
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Gulzhanat Aimagambetova | + 2514 word(s) | 2514 | 2021-06-16 03:46:19 | | | |
| 2 | Nora Tang | + 76 word(s) | 2590 | 2021-06-30 03:35:13 | | |
Video Upload Options
In periodontal diseases, pro-inflammatory cytokines and mediators, including IL-1β, IL-6, TNF-α and PGE2, were produced in subgingival area and then entered systemic circulation. Some authors identified periodontal disease as the cause of more than 18% of all preterm birth cases. Therefore, there is a significant evidence that periodontal pathogens, its enzymes and toxins can induce inflammation in placental tissues and cells.
1. Introduction. Preterm Labor
Preterm labor is defined as a birth before 37 weeks of gestation and occurs in 5–20% of pregnancies [1][2]. It is a leading cause of newborns’ morbidity and mortality, and the second cause of childhood death before the age of 5 years [1][3]. According to data, in the USA the preterm delivery rate is 12–13%, whereas in Europe and other developed countries is up to 9% [4][5]. It is estimated that around 15 million preterm neonates are born annually with the highest rates in Africa, South Asia and North America.
Preterm births account for almost 75 percent of perinatal mortality and more than 50 percent of the long-term morbidity [1][3][5][6][7]. The most common neonatal complications include newborn respiratory distress syndrome (RDS), neural system injury, necrotizing enterocolitis, neonatal jaundice, and infections [2][3]. All the above-mentioned complications lead to prolonged hospitalization, which in turn increases the risk of hospital-acquired infections and death.
Preterm labor is a heterogeneous condition of multifactorial origin influenced by maternal, fetal and environmental factors [8]. Due to the multiple etiologies and risk factors involved, the prediction of preterm labor remains challenging. There are many maternal and/or fetal characteristics associated with preterm birth, including maternal demographic and nutritional status, parity and current pregnancy history, length of the uterine cervix, addictions, infection, and genetic markers (Table 1) The process of preterm labor is thought to be initiated by multiple mechanisms, including infection, immunologically driven processes, placental ischaemia, uterine over distension, bleeding, and other actors [1][5][9][10].
| Risk Factors | OR | 95% CI | Reference |
|---|---|---|---|
| Second trimester cervical length ≤2.50 cm | 6.9 | 4.3–11.1 | [8] |
| Vaginal bleeding in third trimester | 5.9 | 5.1–6.9 | [6] |
| Short interval between pregnancies (<12 months) | 4.2 | 3.0–6.0 | [6] |
| Previous preterm birth with a single newborn | 2.62 | 1.99–3.44 | [8] |
| Vaginal bleeding in first trimester | 2.0 | 1.7–2.3 | [6] |
| Periodontal disease | 2.0 | 1.2–3.2 | [6] |
| Prior cervical conization | 1.7 | 1.24–2.35 | [6] |
| Age younger than 18 | 1.7 | 1.02–3.08 | [6] |
| Low socioeconomic condition | 1.66 | 1.06–2.61 | [8] |
| 1.75 | 1.65–1.86 | [6] | |
| Pregnancy with male fetus | 1.51 | 1.02–2.24 | [8] |
| Asymptomatic bacteriuria | 1.5 | 1.2–1.9 | [6] |
| Bacterial vaginosis | 1.4 | 1.1–1.8 | [6] |
| Family history of preterm birth | 1.35 | 1.12–1.63 | [8] |
| Maternal smoking | 1.27 | 1.21–1.33 | [8] |
| 1.7 | 1.3–2.2 | [6] |
The goal of this review is to evaluate the existing literature sources to identify the role of periodontal infection in the onset of preterm labor. Early identification of pregnancies at highest risk for preterm labor may help in the improvement of the existing and development of new therapeutic management options. It also could serve to improve strategies to prevent neonatal morbidity associated with preterm birth.
2. The Link between Infectious Pathogens and Preterm Labor
There is very strong evidence that infection plays a major role in the pathogenesis of preterm labor. Studies suggest that infection may be responsible for 25–40% of preterm birth cases [5][9][11][12]. The relationship between infection, inflammatory response and preterm labor has been confirmed by multiple findings in preterm labor patients including intrauterine/intra-amniotic and extrauterine maternal infections/inflammations: vaginal infections, urinary tract infections, pneumonia, and periodontal disease [7][12][13][14][15][16][17][18]. The link between infection and preterm birth could also be supported by the fact that antibiotics administered in asymptomatic bacteriuria prevents preterm birth [1][19][20].
Multiple infectious agents could cause the vaginal infection and later via the above-mentioned routes the intrauterine/intra-amniotic infection:Escherichia coli,Enterobacter,group B Streptococcus(GBS)/Streptococcus agalacteae,Staphylococcus epidermidis,Chlamydia trachomatis,Micoplasma hominisandUreaplasma urealiticum,Neisseria gonorrheae,Treponema pallidums,Trichomonas vaginalis, HIV, Hepatitis B and C, as well as bacterial vaginosis (BV). Some of them can be easily ruled out while others might remain undetected and a silent cause of infection.
Women who are tested and found to be positive forU. urealyticumoften have spontaneous preterm labor or preterm premature rupture of membranes (PPROM) Importantly, the earlier the gestational age at preterm labor, the higher the frequency of intrauterine infection [1][5][21]. Although the role of BV itself remains largely unknown, a strong risk for preterm labor was confirmed [5][13][22]. BV has shown to be a cause for spontaneous abortions, highlighting the role of genital infection for adverse pregnancy outcomes (APO) [13].
It is not always clear whether other genital infections likeTrichomonas vaginalis,Treponema pallidum,Neisseria gonorrheae,etc. are responsible for preterm birth [1][5][23].Trichomonas vaginalisseems to be responsible for some preterm labor cases with a relative risk (RR) of about 3 [5]. Conversely,Treponema pallidum,Neisseria gonorrheaeandChlamidia trachomatisare reported to be associated with preterm birth only in the presence of a maternal immune response with RR of 2 [5][24]. Moreover, women with genital infections usually have other risk factors, and many studies have not considered concurrent variables.
Vaginal infections have been found to be linked with an increased risk of PPROM and preterm birth [5]. Being identified as a cause of vaginal infection, microbes access the uterine cavity by an ascending route from the vagina and the cervix [1][5][10]. In turn, intrauterine infection has been identified to be a frequent cause of preterm labor through the spread to the amniotic cavity [1][5]. There are quite a few other routes of its propagation: direct implantation at the time of invasive procedures, retrograde spread through the fallopian tubes, and haematogenous dissemination through the placenta [5][9][25].
Infections of other sites, non-genital, like urinary tract infections, pneumonia, and periodontal infection, are also linked to the preterm labor [5][10]. Some studies suggest an increased risk of preterm labor in periodontal disease [5]. One potential explanation for the relation is that gingival microbes through the bloodstream could reach the uterine cavity and the placenta, resulting in an intra-amniotic infection [5][7][18]. However, the mechanism behind the link between periodontal pathology and preterm labor still remains unclear in some details.
There are two major pathways in biological mechanisms of APO related to oral pathogens defined by the consensus report from the joint European Federation of Periodontology/American Academy of Periodontology workshop on periodontitis and systematic diseases [9][26]: (1) direct mechanisms—oral microorganisms invade the placenta and amniotic cavity via hematogenous dissemination, or in an ascending route via the genitourinary tract; (2) indirect mechanisms, promoted by inflammatory mediators produced in periodontal tissues, in response to the pathogens invasion. These mediators may directly affect the fetal-placental unit or circulate to the liver and increase the systemic inflammatory response, which could later affect the fetal-placental unit [5][7][27].
Vaginal infections lead to the increased concentration of the inflammatory markers that can be identified in cervical and vaginal secretions and have been shown to be significantly associated with the preterm labor rates [9][13][28]. Those include interleukin (IL)-6, IL-8, IL-1β, ferritin and tumor necrosis factor α (TNFα) [5][13]. Endotoxins released by the microorganisms together with the proinflammatory cytokines stimulate the production of prostaglandins (PGs), other inflammatory mediators, and matrix-degrading enzymes. PGs in turn stimulate myometrium leading to increased uterine contractility and onset of preterm labor and PPROM [1][5][7][9][13].
3. Periodontal Pathogens and Pregnancy
Women’s bodies undergo important adaptations in many organ systems and hormonal changes during pregnancy. As well as the other systems, gastrointestinal tract and the oral cavity as a part of it are also under this influence. Vomiting can negatively affect oral hygiene or may cause erosions in the oral cavity [29]. The following oral conditions have been described as affecting pregnant women to a greater degree than their non-pregnant counterparts: dental caries, gingivitis, pregnancy granuloma, and periodontitis [30].
Gingivitis is the most frequent oral disease in pregnancy, with a prevalence of 40–75% according to different sources [29][30][31]. Approximately 50% of women with preexisting gingivitis will face significant exacerbation during pregnancy due to changes in estrogen and progesterone levels combined with oral microbiota alterations and pregnancy-related physiologic immunodeficiency [29][30][31]. Pregnancy gingivitis could be seen very often and usually starts at the first trimester of gestation, worsens as the pregnancy progresses before reaching a peak close to the end of the third trimester and heals spontaneously after birth [29][30][31]. However, in the last weeks of gestation, rates of gingivitis usually decreases and immediately in the postpartum period, the gingival tissues are found to be comparable to those seen during the first trimester of pregnancy.
The decline and exacerbation in oral health during pregnancy depends on multiple factors [29]. During the first trimester of pregnancy, some women may have eating behavior changes like increased consumption of carbohydrates or even pica. Therefore, oral care becomes more important in pregnancy. Recently, a published paper associated gingival changes in pregnancy with increased vascularization and blood flow in conjunction with the pregnancy-related physiologic immunodeficiency and changes in connective tissue metabolism [29][30][31].
As mentioned before, vomiting, especially during the first trimester of pregnancy, increases the acidity in the mouth. Because episodes of vomiting in cases of hyperemesis gravidarum are usually very frequent, the pregnant woman may not pay enough attention to oral care after each event [29][30]. For these reasons, the rate of caries increases in the first trimester of pregnancy. Due to the existence of the listed risk factors, it is important to draw more attention to dental care and health during this period.
The first association between periodontal disease and preterm labor was investigated and reported in 1996 by Offenbacher [32]. In later studies, it has been confirmed that approximately 40% of pregnant women have some form of periodontal disease, and the rate is higher among women of low socioeconomic status [33]. Multiple studies evaluating the link between periodontal pathologies and preterm labor, low birth weight, and preeclampsia, have been published using case-control, cohort, and cross-sectional study designs. From the other side, a strong relationship between preterm birth and periodontitis was confirmed in recently published study, suggesting that oral infections may be considered a risk factor for gestational adversities [34].
Periodontal diseases represent one of the most common chronic infections in humans with a prevalence of 10 to 60% among adult population [9]. Periodontal diseases include many different inflammatory conditions that affect the gingiva, but also the alveolar bone and the periodontal ligament that anchors the tooth to the bone.
Periodontitis is a chronic multifactorial inflammatory disease associated with dysbiotic plaque biofilms and characterized by progressive destruction of the tooth-supporting tissues [35]. It is a relatively common clinical condition, which occurs in more than 30% of people in some populations [35]. The prevalence among pregnant women ranges between 5% and 20% [35].
Periodontitis has been classified by different ways: (1) based on stages defined by severity, complexity and extent and distribution (stage I, II, and III); (2) based on grades that reflect biologic features of the disease including evidence of, or risk for, rapid progression, expected treatment response, and effects on general health (grade A, B, and C) [35]. There are separate classifications of necrotizing periodontal diseases, endo-periodontal lesions, and periodontal abscesses [35].
Periodontal pathology usually begins with a localized inflammation of the gingiva, called gingivitis, caused by dental plaques, microbial biofilms that form on the teeth and gingiva. If left untreated, the inflammation in gingivitis can lead to periodontitis [36]. The inflammatory response in periodontal diseases is found to contribute to the development of certain systemic diseases such as Type II Diabetes mellitus [37], cardiovascular diseases [38][39], rheumatoid arthritis [40], and even oral cancer [41].
Periodontal disease initiates as infection caused by an overgrowth of certain bacterial species in the subgingival sites. The multispecies subgingival biofilm that causes periodontal disease consists mostly of Gram-negative, anaerobic bacteria. In the early stages of biofilm formation, the bacteria that are present mostly belong to the “blue”, “green”, “yellow” and “purple” clusters. Although many virulence factors of the pathogenic bacteria are already known, the exact contribution of each species in the development of periodontitis is still unknown [42].
However, microbial colonization and biofilm formation is just the initial step in the periodontal disease development. The disease develops when the host’s immune system overreacts to the presence of bacteria, a process called dysbiosis [43]. This imbalance is very complex, there are great variances both of the biofilm composition and the host immune reaction profiles which lead to tissue damage due to a heightened inflammatory state [44]. The alveolar bone is resorbed by osteoclasts, ligament fibers are degraded by enzymes called matrix metalloproteinases, and granulation tissue is formed [45].
Approximately, in 50% of cases of premature birth the etiological factors are known and infectious agents responsible for almost 75% of them. However, the other half of cases remain idiopathic [46][47][48].
During pregnancy, due to hormonal changes, there may be a tendency towards development of periodontal disease. In particular, there is an increase of anaerobic gram-negative bacteria such asFusobacterium nucleatum,Treponema denticola,Tannerella forsythia,Campylobacter rectus,Eikenella corrodens, andSelenomonas sputigena[46][47] that could contribute to the incidence of periodontal pathology.
Oral infections might be considered as one of the factors contributing to the preterm labor incidence, since commensal bacterial species of the oral cavity were found to be disseminating to the fetoplacental unit of women with term gestation and APO [46][49]. The following microbes have been found to be strongly associated with APO: Fusobacterium nucleatum,Campylobacter rectus,Porphyromonas gingivalis, andBergeyella spp.[26][46].
Many investigations have shown an association of periodontal disease, prematurity, and low birth weight [18][50][34]. On the other hand, clinical trials have studied the effect of periodontal treatment on APO and showed controversial results [46][51][52]. There are at least two explanations for such contradictory findings: (1) colonization of fetoplacental site by periodontal pathogens appears at the end of the first trimester and its detrimental effect cannot be completely eliminated by antimicrobials, and (2) significant diversity between investigations in regard to the populations’ race, age, and the periodontitis definition accepted for these studies [36][53].
The conclusions of many studies/systematic analyses could be contradictory due to the relative heterogeneity of studied populations according to ethnicity, different risk factors discussed, diversity in socio-economic and education levels, and periodontal status definition.
During pregnancy, periodontal status changes. Due to the physiologic changes in the immune system, pregnant women are more prone to have inflammatory conditions and increased rate of gingival bleeding. It appears that pregnancy might lead to an increase of periodontal disease severity in women suffering from periodontitis before pregnancy [9][10][21][52].
Pregnancy related nausea and vomiting are relatively frequent in early pregnancy. As a consequence, gastric acid would damage periodontal tissue barriers to various pathogens, which could be responsible for placental infection and systemic inflammation leading to preterm birth [54].
Furthermore, hormonal fluctuations during pregnancy have been proposed to initiate changes in the composition of oral biofilm leading to exacerbation of gingival inflammation. Researchers discuss that hematogenous dissemination of microbes and pro-inflammatory mediators from sites of periodontal infection into the placenta, fetal membranes, and amniotic cavity induces pathological processes that lead to APO [55]. However, researchers’ opinions remain controversial as some studies supported this hypothesis, but the others failed to demonstrate improved perinatal outcomes following treatment of periodontal disease in pregnancy [56].
The biological plausibility of association between periodontal disease and APO is based in a hypothetical model of two major pathways (direct and indirect) responsible for the pathologic process [7][57][58][59]. It was discussed in one of the previous sections related to the pathophysiological mechanisms. The pathogenesis of periodontal inflammation and preterm birth including the role of the main inflammatory mediators (IL-1β, IL-6, PGE-2 and TNF-α) are schematically presented on the Figure 1.
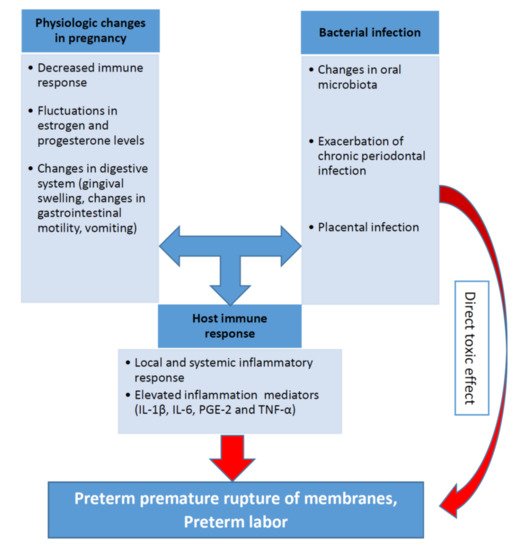
Relationship between pro-inflammatory cytokines, periodontal infection, and preterm birth is recognized in both experimental conditions and animal models. (2015),P. gingivalisinduced preterm birth and low birth weight in pregnant mice and significantly elevated maternal levels of circulating TNF-α, IL-17, On the other hand, periodontal bacteria have been found in normal placentas without APO [60]. In periodontal diseases, pro-inflammatory cytokines and mediators, including IL-1β, IL-6, TNF-α and PGE2, were produced in subgingival area and then entered systemic circulation [5][7][61].
Some authors identified periodontal disease as the cause of more than 18% of all preterm birth cases. Therefore, there is a significant evidence that periodontal pathogens, its enzymes and toxins can induce inflammation in placental tissues and cells [62][63]. Thus, investigations of the association between periodontal disease and APO are highly relevant for clinical medicine [64][65][66].
References
- Glover, A.V.; Manuck, T.A. Screening for spontaneous preterm birth and resultant therapies to reduce neonatal morbidity and mortality: A review. Semin. Fetal Neonatal. Med. 2018, 23, 126–132.
- Berger, R.; Abele, H.; Bahlmann, F.; Bedei, I.; Doubek, K.; Felderhoff-Müser, U.; Fluhr, H.; Garnier, Y.; Grylka-Baeschlin, S.; Helmer, H.; et al. Prevention and Therapy of Preterm Birth. Guideline of the DGGG, OEGGG and SGGG (S2k Level, AWMF Registry Number 015/025, February 2019)—Part 1 with Recommendations on the Epidemiology, Etiology, Prediction, Primary and Secondary Prevention of Preterm Birth. Geburtshilfe und Frauenheilkunde 2019, 79, 800–812.
- Daskalakis, G.; Arabin, B.; Antsaklis, A.; Cabero Roura, L. Preterm Labor: Up to Date. Biomed. Res. Int. 2019, 2019, 4870938.
- Slattery, M.M.; Morrison, J.J. Preterm delivery. Lancet 2002, 360, 1489–1497.
- Goldenberg, R.L.; Culhane, J.F.; Iams, J.D.; Romero, R. Epidemiology and causes of preterm birth. Lancet 2008, 371, 75–84.
- Berger, R.; Abele, H.; Bahlmann, F.; Bedei, I.; Doubek, K.; Felderhoff-Müser, U.; Fluhr, H.; Garnier, Y.; Grylka-Baeschlin, S.; Helmer, H.; et al. Prevention and Therapy of Preterm Birth. Guideline of the DGGG, OEGGG and SGGG (S2k Level, AWMF Registry Number 015/025, February 2019)—Part 1 with Recommendations on the Epidemiology, Etiology, Prediction, Primary and Secondary Prevention of Preterm Birth. Geburtshilfe und Frauenheilkunde 2019, 79, 800–812.
- Ren, H.; Du, M. Role of Maternal Periodontitis in Preterm Birth. Front. Immunol. 2017, 8, 139.
- Glover, A.V.; Manuck, T.A. Screening for spontaneous preterm birth and resultant therapies to reduce neonatal morbidity and mortality: A review. Semin. Fetal Neonatal. Med. 2018, 23, 126–132.
- Figuero, E.; Han, Y.W.; Furuichi, Y. Periodontal diseases and adverse pregnancy outcomes: Mechanisms. Periodontol 2000 2020, 83, 175–188.
- Jajoo, N.S.; Shelke, A.U.; Bajaj, R.S.; Patil, P.P.; Patil, M.A. Association of periodontitis with pre term low birth weight—A review. Placenta 2020, 95, 62–68.
- Gonçalves, L.F.; Chaiworapongsa, T.; Romero, R. Intrauterine infection and prematurity. Ment. Retard. Dev. Disabil. Res. Rev. 2002, 8, 3–13.
- Romero, R.; Espinoza, J.; Kusanovic, J.P.; Gotsch, F.; Hassan, S.; Erez, O.; Chaiworapongsa, T.; Mazor, M. The preterm parturition syndrome. BJOG Int. J. Obstet. Gynaecol. 2006, 113, 17–42.
- Leitich, H. Secondary predictors of preterm labour. Int. J. Obstet. Gynaecol. 2005, 112, 48–50.
- Fidel, P.L.; Romero, R.; Wolf, N.; Cutright, J.; Ramirez, M.; Araneda, H.; Cotton, D.B. Systemic and local cytokine profiles in endotoxin-induced preterm parturition in mice. Am. J. Obstet. Gynecol. 1994, 170, 1467–1475.
- Wang, H.; Hirsch, E. Bacterially-Induced Preterm Labor and Regulation of Prostaglandin-Metabolizing Enzyme Expression in Mice: The Role of Toll-Like Receptor 41. Biol. Reprod. 2003, 69, 1957–1963.
- Kaul, A.K.; Khan, S.; Martens, M.G.; Crosson, J.T.; Lupo, V.R.; Kaul, R. Experimental gestational pyelonephritis induces preterm births and low birth weights in C3H/HeJ mice. Infect. Immun. 1999, 67, 5958–5966.
- Offenbacher, S. Maternal periodontal infections, prematurity, and growth restriction. Clin. Obstet. Gynecol. 2004, 47, 808–821.
- Offenbacher, S.; Boggess, K.A.; Murtha, A.P.; Jared, H.L.; Lieff, S.; McKaig, R.G.; Mauriello, S.M.; Moss, K.L.; Beck, J.D. Progressive Periodontal Disease and Risk of Very Preterm Delivery. Obstet. Gynecol. 2006, 107, 29–36.
- Smaill, F.M.; Vazquez, J.C. Antibiotics for asymptomatic bacteriuria in pregnancy. Cochrane Database Syst. Rev. 2019, 11, CD000490.
- Dautt-Leyva, J.G.; Canizalez-Román, A.; Acosta Alfaro, L.F.; Gonzalez-Ibarra, F.; Murillo-Llanes, J. Maternal and perinatal complications in pregnant women with urinary tract infection caused by Escherichia coli. J. Obstet. Gynaecol. Res. 2018, 44, 1384–1390.
- Fischer, L.A.; Demerath, E.; Bittner-Eddy, P.; Costalonga, M. Placental colonization with periodontal pathogens: The potential missing link. Am. J. Obstet. Gynecol. 2019, 221, 383–392.e3.
- Brabant, G. Vaginose bactérienne et prématurité spontanée [Bacterial vaginosis and spontaneous preterm birth]. J. Gynecol. Obstet. Biol. Reprod. 2016, 45, 1247–1260.
- Goldenberg, R.L.; Culhane, J.F.; Johnson, D.C. Maternal Infection and Adverse Fetal and Neonatal Outcomes. Clin. Perinatol. 2005, 32, 523–559.
- Goldenberg, R.L.; Andrews, W.W.; Yuan, A.C.; Mackay, H.T.; Louis, M.E.S. Sexually Transmitted Diseases and Adverse Outcomes of Pregnancy. Clin. Perinatol. 1997, 24, 23–41.
- Goldenberg, R.L.; Hauth, J.C.; Andrews, W.W. Intrauterine Infection and Preterm Delivery. N. Engl. J. Med. 2000, 342, 1500–1507.
- Sanz, M.; Kornman, K.; Working group 3 of the joint EFP/AAP workshop. Periodontitis and adverse pregnancy outcomes: Consensus report of the Joint EFP/AAP Workshop on Periodontitis and Systemic Diseases. J. Periodontol. 2013, 84, S164–S169.
- Gürsoy, M.; Könönen, E.; Gursoy, U.K.; Tervahartiala, T.; Pajukanta, R.; Sorsa, T. Periodontal Status and Neutrophilic Enzyme Levels in Gingival Crevicular Fluid During Pregnancy and Postpartum. J. Periodontol. 2010, 81, 1790–1796.
- Moosa, Y.; Kwon, D.; De Oliveira, T.; Wong, E.B. Determinants of Vaginal Microbiota Composition. Front. Cell. Infect. Microbiol. 2020, 10.
- Yenen, Z.; Ataçağ, T. Oral care in pregnancy. J. Turk. Gynecol. Assoc. 2019, 20, 264–268.
- Ioannidou, E.; Robinson, P.J. Influence of Pregnancy on the Oral Cavity. Glob. Libr. Women’s Med 2009.
- Silk, H.; Douglass, A.B.; Douglass, J.M.; Silk, L. Oral health during pregnancy. Am. Fam. Physician. 2008, 77, 1139–1144.
- Offenbacher, S.; Katz, V.; Fertik, G.; Collins, J.; Boyd, D.; Maynor, G.; McKaig, R.; Beck, J. Periodontal infection as a possible risk factor for preterm low birth weight. J. Periodontol. 1996, 67, 1103–1113.
- Musskopf, M.L.; Milanesi, F.C.; da Rocha, J.M.; Fiorini, T.; Moreira, C.H.C.; Susin, C.; Rosing, C.K.; Weidlich, P.; Oppermann, R.V. Oral health related quality of life among pregnant women: A randomized controlled trial. Braz. Oral Res. 2018, 32, e002.
- Calixto, N.; Alves, C.; Abreu, L.; Thomaz, E.; Vidal, F.; Gomes-Filho, I.; Lopes, F. Detection of periodontal pathogens in mothers of preterm birth and/or low weight. Med. Oral Patol. Oral Cir. Bucal 2019, 24, e776–e781.
- Papapanou, P.N.; Sanz, M.; Buduneli, N.; Dietrich, T.; Feres, M.; Fine, D.H.; Flemmig, T.F.; Garcia, R.; Giannobile, W.V.; Graziani, F.; et al. Periodontitis: Consensus report of workgroup 2 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J. Periodontol. 2018, 89 (Suppl. 1), S173–S182.
- Kinane, D.; Bouchard, P.; Group E of the European Workshop on Periodontology. Periodontal diseases and health: Consensus Report of the Sixth European Workshop on Periodontology. J. Clin. Periodontol. 2008, 35, 333–337.
- Mealey, B.L.; Rose, L.F. Diabetes mellitus and inflammatory periodontal diseases. Curr. Opin. Endocrinol. Diabetes Obes. 2008, 15, 135–141.
- Kannosh, I.; Staletovic, D.; Toljic, B.; Radunovic, M.; Pucar, A.; Petrovic, S.M.; Grubisa, I.; Lazarevic, M.; Brkic, Z.; Vukcevic, J.K.; et al. The presence of periopathogenic bacteria in subgingival and atherosclerotic plaques–An age related comparative analysis. J. Infect. Dev. Count. 2018, 12, 1088–1095.
- Bahekar, A.A.; Singh, S.; Saha, S.; Molnar, J.; Arora, R. The prevalence and incidence of coronary heart disease is significantly increased in periodontitis: A meta-analysis. Am. Heart J. 2007, 154, 830–837.
- Kaur, S.; White, S.; Bartold, P.M. Periodontal disease and rheumatoid arthritis: A systematic review. J. Dental Res. 2013, 92, 399–408.
- Whitmore, S.E.; Lamont, R.J. Oral Bacteria and Cancer. PLoS Pathog. 2014, 10, e1003933.
- Kornman, K.S.; Page, R.C.; Tonetti, M.S. The host response to the microbial challenge in periodontitis: Assembling the players. Periodontol. 2000 1997, 14, 33–53.
- Hajishengallis, G.; Lamont, R. Beyond the red complex and into more complexity: The polymicrobial synergy and dysbiosis (PSD) model of periodontal disease etiology. Mol. Oral Microbiol. 2012, 27, 409–419.
- Feres, M.; Teles, F.; Teles, R.; Figueiredo, L.C.; Faveri, M. The subgingival periodontal microbiota of the aging mouth. Periodontol. 2000 2016, 72, 30–53.
- Sorsa, T.; Gursoy, U.K.; Nwhator, S.; Hernandez, M.; Tervahartiala, T.; Leppilahti, J.; Gursoy, M.; Könönen, E.; Emingil, G.; Pussinen, P.J.; et al. Analysis of matrix metalloproteinases, especially MMP-8, in gingival crevicular fluid, mouthrinse and saliva for monitoring periodontal diseases. Periodontol. 2000 2016, 70, 142–163.
- Caneiro-Queija, L.; López-Carral, J.; Martin-Lancharro, P.; Limeres-Posse, J.; Diz-Dios, P.; Blanco-Carrion, J. Non-Surgical Treatment of Periodontal Disease in a Pregnant Caucasian Women Population: Adverse Pregnancy Outcomes of a Randomized Clinical Trial. Int. J. Environ. Res. Public Health 2019, 16, 3638.
- Carta, G.; Persia, G.; Falciglia, K.; Iovenitti, P. Periodontal disease and poor obstetrical outcome. Clin. Exp. Obstet. Gynecol. 2004, 31, 47–49.
- López, N.J.; Da Silva, I.; Ipinza, J.N.; Gutiérrez, J. Periodontal Therapy Reduces the Rate of Preterm Low Birth Weight in Women with Pregnancy-Associated Gingivitis. J. Periodontol. 2005, 76, 2144–2153.
- Cruz, I.S.; Herrera, D.; Martin, C.; Herrero, A.; Sanz, M. Association between periodontal status and pre-term and/or low-birth weight in Spain: Clinical and microbiological parameters. J. Periodontal Res. 2013, 48, 443–451.
- Saadaoui, M.; Singh, P.; Al Khodor, S. Oral microbiome and pregnancy: A bidirectional relationship. J. Reprod. Immunol. 2021, 145, 103293.
- Puertas, A.; Magan-Fernandez, A.; Blanc, V.; Revelles, L.; O’Valle, F.; Pozo, E.; León, R.; Mesa, F. Association of periodontitis with preterm birth and low birth weight: A comprehensive review. J. Materm. Fetal Neonatal. Med. 2018, 31, 597–602.
- Offenbacher, S.; Beck, J.D.; Jared, H.L.; Mauriello, S.M.; Mendoza, L.C.; Couper, D.; Stewart, D.D.; Murtha, A.P.; Cochran, D.L.; Dudley, D.J.; et al. Effects of Periodontal Therapy on Rate of Preterm Delivery. Obstet. Gynecol. 2009, 114, 551–559.
- Chambrone, L.; Pannuti, C.M.; Guglielmetti, M.R.; Chambrone, L.A. Evidence grade associating periodontitis with preterm birth and/or low birth weight: II. A systematic review of randomized trials evaluating the effects of periodontal treatment. J. Clin. Periodontol. 2011, 38, 902–914.
- Lee, K.-S.; Song, I.-S.; Kim, E.-S.; Ahn, K.H. Determinants of Spontaneous Preterm Labor and Birth Including Gastroesophageal Reflux Disease and Periodontitis. J. Korean Med. Sci. 2020, 35, e105.
- Liang, S.; Ren, H.; Guo, H.; Xing, W.; Liu, C.; Ji, Y.; Jiang, H.; Zhang, P.; Du, M. Periodontal infection with Porphyromonas gingivalis induces preterm birth and lower birth weight in rats. Mol. Oral Microbiol. 2018, 33, 312–321.
- Rangel-Rincón, L.J.; Vivares-Builes, A.M.; Botero, J.E.; Agudelo-Suárez, A.A. An Umbrella Review Exploring the Effect of Periodontal Treatment in Pregnant Women on the Frequency of Adverse Obstetric Outcomes. J. Évid. Based Dent. Pract. 2018, 18, 218–239.
- Krüger, M.S.D.M.; Casarin, R.P.; Pinto, G.D.S.; Pappen, F.G.; Camargo, M.B.J.; Correa, F.O.B.; Romano, A.R. Maternal periodontal disease and adverse perinatal outcomes: Is there an association? A hospital-based case-control study. J. Matern. Neonatal Med. 2019, 32, 3401–3407.
- Morelli, E.L.; Broadbent, J.M.; Leichter, J.W.; Thomson, W.M. Pregnancy, parity and periodontal disease. Aust. Dent. J. 2018, 63, 270–278.
- Uriza, C.L.; Velosa-Porras, J.; Roa, N.S.; Lara, S.M.Q.; Silva, J.; Ruiz, A.J.; Arregoces, F.M.E. Periodontal Disease, Inflammatory Cytokines, and PGE2 in Pregnant Patients at Risk of Preterm Delivery: A Pilot Study. Infect. Dis. Obstet. Gynecol. 2018, 2018, 1–7.
- Komine-Aizawa, S.; Aizawa, S.; Hayakawa, S. Periodontal diseases and adverse pregnancy outcomes. J. Obstet. Gynaecol. Res. 2019, 45, 5–12.
- Stadelmann, P.; Alessandri, R.; Eick, S.; Salvi, G.E.; Surbek, D.; Sculean, A. The potential association between gingival crevicular fluid inflammatory mediators and adverse pregnancy outcomes: A systematic review. Clin. Oral Investig. 2013, 17, 1453–1463.
- Brikos, C.; O’Neill, L.A. Signalling of toll-like receptors. Handb. Exp. Pharmacol. 2008, 183, 21–50.
- Arce, R.M.; Diaz, P.; Barros, S.; Galloway, P.; Bobetsis, Y.; Threadgill, D.; Offenbacher, S. Characterization of the invasive and inflammatory traits of oral Campylobacter rectus in a murine model of fetoplacental growth restriction and in trophoblast cultures. J. Reprod. Immunol. 2010, 84, 145–153.
- Gao, L.; Xu, T.; Huang, G.; Jiang, S.; Gu, Y.; Chen, F. Oral microbiomes: More and more importance in oral cavity and whole body. Protein Cell 2018, 9, 488–500.
- Manrique-Corredor, E.J.; Orozco-Beltran, D.; Lopez-Pineda, A.; Quesada, J.A.; Gil-Guillen, V.F.; Carratala-Munuera, C. Maternal periodontitis and preterm birth: Systematic review and meta-analysis. Community Dent. Oral Epidemiol. 2019, 47, 243–251.
- Makeeva, I.M.; Ignatko, A.A.; Churganova, A.A.; Lebedev, V.A.; Makeeva, M.K. Periodontal diseases and complicated pregnancy. Stomatology 2019, 98, 70–73.





