- Eming, S.A.; Krieg, T.; Davidson, J.M. Inflammation in wound repair: Molecular and cellular mechanisms. J. Investig. Dermatol. 2007, 127, 514–525.
- Edwards, R.; Harding, K.G. Bacteria and wound healing. Curr. Opin. Infect. Dis. 2004, 17, 91–96.
- Wolcott, R.D.; Rhoads, D.D.; Dowd, S.E. Biofilms and chronic wound inflammation. J. Wound Care 2008, 17, 333–341.
- Foster, D.S.; Jones, R.E.; Ransom, R.C.; Longaker, M.T.; Norton, J.A. The evolving relationship of wound healing and tumor stroma. JCI Insight 2018, 3, e99911.
- Wang, X.; Cheng, F.; Liu, J.; Smått, J.-H.; Gepperth, D.; Lastusaari, M.; Xu, C.; Hupa, L. Biocomposites of copper-containing mesoporous bioactive glass and nanofibrillated cellulose: Biocompatibility and angiogenic promotion in chronic wound healing application. Acta Biomater. 2016, 46, 286–298.
- Pourshahrestani, S.; Zeimaran, E.; Kadri, N.A.; Gargiulo, N.; Jindal, H.M.; Naveen, S.V.; Sekaran, S.D.; Kamarul, T.; Towler, M.R. Potency and cytotoxicity of a novel gallium-containing mesoporous bioactive glass/chitosan composite scaffold as hemostatic agents. ACS Appl. Mater. Interfaces 2017, 9, 31381–31392.
- Mendonca, A.; Rahman, M.S.; Alhalawani, A.; Rodriguez, O.; Gallant, R.C.; Ni, H.; Clarkin, O.M.; Towler, M.R. The effect of tantalum incorporation on the physical and chemical properties of ternary silicon–calcium–phosphorous mesoporous bioactive glasses. J. Biomed. Mater. Res. Part B Appl. Biomater. 2019, 107, 2229–2237.
- Gholipourmalekabadi, M.; Sameni, M.; Hashemi, A.; Zamani, F.; Rostami, A.; Mozafari, M. Silver- and fluoride-containing mesoporous bioactive glasses versus commonly used antibiotics: Activity against multidrug-resistant bacterial strains isolated from patients with burns. Burns 2016, 42, 131–140.
- Zheng, K.; Balasubramanian, P.; Paterson, T.E.; Stein, R.; MacNeil, S.; Fiorilli, S.; Vitale-Brovarone, C.; Shepherd, J.; Boccaccini, A.R. Ag modified mesoporous bioactive glass nanoparticles for enhanced antibacterial activity in 3D infected skin model. Mater. Sci. Eng. C 2019, 103, 109764.
- Paterson, T.E.; Bari, A.; Bullock, A.J.; Turner, R.; Montalbano, G.; Fiorilli, S.; Vitale-Brovarone, C.; MacNeil, S.; Shepherd, J. Multifunctional copper-containing mesoporous glass nanoparticles as antibacterial and proangiogenic agents for chronic wounds. Front. Bioeng. Biotechnol. 2020, 8, 246.
More
Information
Subjects:
Health Care Sciences & Services
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.0K
Revisions:
2 times
(View History)
Update Date:
29 Jun 2021
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




