
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Julien Guy Mahy | + 2107 word(s) | 2107 | 2021-06-28 08:27:04 | | | |
| 2 | Lily Guo | Meta information modification | 2107 | 2021-06-28 09:32:09 | | | | |
| 3 | Lily Guo | Meta information modification | 2107 | 2021-06-28 09:32:37 | | |
Video Upload Options
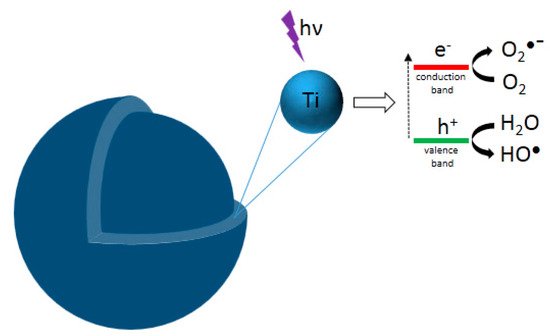
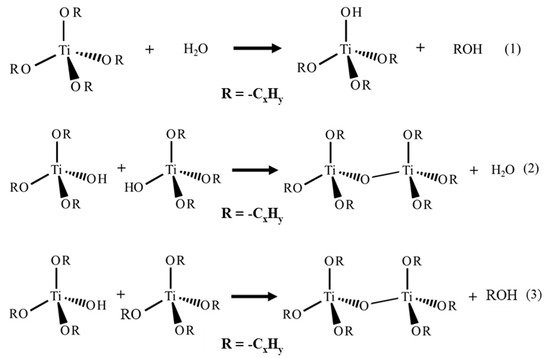
This entry reviews an eco-friendly process for producing TiO2 via colloidal aqueous sol–gel synthesis, resulting in crystalline materials without a calcination step. Three types of colloidal aqueous TiO2 are reviewed: the as-synthesized type obtained directly after synthesis, without any specific treatment; the calcined, obtained after a subsequent calcination step; and the hydrothermal, obtained after a specific autoclave treatment. This eco-friendly process is based on the hydrolysis of a Ti precursor in excess of water, followed by the peptization of the precipitated TiO2. Compared to classical TiO2 synthesis, this method results in crystalline TiO2 nanoparticles without any thermal treatment and uses only small amounts of organic chemicals. Depending on the synthesis parameters, the three crystalline phases of TiO2 (anatase, brookite, and rutile) can be obtained. The morphology of the nanoparticles can also be tailored by the synthesis parameters. The most important parameter is the peptizing agent. Indeed, depending on its acidic or basic character and also on its amount, it can modulate the crystallinity and morphology of TiO2. Colloidal aqueous TiO2 photocatalysts are mainly being used in various photocatalytic reactions for organic pollutant degradation. The as-synthesized materials seem to have equivalent photocatalytic efficiency to the photocatalysts post-treated with thermal treatments and the commercial Evonik Aeroxide P25, which is produced by a high-temperature process. Indeed, as-prepared, the TiO2 photocatalysts present a high specific surface area and crystalline phases. Emerging applications are also referenced, such as elaborating catalysts for fuel cells, nanocomposite drug delivery systems, or the inkjet printing of microstructures. Only a few works have explored these new properties, giving a lot of potential avenues for studying this eco-friendly TiO2 synthesis method for innovative implementations.
1. Introduction
2. Morphology
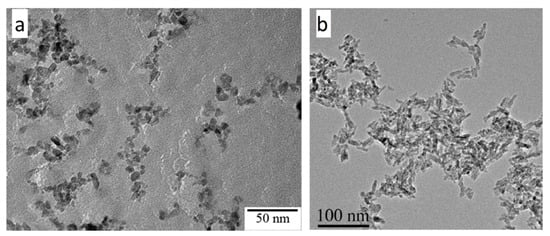
2.1. Morphology of As-Synthesized Aqueous TiO2
2.2. Morphology of Aqueous TiO2 after Calcination Treatment
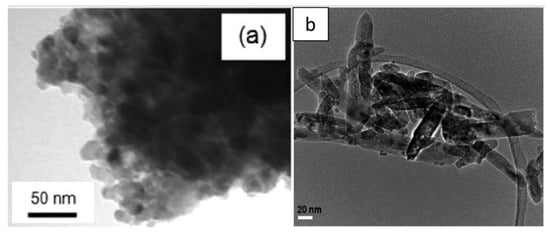
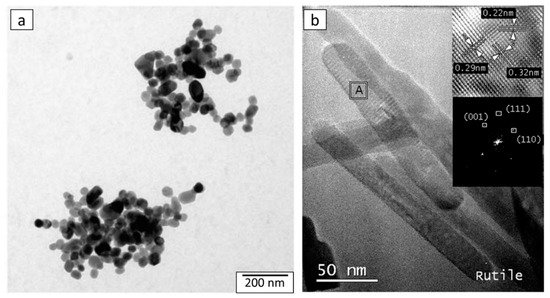
2.3. Morphology of Aqueous TiO2 after Hydrothermal Treatment
References
- Oturan, M.A.; Aaron, J.-J. Advanced Oxidation Processes in Water/Wastewater Treatment: Principles and Applications. A Review. Crit. Rev. Environ. Sci. Technol. 2014, 44, 2577–2641.
- Nakata, K.; Fujishima, A. TiO2 photocatalysis: Design and applications. J. Photochem. Photobiol. C Photochem. Rev. 2012, 13, 169–189.
- Hermawan, A.; Hanindriyo, A.T.; Ramadhan, E.R.; Asakura, Y.; Hasegawa, T.; Hongo, K.; Inada, M.; Maezono, R.; Yin, S. Octahedral morphology of NiO with (111) facet synthesized from the transformation of NiOHCl for the NOx detection and degradation: Experiment and DFT calculation. Inorg. Chem. Front. 2020, 7, 3431–3442.
- Ong, C.B.; Ng, L.Y.; Mohammad, A.W. A review of ZnO nanoparticles as solar photocatalysts: Synthesis, mechanisms and applications. Renew. Sustain. Energy Rev. 2018, 81, 536–551.
- Ma, R.; Zhang, S.; Wen, T.; Gu, P.; Li, L.; Zhao, G.; Niu, F.; Huang, Q.; Tang, Z.; Wang, X. A critical review on visible-light-response CeO2-based photocatalysts with enhanced photooxidation of organic pollutants. Catal. Today 2019, 335, 20–30.
- Chiam, S.-L.; Pung, S.-Y.; Yeoh, F.-Y. Recent developments in MnO2-based photocatalysts for organic dye removal: A review. Environ. Sci. Pollut. Res. 2020, 27, 5759–5778.
- Pelaez, M.; Nolan, N.T.; Pillai, S.C.; Seery, M.; Falaras, P.; Kontos, A.G.; Dunlop, P.S.; Hamilton, J.W.; Byrne, J.; O’Shea, K.; et al. A review on the visible light active titanium dioxide photocatalysts for environmental applications. Appl. Catal. B Environ. 2012, 125, 331–349.
- Malengreaux, C.M.; Douven, S.; Poelman, D.; Heinrichs, B.; Bartlett, J.R. An ambient temperature aqueous sol–gel processing of efficient nanocrystalline doped TiO2-based photocatalysts for the degradation of organic pollutants. J. Sol Gel Sci. Technol. 2014, 71, 557–570.
- Espino-Estévez, M.; Fernández-Rodríguez, C.; González-Díaz, O.M.; Araña, J.; Espinós, J.; Ortega-Méndez, J.; Doña-Rodríguez, J.M. Effect of TiO2–Pd and TiO2–Ag on the photocatalytic oxidation of diclofenac, isoproturon and phenol. Chem. Eng. J. 2016, 298, 82–95.
- Vaiano, V.; Iervolino, G.; Sannino, D.; Murcia, J.J.; Hidalgo, M.C.; Ciambelli, P.; Navío, J.A. Photocatalytic removal of patent blue V dye on Au-TiO2 and Pt-TiO2 catalysts. Appl. Catal. B Environ. 2016, 188, 134–146.
- Di Paola, A.; Marci, G.; Palmisano, L.; Schiavello, M.; Uosaki, K.; Ikeda, A.S.; Ohtani, B. Preparation of Polycrystalline TiO2Photocatalysts Impregnated with Various Transition Metal Ions: Characterization and Photocatalytic Activity for the Degradation of 4-Nitrophenol. J. Phys. Chem. B 2002, 106, 637–645.
- Rauf, M.; Meetani, M.; Hisaindee, S. An overview on the photocatalytic degradation of azo dyes in the presence of TiO2 doped with selective transition metals. Desalination 2011, 276, 13–27.
- Bodson, C.J.; Heinrichs, B.; Tasseroul, L.; Bied, C.; Mahy, J.G.; Man, M.W.C.; Lambert, S.D. Efficient P- and Ag-doped titania for the photocatalytic degradation of waste water organic pollutants. J. Alloys Compd. 2016, 682, 144–153.
- Di Valentin, C.; Pacchioni, G.; Selloni, A.; Livraghi, S.; Giamello, E. Characterization of Paramagnetic Species in N-Doped TiO2 Powders by EPR Spectroscopy and DFT Calculations. J. Phys. Chem. B 2005, 109, 11414–11419.
- Gilma, G.O.; Carlos, A.P.M.; Fernando, M.O.; Edgar, A.P.-M. Photocatalytic degradation of phenol on TiO2 and TiO2/Pt sensitized with metallophthalocyanines. Catal. Today 2005, 107–108, 589–594.
- Mahy, J.G.; Paez, C.A.; Carcel, C.; Bied, C.; Tatton, A.S.; Damblon, C.; Heinrichs, B.; Man, M.W.C.; Lambert, S.D. Porphyrin-based hybrid silica-titania as a visible-light photocatalyst. J. Photochem. Photobiol. A Chem. 2019, 373, 66–76.
- Xie, H.; Gao, G.; Tian, Z.; Bing, N.; Wang, L. Synthesis of TiO2 nanoparticles by propane/air turbulent flame CVD process. Particuology 2009, 7, 204–210.
- Djenadic, R.; Winterer, M. Chemical Vapor Synthesis of Nanocrystalline Oxides. In 2D Nanoelectronics; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 2012; pp. 49–76.
- Inturi, S.N.R.; Boningari, T.; Suidan, M.; Smirniotis, P.G. Flame Aerosol Synthesized Cr Incorporated TiO2for Visible Light Photodegradation of Gas Phase Acetonitrile. J. Phys. Chem. C 2013, 118, 231–242.
- Dar, M.I.; Chandiran, A.K.; Graetzel, M.; Nazeeruddin, M.K.; Shivashankar, S.A. Controlled synthesis of TiO2 nanoparticles and nanospheres using a microwave assisted approach for their application in dye-sensitized solar cells. J. Mater. Chem. A 2013, 2, 1662–1667.
- Zhang, D.; Qi, L.; Ma, J.; Cheng, H. Formation of crystalline nanosized titania in reverse micelles at room temperature. J. Mater. Chem. 2002, 12, 3677–3680.
- Nian, J.-N.; Teng, H. Hydrothermal Synthesis of Single-Crystalline Anatase TiO2Nanorods with Nanotubes as the Precursor. J. Phys. Chem. B 2006, 110, 4193–4198.
- Simon, P.; Pignon, B.; Miao, B.; Coste-Leconte, S.; Leconte, Y.; Marguet, S.; Jegou, P.; Bouchet-Fabre, B.; Reynaud, C.; Herlin-Boime, N. N-Doped Titanium Monoxide Nanoparticles with TiO Rock-Salt Structure, Low Energy Band Gap, and Visible Light Activity. Chem. Mater. 2010, 22, 3704–3711.
- Gratzel, M. Sol-Gel Processed TiO2 Films for Photovoltaic Applications. J. Sol Gel Sci. Technol. 2001, 22, 7–13.
- Carp, O. Photoinduced reactivity of titanium dioxide. Prog. Solid State Chem. 2004, 32, 33–177.
- Huang, T.; Huang, W.; Zhou, C.; Situ, Y.; Huang, H. Superhydrophilicity of TiO2/SiO2 thin films: Synergistic effect of SiO2 and phase-separation-induced porous structure. Surf. Coat. Technol. 2012, 213, 126–132.
- Guan, K. Relationship between photocatalytic activity, hydrophilicity and self-cleaning effect of TiO2/SiO2 films. Surf. Coat. Technol. 2005, 191, 155–160.
- Antonelli, D.M.; Ying, J. Synthesis of Hexagonally Packed Mesoporous TiO2 by a Modified Sol–Gel Method. Angew. Chem. Int. Ed. 1995, 34, 2014–2017.
- Braconnier, B.; Páez, C.A.; Lambert, S.; Alié, C.; Henrist, C.; Poelman, D.; Pirard, J.-P.; Cloots, R.; Heinrichs, B. Ag- and SiO2-doped porous TiO2 with enhanced thermal stability. Microporous Mesoporous Mater. 2009, 122, 247–254.
- Anderson, C.; Bard, A.J. An Improved Photocatalyst of TiO2/SiO2 Prepared by a Sol-Gel Synthesis. J. Phys. Chem. 1995, 99, 9882–9885.
- Brinker, G.W.; Jeffrey, C.S. Sol-gel science. In The Physics and Chemistry of Sol-Gel Processing; Academic Press: Cambridge, MA, USA, 2013.
- Schubert, U. Chemical modification of titanium alkoxides for sol–gel processing. J. Mater. Chem. 2005, 15, 3701–3715.
- Jan, W.G. Encyclopedic Dictionary of Polymers. Encycl. Dict. Polym. 2011.
- Mahmoud, H.A.; Narasimharao, K.; Ali, T.T.; Khalil, K.M.S. Acidic Peptizing Agent Effect on Anatase-Rutile Ratio and Photocatalytic Performance of TiO2 Nanoparticles. Nanoscale Res. Lett. 2018, 13, 48.
- Yamanaka, S.; Nishihara, T.; Hattori, M.; Suzuki, Y. Preparation and properties of titania pillared clay. Mater. Chem. Phys. 1987, 17, 87–101.
- Anderson, M.A.; Gieselmann, M.J.; Xu, Q. Titania and alumina ceramic membranes. J. Membr. Sci. 1988, 39, 243–258.
- Doeuff, S.; Henry, M.; Sanchez, C.; Livage, J. Hydrolysis of titanium alkoxides: Modification of the molecular precursor by acetic acid. J. Non Cryst. Solids 1987, 89, 206–216.
- Mahshid, S.; Askari, M.; Ghamsari, M.S. Synthesis of TiO2 nanoparticles by hydrolysis and peptization of titanium isopropoxide solution. J. Mater. Process. Technol. 2007, 189, 296–300.
- Bischoff, B.L.; Anderson, M.A. Peptization Process in the Sol-Gel Preparation of Porous Anatase (TiO2). Chem. Mater. 1995, 7, 1772–1778.
- Cassaignon, S.; Koelsch, M.; Jolivet, J.-P. From TiCl3 to TiO2 nanoparticles (anatase, brookite and rutile): Thermohydrolysis and oxidation in aqueous medium. J. Phys. Chem. Solids 2007, 68, 695–700.
- Mahy, J.G.; Deschamps, F.; Collard, V.; Jérôme, C.; Bartlett, J.; Lambert, S.D.; Heinrichs, B. Acid acting as redispersing agent to form stable colloids from photoactive crystalline aqueous sol–gel TiO2 powder. J. Sol Gel Sci. Technol. 2018, 87, 568–583.
- Mahshid, S.; Askari, M.; Ghamsari, M.S.; Afshar, N.; Lahuti, S. Mixed-phase TiO2 nanoparticles preparation using sol–gel method. J. Alloys Compd. 2009, 478, 586–589.
- Mahy, J.G.; Lambert, S.D.; Léonard, G.L.-M.; Zubiaur, A.; Olu, P.-Y.; Mahmoud, A.; Boschini, F.; Heinrichs, B. Towards a large scale aqueous sol-gel synthesis of doped TiO2: Study of various metallic dopings for the photocatalytic degradation of p-nitrophenol. J. Photochem. Photobiol. A Chem. 2016, 329, 189–202.
- Mahy, J.G.; Cerfontaine, V.; Poelman, D.; Devred, F.; Gaigneaux, E.M.; Heinrichs, B.; Lambert, S.D. Highly Efficient Low-Temperature N-Doped TiO2 Catalysts for Visible Light Photocatalytic Applications. Materials 2018, 11, 584.
- Malengreaux, C.M.; Pirard, S.L.; Léonard, G.; Mahy, J.G.; Herlitschke, M.; Klobes, B.; Hermann, R.; Heinrichs, B.; Bartlett, J.R. Study of the photocatalytic activity of Fe3+, Cr3+, La3+ and Eu3+ single-doped and co-doped TiO2 catalysts produced by aqueous sol-gel processing. J. Alloys Compd. 2017, 691, 726–738.
- Ghamsari, M.S.; Radiman, S.; Hamid, M.A.A.; Mahshid, S.; Rahmani, S. Room temperature synthesis of highly crystalline TiO2 nanoparticles. Mater. Lett. 2013, 92, 287–290.
- Vorkapic, D.; Matsoukas, T. Effect of Temperature and Alcohols in the Preparation of Titania Nanoparticles from Alkoxides. J. Am. Ceram. Soc. 2005, 81, 2815–2820.
- Wang, J.; Han, X.; Liu, C.; Zhang, W.; Cai, R.; Liu, Z. Adjusting the Crystal Phase and Morphology of Titania via a Soft Chemical Process. Cryst. Growth Des. 2010, 10, 2185–2191.
- Xu, Q.; Anderson, M.A. Synthesis of porosity controlled ceramic membranes. J. Mater. Res. 1991, 6, 1073–1081.
- Periyat, P.; Saeed, P.; Ullattil, S. Anatase titania nanorods by pseudo-inorganic templating. Mater. Sci. Semicond. Process. 2015, 31, 658–665.
- Cesconeto, F.R.; Borlaf, M.; Nieto, M.I.; de Oliveira, A.P.N.; Moreno, R. Synthesis of CaTiO3 and CaTiO3/TiO2 nanoparticulate compounds through Ca2+/TiO2 colloidal sols: Structural and photocatalytic characterization. Ceram. Int. 2018, 44, 301–309.
- Sugimoto, T.; Zhou, X.; Muramatsu, A. Synthesis of uniform anatase TiO2 nanoparticles by gel–sol method 4. Shape control. J. Colloid Interface Sci. 2003, 259, 53–61.
- Sugimoto, T.; Zhou, X.; Muramatsu, A. Synthesis of uniform anatase TiO2 nanoparticles by gel–sol method 3. Formation process and size control. J. Colloid Interface Sci. 2003, 259, 43–52.
- Hore, S.; Palomares, E.; Smit, H.; Bakker, N.J.; Comte, P.; Liska, P.; Thampi, K.R.; Kroon, J.M.; Hinsch, A.; Durrant, J.R. Acid versus base peptization of mesoporous nanocrystalline TiO2 films: Functional studies in dye sensitized solar cells. J. Mater. Chem. 2004, 15, 412–418.
- Li, H.; Afanasiev, P. On the selective growth of titania polymorphs in acidic aqueous medium. Mater. Res. Bull. 2011, 46, 2506–2514.




