
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Angelo Cignarelli | + 5678 word(s) | 5678 | 2021-06-09 06:15:05 | | | |
| 2 | Nora Tang | Meta information modification | 5678 | 2021-06-28 03:57:49 | | |
Video Upload Options
Erectile dysfunction (ED) is a common underestimated complication of diabetes mellitus that affects more than 50% of people with diabetes. Diabetes dramatically raises the risk of developing ED by 2.5-fold. Despite that several studies have explained the pathogenetic mechanisms involved in the generation of erectile failure, few studies to date have described the efficacy of glucose-lowering medications in the restoration of normal sexual activity. Herein, we will present current knowledge about the main starters of the pathophysiology of diabetic ED and explore the role of different anti-diabetes therapies in the potential remission of ED, highlighting specific pathways whose activation or inhibition could be fundamental for sexual care in a diabetes setting.
1. Introduction
Erectile dysfunction (ED) is a common underestimated complication of diabetes mellitus that affects more than 50% of people with diabetes [1][2]. Diabetes dramatically raises the risk of developing ED by 2.5-fold. Additionally, ED represents an early sentinel of a cardiovascular event preceding a coronary event by at least three years [3], and thus warrants remarkable consideration by clinicians so as to prevent one of the main causes of death among subjects with type 2 diabetes (T2D) [4].
Several mechanisms related to the onset of diabetes may explain the high prevalence of ED among subjects with T2D. Hyperglycaemia and insulin resistance promote several biochemical derangements in the vascular and neurological systems, leading to an improper induction of erection. Moreover, obesity and visceral fat accumulation, typically observed in subjects with T2D, collectively represent one of the main risk factors for secondary hypogonadism [5]. Indeed, almost 40% of subjects with T2D are obese [6], and roughly 50% of these individuals have a haemoglobin A1c (HbA1c) concentration higher than 7% [7]. Therefore, the association of visceral obesity with the hyperglycaemic/dyslipidaemic milieu and the combination of low circulating testosterone levels and the development endothelial dysfunction, macrovascular and microvascular disease, and diabetic neuropathy can significantly alter the fine mechanisms involved in regular erectile function.
Several hypoglycaemic agents are commonly prescribed to subjects with T2D to reduce HbA1c levels and, thus, the incidence and progression of diabetic complications. Therefore, a significant impact of these drugs on erectile function is to be expected. However, substantial differences in the mechanism of action, glucose-lowering efficacy and effects on body weight and other cardiovascular risk factors of each drug may explain a potential variable impact on erectile function.
This review sought to report on the selective effects demonstrated by glucose-lowering medications on erectile function and explore the potential mechanisms involved.
2. Pathophysiology of ED
After the release of NO, vascular smooth muscle responds with a cyclic guanosine monophosphate (cGMP)-mediated dilatation of the corpora cavernosa, facilitating the supply of blood. However, a lack of NO due to endothelial dysfunction triggers insufficient relaxation of the vascular smooth muscle of the corpora cavernosa, resulting in ED [8]. In this setting, several biological contributors are known to interfere with sexual performance, particularly in the context of metabolic illness (e.g., diabetes, obesity). We henceforth discuss current advances concerning the effects of endogenous mediators and related molecular mechanisms that affect erectile functionality and suggest potential therapeutic strategies that may restore metabolic control along with improved sexual function.
2.1 Hyperglycemia
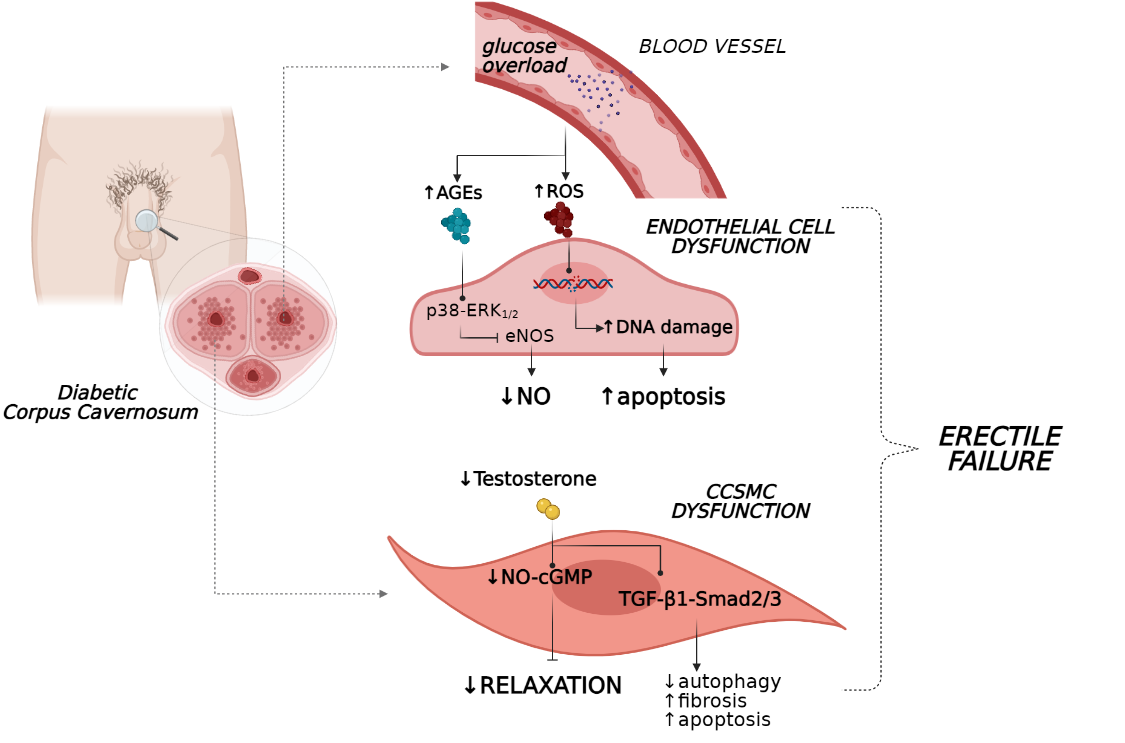
A higher glucose level is a hallmark of T2D and contributes to various metabolic derangements that promote endothelial dysfunction and vascular complications. Long-term hyperglycaemia reportedly leads to the increased generation of advanced glycation end-products (AGEs) and reactive oxygen species (ROS) [9][10][11][12], which may accelerate endothelial dysfunction through the impairment of endothelial NO synthase (eNOS) activity and NO production. As a result, an imbalance between vasoactive mediators and vasoconstrictors occurs, modifying the vascular permeability and resulting in a disruption of endothelial integrity. In this context, endothelial walls lose their sensitivity to mediators of vasodilation and increase their responses to vasoconstrictor stimuli, leading to in impaired vascular and smooth muscle relaxation, generating clinical ED [13].
The formation of AGE products represents a real insult that hampers endothelium functionality due to the abrogation of signalling pathways regulating NO release [14][15]. Indeed, when the production of AGEs was abrogated in vivo with tight glycaemic control, mice with type 1 diabetes (T1D) showed restored erectile parameters in association with an amelioration of histological features of the corpora cavernosum [16]. Additionally, in a recent study on the human coronary artery, endothelial cells stimulation with AGEs induced reductions in mRNA and protein levels of NOS, phospho-eNOS (Ser1177), and These in vitro results confirmed previous findings where AGE levels were found to positively correlate with endothelial dysfunction in diabetes [17], typically in penile tissue [18].
In this scenario, both hyperglycaemia and AGEs are known to exert a detrimental effect on endothelial function by impairing the angiopoitin-Tie-2 system. A previous in vitro study revealed that high glucose concentrations suppress the ability of Ang-1 to activate the Tie-2 receptor with the abrogation of PI3K/Akt signalling, thus reducing vascular protection [19]. Furthermore, hyperglycaemia together with AGEs impaired the intracellular signalling cascade induced by Ang-1 in a FoxO1-dependent manner, with a subsequent increase in Ang-2 production, thus enhancing the responsiveness of endothelial cells to inflammatory or angiogenic cytokines [20]. Considering the critical role of the Ang-1/Ang-2 system in the preservation of endothelial function, it is reasonable that a glucose-induced failure of this axis could also affect the vascular bed of penile tissue thus favouring ED development.
The poor endothelium-dependent vasorelaxation in ED is also fostered by ROS. These factors show a deleterious effect on cavernosal smooth muscle reactivity, interfering with eNOS bioavailability. Indeed, several studies have demonstrated that the restoration of the anti-oxidant system via either pharmacological compounds or genetic methods re-established erectile function through an increase in NO production [21][22] and appeared to potentiate the efficacy of current pharmacological medications for sexual dysfunction [23].
Together, these processes caused direct injury to the endothelial system of the penis vasculature due to the accumulation of peroxide lipids species, DNA and protein modifications (i.e., nitration of tyrosine) [13], leading to aggravation of endothelial dysfunction and interference with erectile vasorelaxation (Figure 1). Additionally, when penile tissue malfunction persists, increased apoptosis of endothelial cells occurs together with a reduction in their regeneration, weakening the homeostasis of the vascular bed [24]. In this scenario, the persistence of hyperglycaemia also appears to have a critical role in erectile performance. This phenomenon appears to be associated with a ‘metabolic memory’ as was recently observed in men with T2D and ED, in which early exposure to sustained hyperglycaemia has long-term disadvantageous effects on erectile function, which persist even after patients achieved better glycaemic control [25].
Together, these results suggest that not only glucose overload but also the duration of exposure to a hyperglycaemic state under diabetic conditions could favour ED development to different extents.
2.2. Hypogonadism
Hypogonadotropic hypogonadism is common in men with T2D, with a prevalence of up to 40%. The major molecular player in the impaired hypothalamic–pituitary–testicular (HPT) axis is an increase in body fat mass. Longitudinal results from the European Male Ageing Study reported that obesity has a detrimental effect on testosterone release and indicated that modifications to the HPT axis were reversible following weight reduction [26].
In a multicentre population-based study, significant relationships between total testosterone level and worse sexual functioning and between free testosterone level and ED in ageing men were found [27]. Indeed, testosterone regulates sexual function and biological processes underlying erection via both central and peripheral mechanisms [28]. Therefore, the deprivation of circulating levels of this hormone compromises sexual drive and function, leading to ED development.
Compelling in vivo evidence suggests that androgens regulate corporeal haemodynamics and participate in the maintenance of penile tissue integrity. As a matter of fact, androgens tightly control endothelial cell proliferation and survival [29] and the penis vasculature via both direct and indirect mechanisms in humans [30][31]. Particularly, testosterone evokes vasorelaxation by stimulating NOS production and endothelium-dependent hyperpolarisation factors (EDHFs) [32]. Therefore, this hormone is essential for “erectogenesis” since its deprivation leads to enhanced endothelial cell apoptosis and decreased production of eNOS [33] and NO levels, as was recently observed in both human and rat studies (Figure 1) [34][35].
The vasoprotective effectiveness of androgens was also confirmed after testosterone replacement therapy (TRT), where endothelial function was completely restored and the progression of ED was significantly counteracted both for a short time [36] and in the long-term in men with hypogonadism [37]. Accordingly, men affected by hypogonadism with veno-occlusive dysfunction demonstrated improved penile haemodynamics after TRT with decreased end-diastolic velocity of the cavernous artery, thus preventing impotency [38]. Notably, the molecular processes driving ED amelioration after testosterone replacement were further investigated in vivo. Indeed, T1D rats with an experience of ED reverted hypogonadism after exposure to testosterone supplementation in association with restored expression of neuronal NOS (nNOS) and phosphodiesterase type 5 (PDE5) and ameliorated penile sensitivity to vasorelaxation stimuli [39].
The activity of these compounds, however, was completely lost in the presence of severe androgen deprivation. Indeed, under low testosterone levels, a typical condition of hypogonadal patients, reduced expression of PDE5 within penile tissue was observed, which, in turn, resulted in a failure of PDE5i-based therapy. When testosterone levels were normalised with TRT, PDE5 expression was restored, thus ameliorating PDE5i efficacy with final recovery of penile functionality and erectile performance [40][41]. Indeed, in a recent review, conflicting results emerged on the presence of a putative androgen response element (ARE) within the human and rat PDE5A gene sequence; furthermore, other studies showed that reduced PDE5 expression observed in castrated animals is due to a reduction of smooth muscle content rather than by direct effects of reduced androgen levels [42].
Despite the aforementioned results that described direct modulation of vasodilator genes by testosterone, emerging ex vivo data suggest that androgens regulate the physiological function of SMCs in human corpora cavernosum regardless of NO-cGMP dilator pathways. In particular, supraphysiological levels of testosterone evoked relaxation responses of SMCs in explants of human corpora cavernosum from patients with ED probably via ‘nongenomic mechanisms’ [43][44]. In particular, the treatment of precontracted cavernosal tissue with testosterone led to cell relaxation within a few minutes, even though both NO synthesis and androgen receptors were inhibited, suggesting the involvement of noncanonical mechanisms [43][44].
Noteworthy, inadequate testosterone bioavailability, beyond the detrimental effects of endothelial integrity, impaired normal erectile organ structure by favouring the loss of corpus cavernosum SMCs (CCSMCs) and increasing collagen deposition in the penis [45]. In this setting, the TGF-β1–Smad2/3 signalling pathway appears to mediate fibrotic processes of penile tissue in association with reduced autophagy and increased apoptosis of CCSMCs, as shown in a castrated rat model (Figure 1) [46]. These events were reverted by testosterone administration, which preserved regular erectile function and facilitated histology stabilisation of the penile organ [46]. In concert, these findings suggest that testosterone deprivation under eugonadal conditions contribute to the impairment of penile tissue and function.
3. Glucose-Lowering Medications
The efficacy of current glucose-lowering medications on erectile functionality has not been extensively investigated. In particular, improvements in ED after anti-diabetes drug therapy may be ascribed to indirect mechanisms such as the reduction of hyperglycaemia, excess body weight, high blood pressure and the amelioration of other detrimental factors. Notwithstanding, a direct effect of glucose-lowering agents on both endothelial and smooth muscle cells is reasonable.
The pharmacotherapy of diabetes was recently enriched due to the availability of novel agents [i.e., glucagon-like peptide-1 receptor agonists (GLP-1RAs), sodium–glucose cotransporter-2 inhibitors (SGLT2is), dipeptidyl peptidase-4 inhibitors (DPP4is)] able to correct hyperglycaemia with less hypoglycaemic events. Several studies have claimed that the protective effects on the vascular wall of these drugs are exerted via indirect mechanisms such as the reduction of glycaemia, blood pressure and body weight. Whether these anti-hyperglycaemic agents preserve penile endothelial cell viability and function, thus preventing diabetic-related erectile failure, however, is not known. Herein, we discuss the current knowledge on the effects of anti-diabetes agents on the restoration of sexual performance.
3.1. Insulin
Insulin regulates several aspects of energy metabolism, including the absorption of nutrients in metabolically active organs (i.e., liver, skeletal muscle, adipose tissue) and protein synthesis, as well as reduction of HbA1c (−1.16 ± 0.84%) and body weight gain (+0.27 ± 3.38 kg) [47]. In particular, subcutaneous administration of insulin in T1D rats resolved the puzzle of higher intracavernosal pressure together with the restoration of sex hormone receptor expression levels [48]. By contrast, in another study, T1D rats exposed to multiple insulin injections showed an aggravation of erectile parameters as compared with control rats, principally due to a nitrergic degeneration (↓ NOS) and a decrease in testosterone levels, which were not reversed by insulin [49]. Moreover, a parallel arm, open-label, randomized, controlled study conducted in T2D men with ED demonstrated that the use of continuous subcutaneous insulin infusion, as compared to multiple daily injection therapy, for six months led to increased erectile function, with a reduction in ED severity or ED resolution associated with better glycaemic control [49].
Nevertheless, the complete recovery of erectile function by insulin treatment appears to be obtained by combining insulin administration with other therapeutic approaches. For instance, Zhou et al. demonstrated that ED improved after insulin treatment only exclusively via a cell-regeneration process driven by adipose stem cells (ASCs) [50]. In particular, ADSC injection alone per se led to a partial retrieval of penis activity (i.e., ↓ cell apoptosis, restored cavernous endothelium, etc.), but the favourable changes in penile tissue with functional recovery of erection were achieved only after insulin administration, which led to a reduction in AGE products within the penis compartment [50].
Overall, these results provide remarkable evidence that improvements in glycaemic control with insulin therapy could assist in the recovery from diabetes-associated ED. Recent findings, however, suggest that single monotherapy does not sustain the full restoration of penile activity to a near-normal status. A synergistic action of insulin and inoculations of ASCs could be a hopeful approach to warrant a complete remission of ED.
3.2. Sulfonylureas
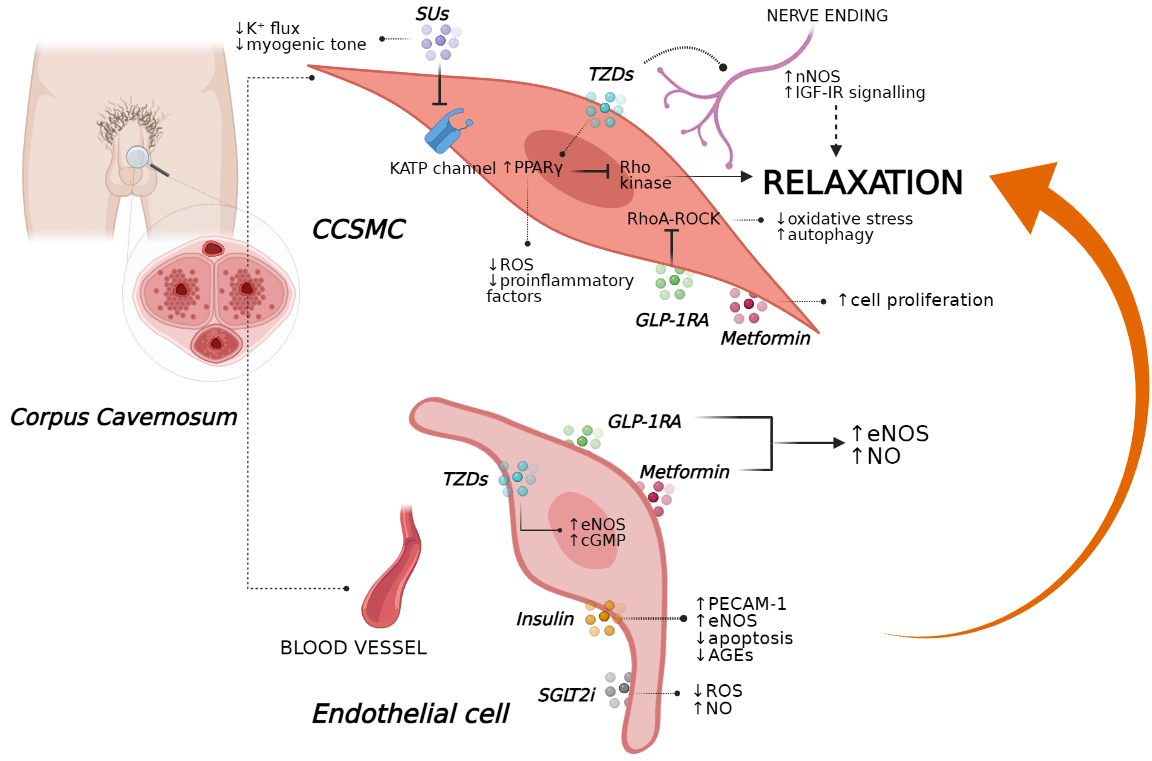
For several years, sulfonylureas (SUs) have been the most commonly prescribed medications for the treatment of T2D, being able to ameliorate glycaemic control reducing HbA1c levels (−1.0 ± 1.1%), despite induction of weight gain (+ 1.2 ± 2.3 kg) [51][52]. SUs are known to act as insulin secretagogues through the activation of SU receptor 1 on the cell surface of pancreatic β-cells. Following SU receptor 1 activation by these compounds, the inhibition of adenosine triphosphate (ATP)-sensitive K+ channels (KATPs) occurs, determining cell membrane depolarisation and resulting in exocytosis of insulin-containing granules [53]. Notably, the expression of KATPs was also found in different extra-pancreatic tissues, including the corporal smooth muscles, which physiologically regulate muscle tone and relaxation of penile resistance arteries to promote erection [54].
Insuk et al. demonstrated the presence of a subtype of KATPs within human corporal smooth muscle, i.e., Kir6.1–Kir6.2, whose location was found near to the SUR2B subunit [55]. In the same study, K+ flux within the human corpora cavernosum after stimulation with selective KATP activators was completely counteracted in the presence of non-selective SUs (i.e., glibenclamide and glimepiride), resulting in an inhibition of muscle vasodilatation [55][56][57].
Similarly, Rubio et al. observed that SUs (e.g., glibenclamide) block the myogenic tone and prevent muscle relaxation evoked by KATP openers; however, this effect was lost when SUs were administered together with PDE5i (i.e., sildenafil), indicating that KATP channels could not be involved in the relaxations induced by cGMP-elevating agents [58][59]. By contrast, in a cross-sectional controlled clinical study, glibenclamide-based therapy significantly triggered an increase in total and free testosterone levels, SHBG levels, sex drive, and erectile function in individuals with T2D [60]. In this setting, SUs appeared to sustain sex drive by direct stimulation of testosterone synthesis in men with T2D and hypogonadism [61][62].
Noteworthy, several retrospective studies have revealed that men with severe hypoglycaemia use illegal sexual enhancement products together with undeclared medication errors based on SU therapy (i.e., glibenclamide, glyburide), thus highlighting a possible positive effect of these insulinotropic drugs on sexual function [63][64]. Whether the potential therapeutic effects of SUs against ED development could be explained by the prevention of endothelial and vascular functionality is still poorly understood. In a recent randomised control trial, glibenclamide did not alter endothelial function when administered to men with T2D without cardiovascular complications as well as hypertensive diabetics, in whom its administration was found to worsen blood pressure [65][66]. Nevertheless, a novel SUs compound (I4) displayed greater alleviating effects on cardiovascular complications than glimepiride in T2D rats and significantly improved in vitro NO synthesis and NOS activity with final amelioration of endothelial dysfunction markers (Figure 2) [67].
In concert, these data highlight that further studies are needed to elucidate the controversial effects of SUs on endothelial cell functions and, consequently, on ED. Differences in SU selectivity could be the rationale behind the different outcomes observed.
3.3. Metformin
In addition to its well-known glucose-lowering effect (HbA1c reduction of −1.26 ± 0.02%), metformin favours some body weight loss (−2.5 kg) with a reduction in visceral fat area in association with multiple endocrine variations [2][68][69][70]. Interestingly, several in vitro studies have also ascribed anti-inflammatory and anti-atherogenic properties to this compound in terms of the prevention of oxidative stress and the amelioration of vascular and endothelial functions under stressor stimuli as ROS and inflammatory cytokines [71][72]. Particularly, novel insights have revealed that metformin restores the expression of two essential mediators of vasodilatory responses (nNOS and eNOS) within penile tissue of obese rats, thus suggesting potential implications in the treatment of ED [73]. Additionally, under metformin-based therapy, obese mice experienced a restored corpus cavernosum erectile response, together with attenuated norepinephrine-dependent sympathetic activities and decreased intracavernosal pressure [74].
As previously discussed, hyperglycaemia is a strong pathogenetic factor of penile dysfunction in individuals with T2D [1][75][76]. The extreme consequence of long-term exposure to high glucose levels is the accumulation of products of nonenzymatic glycation and angiotensin II, which accelerate the endothelial injury through oxidative stress and autophagy. In this scenario, metformin therapy appears to have a favourable effect against angiotensin II–induced ED in terms of the restoration of normal intracavernosal muscle tone and increased muscle relaxation according to an increase in eNOS activity as observed in a hypertensive rat model [77].
In a rat model, metformin therapy combined with icariside II, a PDE5i, restored the proliferation of CCSMCs and ameliorated NOS activity, leading to improvements in penile erectile function (Figure 2) In particular, metformin is known to favour AMP breakdown by inhibiting AMPD [78][79], a cytosolic enzyme regulating the signalling of adenosine, a key neuronal mediator of penile vasculature function and a potent endogenous vasodilator [80]. Additionally, metformin reverted metabolic syndrome in mice being fed a high-fat diet in association with an amelioration of erectile function, probably through the regulation of adenosine signalling [81]. Notwithstanding, a prospective, randomised, double-blind, placebo-controlled pilot study observed that non-diabetic men with ED, insulin resistance and a prior history of poor response to sildenafil had improved erectile function after metformin therapy in combination with sildenafil [82].
Metformin intervention also appears to prevent testicular spermatogenic dysfunction observed under hypercaloric conditions. exhibited an amelioration of semen quality and restored endogenous hormone levels (i.e., testosterone) after metformin treatment, supporting the hypothesis that this compound could also preserve reproductive functions in the setting of ED [83]. Finally, data from patients with metabolic syndrome have demonstrated an association between metformin administration and increased androgen levels associated with an improvement in semen characteristics [84]. Nevertheless, further investigations are necessary to identify the molecular mechanisms by which metformin could act on the reproductive system, thus counteracting ED development.
3.4. Acarbose (ACA)/α-glucosidase inhibitors
ACA acts as a reversible inhibitor of pancreatic α-amylase and hydrolase, which are involved in carbohydrate catabolism, thus delaying glucose absorption in the gut and thus determining a weight-loss (−3.3 ± 3.7 kg) [85][86]. The growing number of side effects involving the gastrointestinal tract (e.g., diarrhoea, abdominal pain), however, has made it difficult to use these molecules in the clinical practice of T2D. The anti-hyperglycaemic action of ACA is slightly lower to that of metformin and SUs in terms of the reduction in HbA1c levels (−1.86 ± 1.10%) [86][87]. In addition, this drug could also reduce the incidence of newly diagnosed diabetes among individuals with impaired glucose tolerance [88]. In an international, multicentre double-blind, placebo-controlled randomised trial, ACA-based therapy demonstrated a cardioprotective role [89], possibly caused by the prevention of endothelial cell dysfunction [90] in both individuals with impaired glucose tolerance and new-onset T2D.
Conflicting data on the efficacy of ACA against diabetic-related ED exist, even though this drug has shown anti-oxidant and anti-inflammatory properties [91][92][93]. An in vivo study revealed that ACA attenuates vascular barrier dysfunction and improves the hyperglycaemia-induced inflammasome, thus highlighting its potential role in the remission of vascular pathology under diabetic conditions [94]. Recent in vivo findings in T1D rats have revealed that the benefits of ACA on ED were potentiated were animals fed with moringa seed and a leaf-fortified diet as reported by expression analysis of some ED-related biomarkers in the penile tissue (i.e., acetylcholinesterase (AchE), monoamine oxidases (MAO), adenosine deaminase (ADA) , angiotensin I converting enzyme (ACE), thiobarbituric acid reactive substances (TBARS))
Taken together, these findings support the concept that a combination therapy based on lifestyle modifications plus ACA treatment could help to restore a normal sexual function, probably by reducing inflammatory responses and enhancing antioxidant pathways within the penile tissue. However, investigations in this regard are still too scarce to suggest use of α-glucosidase inhibitors in ED.
3.5. Thiazolidinediones
In the last decade, the pharmacological management of T2D has been improved by the discovery of new insulin sensitiser molecules such as thiazolidinediones (TZDs), also called ‘glitazones’, which able to ameliorate insulin sensitivity (HbA1c reduction of −2.0 ± 0.4%) [95] and pancreatic β-cells function by stimulating the activity of PPARγ, a critical target of the regulation of energy homeostasis. Among these compounds, rosiglitazone and pioglitazone are currently approved by the United States Food and Drug Administration as monotherapy or for use in combination with metformin or SUs, even though they are not appropriate for the correction of body weight (−0.03 ± 1.52 kg) [96]. Indeed, the expression of PPARγ was also identified in vascular SMCs, where its activation by agonists reduced the release of ROS and pro-inflammatory molecules and prevented the occurrence of vascular damage typically observed in a hypertensive state [97]. In this scenario, pioglitazone modulated the vasculature contractility in vivo by interfering with endothelin-1–induced vasoactive effects, thus warranting the protection of vessel structure and function [98].
Notwithstanding, in vivo results reported that pioglitazone could enhance the response to oxidative stress as observed in the setting of corporal veno-occlusive dysfunction. In particular, low-dose pioglitazone attenuated fibrotic processes and oxidative stress within the corpora cavernosum of rats with T2D regardless of glycaemic control [99]. Then, the same authors highlighted that the amelioration of corporal compliance by pioglitazone in an ageing-related corporal veno-occlusive dysfunction model was due to the increase in penile relaxation through the inactivation of the Rho-kinase system, an important inhibitor of SMC relaxation and penile erection [100]. However, this molecule was unable to affect the ratio of collagen to SMCs within penile tissue; therefore, the remission of ED occurred by counteracting anti-erectile agents, but not fibrotic factors [100].
Additionally, animals with similar cavernosal nerve trauma experienced dose-dependent improvements in intracavernosal pressure after pioglitazone-based therapy together with increased expression levels of eNOS, nNOS and cGMP, culminating in the recovery of normal erectile performance (Figure 1) [101]. These findings were also confirmed by in vitro results, showing that pioglitazone resulted in the inhibition of Ca+2 influx into penile SMCs by blocking L-type voltage-dependent calcium channels [102] and upregulating eNOS within vascular endothelial cells, thus triggering vasorelaxation [103].
Pioglitazone appears to preserve erectile activity when the corporal cavernosum undergoes neuronal failure. This was demonstrated in rats with bilateral cavernosal nerve crush injury [104]. Currently, the mechanism underlying these effects are still poorly investigated, even though some studies have established that the prevention of erectile failure by pioglitazone administration could occur via both anti-inflammatory and insulin-like growth factor Indeed, when IGF-I receptors (IGF-IRs) were inhibited by a selective antagonist in vivo, pioglitazone failed to show neuroprotective activity for peripheral nerve regeneration within penile tissues in a rat model with bilateral cavernous nerve injury
Although the androgenic actions of pioglitazone were poorly analysed, a double-blinded study contended that treatment with pioglitazone in men with moderate-to-severe ED and lower sildenafil responsiveness resulted in the improvement of erectile function scores and increased sildenafil response, without changes in testosterone, glucose levels and other clinical parameters [105].
Conversely, in vivo studies reported that sildenafil abolished the vasorelaxant effects of both pioglitazone and rosiglitazone, interfering with potential beneficial effects of these antidiabetic drugs on ED and probably exerting an aberrant activity on certain ATP-dependent ion transporters through the enhancement of glucose-/glycolysis-dependent contraction-facilitating processes [106].
In concert, these results provide evidence that TZDs, particularly pioglitazone, could act as a regenerative agent to facilitate functional recovery of erectile activity and as a anti-vasoconstrictor molecule in the presence of severe cavernosal impairment. Nevertheless, further studies should elucidate the interactions of TZDs with drugs commonly used in sexual dysfunction that could compromise treatment outcomes and compliance.
3.6. GLP-1RAs and DPP-4i
Indeed, endothelial cells, including human coronary artery endothelial cells and human umbilical vein endothelial cells, express the GLP-1 receptor (GLP-1R) [107][108]. In addition, several in vivo and in vitro studies have suggested that GLP-1 and its analogues directly control endothelial function [109][110] by stimulating the AMPK–eNOS axis and NO production [111][112]. Recently, Cai et al. found that GLP-1 is able to prevent oxidative stress-induced dysfunction and autophagy in human endothelial cells, and that the protective effects of GLP-1 might be dependent upon downstream restoration of the epigenetic factor histone deacetylase 6, a downstream molecular effector of EKR1/2 induced by oxidant injury, suggesting the potential therapeutic application of GLP-1 in the prevention and treatment of endothelial damage induced by oxidative stress in subjects with T2D [113]. This liraglutide-mediated improvement in endothelial function might be due to changes in the expression levels of genes related to inflammatory pathways,
Importantly, endothelial dysfunction represents a key event not only in the development of atherosclerosis, but also of ED since impaired NO production by the endothelium and/or increased inactivation of NO by ROS is fundamental in the induction of an erection [114]. In this regard, Yuan et al. recently demonstrated that liraglutide could improve erection function in diabetes-induced ED by regulating smooth muscle dysfunction, oxidative stress and autophagy, independently of a glucose-lowering effect, in a rat model of T1D and in CCSMCs [115]. In addition, GLP-1R inhibition abolished the beneficial functions of liraglutide treatment [115].
Since the GLP-1R is also expressed in the testes, the direct impact of GLP-1RAs on the reproductive system could also be derived from activation of the testicular GLP-1R. Indeed, when GLP-1R was genetically ablated in vivo, mice exhibited impaired glucose tolerance in association with significant decreases in adrenal, testis and seminal vesicle weights [116]. The impact of GLP-1 agonism on testes was also assessed in a high-fat diet-induced obesity mouse model characterised by reduced serum levels of testosterone, impairment of sperm quality and increased inflammation of the testes. Meanwhile, exenatide treatment reduced body weight, as well as the expression of pro-inflammatory cytokines, and improved the quality of sperm to the level of controls [117].
Considering human studies, a recent retrospective study has evidenced that liraglutide, in addition to lifestyle changes and metformin and testosterone therapy, allowed not only reaching glycemic target and lowering body weight, but also obtaining a considerable improvement of ED in diabetic obese men with overt hypogonadism [118]. Similarly, short-term combined treatment using exenatide and metformin was found to be superior to glimepiride and metformin in the correction of sexual dysfunction in patients with obesity and T2D [119]. In a case report, the use of liraglutide was found to cause interrupted sperm production in a 35-years-old man experiencing primary and idiopathic infertility, which was completely restored after five months of liraglutide withdrawal [120]. Therefore, further experimental studies are needed to clarify the effects of incretin mimetics on the male reproductive system.
It should be noted that the exact molecular processes by which GLP-1 may restore erectile performance are however still unknown. New insights have highlighted the importance of the Ras homolog gene family (RhoA)–Rho-associated protein kinase (ROCK) pathway in maintaining a flaccid penile state; notably, the inhibition of RhoA–ROCK signalling is able to improve ED, even when associated with hard-to-treat causes, such as diabetes, suggesting that RhoA–ROCK could represent a new therapeutic target [121]. Indeed, as previously discussed, liraglutide exerted protective effects on ED associated with the regulation of smooth muscle dysfunction, ROS production, and autophagy by regulating the RhoA–ROCK pathway Another potential molecular mechanism by which GLP-1RAs might improve ED involves the combined downregulation of NADPH oxidase and increased NO production via eNOS and nNOS: in an animal model of methylglyoxal-induced corpus cavernosum dysfunction, chronic treatment with exendin-4 exerted a protective effect on this pathway independently of its glucose-lowering effect [122].
Besides the insulinotropic ability of DPP4is, these drugs could interfere with the degradation of key players that regulate the trafficking of circulating endothelial progenitor cells released from bone marrow [123], including SDF-1α [124], substance P [125][126], and pituitary adenylate cyclase–activating polypeptide (PACAP), a peptide isolated in the hypothalamus that enhances gonadotropin release and improves sex steroid levels. PACAP also has vasorelaxant effects, and, similarly to substance P, improves vascular endothelial growth factor levels [127][128]. Interestingly, vildagliptin, a DPP4i, was recently shown to inhibit the development of endothelial dysfunction and to prevent atherogenesis in non-diabetic apolipoprotein E–deficient mice [129]. In addition, Ma et al. reported that saxagliptin, another DPP4i, suppressed oxidised low-density lipoprotein cholesterol (ox-LDL)-induced endothelial dysfunction in human vascular endothelial cells, by inactivating JNK, AP-1 and NF-κB
However, similarly to GLP-1RA, a case report described a deterioration of semen quality following three months of sitagliptin therapy in a subject with T2D, with semen quality recovering upon discontinuation of the drug [130]. However, similarly to GLP-1RA, a case report described a deterioration of semen quality following three months of sitagliptin therapy in an individual with T2D, with semen quality recovering upon discontinuation of the drug [130].
Taken together, these data highlight that incretin-based therapies have the potential to be included in the pharmacotherapy of ED regardless of their glucose regulatory effects. Despite the paucity of mechanistic studies in humans, incretin mimetics could evoke antioxidant responses via the RhoA–ROCK pathway, thus improving the homeostasis of CCSMCs and erectile activity. On the other hand, DPP4is could enhance the signalling pathways regulating endothelial function and vascular repair via SDF-1α, substance P, and PACAP, thus participating in the improvement of erectile performance.
3.7. SGLT2is
SGLT2is, also called gliflozins, represent another class of anti-hyperglycaemic agents currently used in T2D treatment; these medications are able to reduce hyperglycaemia (Hb1Ac reduction of −0.8 ± 1.4%) through the inhibition of glucose reabsorption in the proximal convoluted tubule of the kidney and the increase of glycosuria [131][132][133].
Large randomised trials of SGLT2is have shown reductions in cardiovascular events (particularly rates of hospitalisation for heart failure) in patients with T2D and in those with heart failure with reduced ejection fraction with or without diabetes [134]. Positive effects on body weight (−2.8 ± 4.9 kg) and the cardiovascular system manifest rapidly and, for this reason, are unlikely to be related only to the improvement in glycaemic control [133]. Indeed, SGLT-2i have also shown positive effects on multiple cardiovascular disease risk factors, including high blood pressure and obesity, as well as on serum uric acid and albuminuria levels [135][136].
To date, numerous in vitro and in vivo studies have investigated the molecular mechanisms through which SGLT2is exert positive cardiovascular effects. In this regard, it has been recently demonstrated that empagliflozin, a selective SGLT2i, not only reduced glucose levels, but also attenuated endothelial dysfunction and atherogenesis and improved cardiac remodelling in diabetic apolipoprotein E–deficient mice and in an experimental model of metabolic syndrome, the obese ZSF1 rat [137][138]. In addition, mice with T2D treated with dapagliflozin, another widely prescribed SGLT2i, for eight weeks displayed significantly less arterial stiffness, improvements in endothelial and vascular smooth muscle dysfunctions and reductions in circulating markers of inflammation as compared with nontreated diabetic mice [139].
Data obtained from experimental models have been confirmed in human studies, as evidenced by a recent pilot study in which acute treatment of subjects with T2D with dapagliflozin significantly improved systemic endothelial function and reduced both renal resistive index and aortic stiffness [140]. In particular, Uthman et al. showed that empagliflozin and dapagliflozin were able to restore NO bioavailability, by reducing ROS generation, in tumour necrosis factor–α-stimulated human coronary artery endothelial cells and human umbilical vein endothelial cells, without affecting eNOS expression and signalling, barrier function, or the expression of adhesion molecules such as VCAM-1 and ICAM-1 [141]. Data obtained from experimental models have been confirmed in human studies, as evidenced by a recent pilot study in which acute treatment of individuals with T2D with dapagliflozin significantly improved systemic endothelial function and reduced both renal resistive index and aortic stiffness [140]. In particular, Uthman et al. showed that empagliflozin and dapagliflozin were able to restore NO bioavailability, by reducing ROS generation, in tumour necrosis factor–α-stimulated human coronary artery endothelial cells and human umbilical vein endothelial cells, without affecting eNOS expression and signalling, barrier function, or the expression of adhesion molecules such as VCAM-1 and ICAM-1 [141].
Since endothelial dysfunction represents a key event in the development of ED, as detailed above, SGLT2is are likely to favourably affect ED as well. The effects of chronic treatment with empagliflozin on ED have been investigated in a T2D rat model, the Goto–Kakizaki rat, in the presence or absence of acute intravenous injection of sildenafil [142]. However, further investigations are needed to understand whether the positive effects of empagliflozin on ED are due to better glycaemic control and/or to a reduction in diabetes-associated inflammatory state, and/or alternatively to a direct effect on penile endothelial cells.
4. Conclusions
To date, several glucose-lowering medications are available to treat subjects with T2D. Importantly, several experimental and clinical data have highlighted the ability of glucose-lowering drugs to improve endothelial dysfunction and preserve endothelial cell viability. In addition, since endothelial dysfunction represents a key event in the development of atherosclerosis, as well as of ED, the potential role of these agents in ED and the related molecular mechanisms are under investigation. Several lines of experimental evidence suggest potential effects of specific glucose-lowering medications in the therapy of ED. Furthermore, another important aspect relates to the need to evaluate the pharmacological interactions between glucose-lowering agents and other drugs used in the treatment of sexual dysfunctions.
References
- Corona, G.; Giorda, C.B.; Cucinotta, D.; Guida, P.; Nada, E.; Aglialoro, A.; Albanese, V.; Albano, S.; Antonangelo, C.; Baccetti, F.; et al. Sexual dysfunction at the onset of type 2 diabetes: The interplay of depression, hormonal and cardiovascular factors. J. Sex. Med. 2014, 11, 2065–2073.
- Kouidrat, Y.; Pizzol, D.; Cosco, T.; Thompson, T.; Carnaghi, M.; Bertoldo, A.; Solmi, M.; Stubbs, B.; Veronese, N. High prevalence of erectile dysfunction in diabetes: A systematic review and meta-analysis of 145 studies. Diabet. Med. 2017, 34, 1185–1192.
- Gandaglia, G.; Briganti, A.; Jackson, G.; Kloner, R.A.; Montorsi, F.; Montorsi, P.; Vlachopoulos, C. A systematic review of the association between erectile dysfunction and cardiovascular disease. Eur. Urol. 2014, 65, 968–978.
- Baena-Díez, J.M.; Peñafiel, J.; Subirana, I.; Ramos, R.; Elosua, R.; Marín-Ibañez, A.; Guembe, M.J.; Rigo, F.; Tormo-Díaz, M.J.; Moreno-Iribas, C.; et al. Risk of Cause-Specific Death in Individuals With Diabetes: A Competing Risks Analysis. Diabetes Care 2016, 39, 1987–1995.
- Corona, G.; Maseroli, E.; Rastrelli, G.; Francomano, D.; Aversa, A.; Hackett, G.I.; Ferri, S.; Sforza, A.; Maggi, M. Is late-onset hypogonadotropic hypogonadism a specific age-dependent disease, or merely an epiphenomenon caused by accumulating disease-burden? Minerva Endocrinol. 2016, 41, 196–210.
- Fried, M.; Yumuk, V.; Oppert, J.-M.; Scopinaro, N.; Torres, A.J.; Weiner, R.; Yashkov, Y.; Frühbeck, G. Interdisciplinary European Guidelines on Metabolic and Bariatric Surgery. Obes. Facts 2013, 6, 449–468.
- Manicardi, V.; Adinolfi, V.; Aricò, N.; Botta, A.; Clemente, G.; Fava, D.; La Penna, G.; Lapice, E.; Miranda, C.; Nicolucci, A.; et al. Annali AMD 2020. 2020. Available online: (accessed on 5 June 2021).
- Malavige, L.S.; Levy, J.C. Erectile dysfunction in diabetes mellitus. J. Sex. Med. 2009, 6, 1232–1247.
- Nowotny, K.; Jung, T.; Höhn, A.; Weber, D.; Grune, T. Advanced glycation end products and oxidative stress in type 2 diabetes mellitus. Biomolecules 2015, 5, 194–222.
- Afanas’Ev, I. Signaling of reactive oxygen and nitrogen species in diabetes mellitus. Oxid. Med. Cell. Longev. 2010, 3, 361–373.
- D’Oria, R.; Schipani, R.; Leonardini, A.; Natalicchio, A.; Perrini, S.; Cignarelli, A.; Laviola, L.; Giorgino, F. The Role of Oxidative Stress in Cardiac Disease: From Physiological Response to Injury Factor. Oxid. Med. Cell. Longev. 2020, 2020, 5732956.
- Incalza, M.A.; D’Oria, R.; Natalicchio, A.; Perrini, S.; Laviola, L.; Giorgino, F. Oxidative stress and reactive oxygen species in endothelial dysfunction associated with cardiovascular and metabolic diseases. Vasc. Pharm. 2018, 100, 1–19.
- Castela, Â.; Costa, C. Molecular mechanisms associated with diabetic endothelial-erectile dysfunction. Nat. Rev. Urol. 2016, 13, 266–274.
- Carneiro, F.S.; Giachini, F.R.C.; Carneiro, Z.N.; Lima, V.V.; Ergul, A.; Webb, R.C.; Tostes, R.C. Erectile dysfunction in young non-obese type II diabetic goto-kakizaki rats is associated with decreased eNOS phosphorylation at ser1177. J. Sex. Med. 2010, 7, 3620–3634.
- Musicki, B.; Kramer, M.F.; Becker, R.E.; Burnett, A.L. Inactivation of phosphorylated endothelial nitric oxide synthase (Ser-1177) by O-GlcNAc in diabetes-associated erectile dysfunction. Proc. Natl. Acad. Sci. USA 2005, 102, 11870–11875.
- Wang, L.; Tian, W.; Uwais, Z.; Li, G.; Li, H.; Guan, R.; Gao, Z.; Xin, Z. AGE-Breaker ALT-711 Plus Insulin Could Restore Erectile Function in Streptozocin-Induced Type 1 Diabetic Rats. J. Sex. Med. 2014, 11, 1452–1462.
- Yeh, C.H.; Sturgis, L.; Haidacher, J.; Zhang, X.N.; Sherwood, S.J.; Bjercke, R.J.; Juhasz, O.; Crow, M.T.; Tilton, R.G.; Denner, L. Requirement for p38 and p44/p42 mitogen-activated protein kinases in RAGE-mediated nuclear factor-κB transcriptional activation and cytokine secretion. Diabetes 2001, 50, 1495–1504.
- Seftel, A.D.; Vaziri, N.D.; Zhemnin, N.I.; Razmjouei, K.; Fogarty, J.; Hampel, N.; Polak, J.; Wang, R.Z.; Ferguson, K.; Block, C.; et al. Advanced glycation end products in human penis: Elevation in diabetic tissue, site of deposition, and possible effect through iNos or eNos. Urology 1997, 50, 1016–1026.
- Singh, H.; Brindle, N.P.J.; Zammit, V.A. High glucose and elevated fatty acids suppress signaling by the endothelium protective ligand angiopoietin-1. Microvasc. Res. 2010, 79, 121–127.
- Puddu, A.; Sanguineti, R.; Maggi, D.; Nicolò, M.; Traverso, C.E.; Cordera, R.; Viviani, G.L. Advanced Glycation End-Products and Hyperglycemia Increase Angiopoietin-2 Production by Impairing Angiopoietin-1-Tie-2 System. J. Diabetes Res. 2019, 2019.
- Deng, W.; Bivalacqua, T.J.; Champion, H.C.; Hellstrom, W.J.; Murthy, S.N.; Kadowitz, P.J. Superoxide dismutase—A target for gene therapeutic approach to reduce oxidative stress in erectile dysfunction. Methods Mol. Biol. 2010, 610, 213–227.
- Angulo, J.; Peiró, C.; Cuevas, P.; Gabancho, S.; Fernández, A.; González-Corrochano, R.; La Fuente, J.M.; Baron, A.D.; Chen, K.S.; de Tejada, I.S. The novel antioxidant, AC3056 (2,6-di-t-butyl-4-((Dimethyl-4-Methoxyphenylsilyl)Methyloxy)Phenol), reverses erectile dysfunction in diabetic rats and improves NO-mediated responses in penile tissue from diabetic men. J. Sex. Med. 2009, 6, 373–387.
- Goswami, S.K.; Gangadarappa, S.K.; Vishwanath, M.; Razdan, R.; Jamwal, R.; Bhadri, N.; Inamdar, M.N. Antioxidant Potential and Ability of Phloroglucinol to Decrease Formation of Advanced Glycation End Products Increase Efficacy of Sildenafil in Diabetes-Induced Sexual Dysfunction of Rats. Sex. Med. 2016, 4, e106–e114.
- Costa, C.; Virag, R. The endothelial-erectile dysfunction connection: An essential update. J. Sex. Med. 2009, 6, 2390–2404.
- Hui, J.; Chen, S.; Zhang, H.; Yang, C.; Wei, A.; He, S. Effects of “metabolic memory” on erectile function in diabetic men: A retrospective case-control study. Andrology 2021, 9, 288–296.
- Camacho, E.M.; Huhtaniemi, I.T.; O’Neill, T.W.; Finn, J.D.; Pye, S.R.; Lee, D.M.; Tajar, A.; Bartfai, G.; Boonen, S.; Casanueva, F.F.; et al. Age-associated changes in hypothalamic-pituitary-testicular function in middle-aged and older men are modified by weight change and lifestyle factors: Longitudinal results from the European Male Ageing Study. Eur. J. Endocrinol. 2013, 168, 445–455.
- O’Connor, D.B.; Lee, D.M.; Corona, G.; Forti, G.; Tajar, A.; O’Neill, T.W.; Pendleton, N.; Bartfai, G.; Boonen, S.; Casanueva, F.F.; et al. The relationships between sex hormones and sexual function in middle-aged and older European men. J. Clin. Endocrinol. Metab. 2011, 96, E1577–E1587.
- Traish, A.M.; Goldstein, I.; Kim, N.N. Testosterone and Erectile Function: From Basic Research to a New Clinical Paradigm for Managing Men with Androgen Insufficiency and Erectile Dysfunction. Eur. Urol. 2007, 52, 54–70.
- Torres-Estay, V.; Carreño, V.D.; Fuenzalida, P.; Watts, A.; San Francisco, I.F.; Montecinos, V.P.; Sotomayor, P.C.; Ebos, J.; Smith, G.J.; Godoy, A.S. Androgens modulate male-derived endothelial cell homeostasis using androgen receptor-dependent and receptor-independent mechanisms. Angiogenesis 2017, 20, 25–38.
- Torres-Estay, V.; Carreño, V.D.; San Francisco, I.F.; Sotomayor, P.; Godoy, A.S.; Smith, G.J. Androgen receptor in human endothelial cells. J. Endocrinol. 2015, 224, R131–R137.
- Schultheiss, D.; Badalyan, R.; Pilatz, A.; Gabouev, A.I.; Schlote, N.; Wefer, J.; Von Wasielewski, R.; Mertsching, H.; Sohn, M.; Stief, C.G.; et al. Androgen and estrogen receptors in the human corpus cavernosum penis: Immunohistochemical and cell culture results. World J. Urol. 2003, 21, 320–324.
- Aversa, A.; Isidori, A.M.; De Martino, M.U.; Caprio, M.; Fabbrini, E.; Rocchietti-March, M.; Frajese, G.; Fabbri, A. Androgens and penile erection: Evidence for a direct relationship between free testosterone and cavernous vasodilation in men with erectile dysfunction. Clin. Endocrinol. 2000, 53, 517–522.
- Yamamoto, H.; Sasaki, S.; Tatsura, H.; Umemoto, Y.; Kubota, H.; Kamiya, H.; Kawai, T.; Kang, K.; Kohri, K. Penile apoptosis in association with p53 under lack of testosterone. Urol. Res. 2004, 32, 9–13.
- Skogastierna, C.; Hotzen, M.; Rane, A.; Ekström, L. A Supraphysiological dose of testosterone induces nitric oxide production and oxidative stress. Eur. J. Prev. Cardiol. 2014, 21, 1049–1054.
- Kumar, R.; Malhotra, N.; Jacob, J.J. Targeting testosterone measurements to patients with type 2 diabetes mellitus and moderate to severe symptomatic erectile dysfunction. Diabetes Res. Clin. Pract. 2018, 137, 221–223.
- Makhsida, N.; Shah, J.; Yan, G.; Fisch, H.; Shabsigh, R. Hypogonadism and metabolic syndrome: Implications for testosterone therapy. J. Urol. 2005, 174, 827–834.
- Saad, F.; Caliber, M.; Doros, G.; Haider, K.S.; Haider, A. Long-term treatment with testosterone undecanoate injections in men with hypogonadism alleviates erectile dysfunction and reduces risk of major adverse cardiovascular events, prostate cancer, and mortality. Aging Male 2020, 23, 81–92.
- Efesoy, O.; Çayan, S.; Akbay, E. The Effect of Testosterone Replacement Therapy on Penile Hemodynamics in Hypogonadal Men With Erectile Dysfunction, Having Veno-Occlusive Dysfunction. Am. J. Mens. Health 2018, 12, 634–638.
- Zhang, X.H.; Filippi, S.; Morelli, A.; Vignozzi, L.; Luconi, M.; Donati, S.; Forti, G.; Maggi, M. Testosterone restores diabetes-induced erectile dysfunction and sildenafil responsiveness in two distinct animal models of chemical diabetes. J. Sex. Med. 2006, 3, 253–266.
- Aversa, A.; Francomano, D.; Lenzi, A. Does testosterone supplementation increase PDE5-inhibitor responses in difficult-to-treat erectile dysfunction patients? Expert Opin. Pharm. 2015, 16, 625–628.
- Zhu, J.; Zhang, W.; Ou, N.; Song, Y.; Kang, J.; Liang, Z.; Hu, R.; Yang, Y.; Liu, X. Do testosterone supplements enhance response to phosphodiesterase 5 inhibitors in men with erectile dysfunction and hypogonadism: A systematic review and meta-analysis. Transl. Urol. 2020, 9, 591–600.
- Lin, C.S.; Xin, Z.; Namiki, M.; Albersen, M.; Muller, D.; Lue, T.F. Direct androgen regulation of PDE5 gene or the lack thereof. Int. J. Impot. Res. 2013, 25, 81–85.
- den Broeck, T.; Soebadi, M.A.; Falter, A.; Raets, L.; Duponselle, J.; Lootsma, J.; Heintz, A.; Philtjens, U.; Hofkens, L.; Gonzalez-Viedma, A.; et al. Testosterone Induces Relaxation of Human Corpus Cavernosum Tissue of Patients With Erectile Dysfunction. Sex. Med. 2020, 8, 114–119.
- Jones, R.D.; English, K.M.; Hugh Jones, T.; Channer, K.S. Testosterone-induced coronary vasodilatation occurs via a non-genomic mechanism: Evidence of a direct calcium antagonism action. Clin. Sci. 2004, 107, 149–158.
- Wang, X.J.; Xu, T.Y.; Xia, L.L.; Zhong, S.; Zhang, X.H.; Zhu, Z.W.; Chen, D.R.; Liu, Y.; Fan, Y.; Xu, C.; et al. Castration impairs erectile organ structure and function by inhibiting autophagy and promoting apoptosis of corpus cavernosum smooth muscle cells in rats. Int. Urol. Nephrol. 2015, 47, 1105–1115.
- Cui, K.; Li, R.; Chen, R.; Li, M.; Wang, T.; Yang, J.; Chen, Z.; Wang, S.; Liu, J.; Rao, K. Androgen deficiency impairs erectile function in rats through promotion of corporal fibrosis. Andrologia 2018, 50, e12797.
- Arakaki, R.F.; Blevins, T.C.; Wise, J.K.; Liljenquist, D.R.; Jiang, H.H.; Jacobson, J.G.; Martin, S.A.; Jackson, J.A. Comparison of insulin lispro protamine suspension versus insulin glargine once daily added to oral antihyperglycaemic medications and exenatide in type 2 diabetes: A prospective randomized open-label trial. Diabetes Obes. Metab. 2014, 16, 510–518.
- Shirai, M.; Yamanaka, M.; Shiina, H.; Igawa, M.; Ogishima, T.; Fujime, M.; Ishii, N.; Okuyama, A.; Lue, T.F.; Dahiya, R. Androgen, estrogen, and progesterone receptor gene regulation during diabetic erectile dysfunction and insulin treatment. Urology 2004, 64, 1244–1249.
- Kesavadev, J.; Sadasivan Pillai, P.B.; Shankar, A.; Warrier, R.S.; Ramachandran, L.; Jothydev, S.; Krishnan, G. Exploratory CSII Randomized Controlled Trial on Erectile Dysfunction in T2DM Patients (ECSIITED). J. Diabetes Sci. Technol. 2018, 12, 1252–1253.
- Zhou, F.; Hui, Y.; Xu, Y.; Lei, H.; Yang, B.; Guan, R.; Gao, Z.; Xin, Z.; Hou, J. Effects of adipose-derived stem cells plus insulin on erectile function in streptozotocin-induced diabetic rats. Int. Urol. Nephrol. 2016, 48, 657–669.
- Goldner, M.G.; Knatterud, G.L.; Prout, T.E. Effects of hypoglycemic agents on vascular complications in patients with adult-onset diabetes. 3. Clinical implications of UGDP results. Clin. Trial 1971, 218, 1400–1410.
- Koren, S.; Shemesh-Bar, L.; Tirosh, A.; Peleg, R.K.; Berman, S.; Hamad, R.A.; Vinker, S.; Golik, A.; Efrati, S. The effect of sitagliptin versus glibenclamide on arterial stiffness, blood pressure, lipids, and inflammation in type 2 diabetes mellitus patients. Diabetes Technol. 2012, 14, 561–567.
- Burke, M.A.; Mutharasan, R.K.; Ardehali, H. The sulfonylurea receptor, an atypical ATP-binding cassette protein, and its regulation of the KATP channel. Circ. Res. 2008, 102, 164–176.
- Lee, S.W.; Wang, H.Z.; Christ, G.J. Characterization of ATP-sensitive potassium channels in human corporal smooth muscle cells. Int. J. Impot. Res. 1999, 11, 179–188.
- Insuk, S.O.; Chae, M.R.; Choi, J.W.; Yang, D.K.; Sim, J.H.; Lee, S.W. Molecular basis and characteristics of KATP channel in human corporal smooth muscle cells. Int. J. Impot. Res. 2003, 15, 258–266.
- de Miranda Cará, A.; Fregonesi, A.; Antunes, E.; De Nucci, G.; Rodrigues Netto, N. Role of adenosine triphosphate-dependent potassium channels in canine penile erection. Urology 2004, 64, 603–607.
- Sehra, D.; Sehra, S.; Sehra, S.T. Sulfonylureas: Do we need to introspect safety again? Expert Opin. Drug Saf. 2011, 10, 851–861.
- Ruiz Rubio, J.L.; Hernández, M.; Rivera de los Arcos, L.; Benedito, S.; Recio, P.; García, P.; García-Sacristán, A.; Prieto, D. Role of ATP-sensitive K+ channels in relaxation of penile resistance arteries. Urology 2004, 63, 800–805.
- Hsieh, G.; Kolasa, T.; Sullivan, J.; Brioni, J. Dual mechanism of action of nicorandil on rabbit corpus cavernosal smooth muscle tone. Int. J. Impot. Res. 2001, 13, 240–246.
- Al-Kuraishy, H.M.; Al-Gareeb, A.I. Erectile Dysfunction and Low Sex Drive in Men with Type 2 DM: The Potential Role of Diabetic Pharmacotherapy. J. Clin. Diagn. Res. 2016, 10, FC21–FC26.
- Wong, L.; Chen, H.; Lai, S.; Yang, H.; Kuang, J.; Pei, J. Effects of sulfonylurea as initial treatment on testosterone of middle-aged men with type 2 diabetes: A 16-week, pilot study. J. Diabetes Investig. 2015, 6, 454–459.
- Brock, G.; Heiselman, D.; Maggi, M.; Kim, S.W.; Rodríguez Vallejo, J.M.; Behre, H.M.; McGettigan, J.; Dowsett, S.A.; Hayes, R.P.; Knorr, J.; et al. Effect of Testosterone Solution 2% on Testosterone Concentration, Sex Drive and Energy in Hypogonadal Men: Results of a Placebo Controlled Study. J. Urol. 2016, 195, 699–705.
- Chan, T.Y.K. Outbreaks of severe hypoglycaemia due to illegal sexual enhancement products containing undeclared glibenclamide. Pharm. Drug Saf. 2009, 18, 1250–1251.
- Kao, S.L.; Chan, C.L.; Tan, B.; Lim, C.C.T.; Dalan, R.; Gardner, D.; Pratt, E.; Lee, M.; Lee, K.O. An Unusual Outbreak of Hypoglycemia. N. Engl. J. Med. 2009, 360, 734–736.
- Yosefy, C.; Magen, E.; Kiselevich, A.; Priluk, R.; London, D.; Volchek, L.; Viskoper, R.J. Rosiglitazone improves, while glibenclamide worsens blood pressure control in treated hypertensive diabetic and dyslipidemic subjects via modulation of insulin resistance and sympathetic activity. J. Cardiovasc. Pharm. 2004, 44, 215–222.
- Cosenso-Martin, L.N.; Giollo-Júnior, L.T.; Fernandes, L.A.B.; Cesarino, C.B.; Nakazone, M.A.; de Nassau Machado, M.; Yugar-Toledo, J.C.; Vilela-Martin, J.F. Effect of vildagliptin versus glibenclamide on endothelial function and arterial stiffness in patients with type 2 diabetes and hypertension: A randomized controlled trial. Acta Diabetol. 2018, 55, 1237–1245.
- Ma, L.; Lu, N.; Wu, G. Antiplatelet aggregation and endothelial protection of I4, a new synthetic anti-diabetes sulfonylurea compound. Platelets 2015, 26, 342–348.
- Harden, K.A.; Cowan, P.A.; Velasquez-Mieyer, P.; Patton, S.B. Effects of lifestyle intervention and metformin on weight management and markers of metabolic syndrome in obese adolescents. J. Am. Acad. Nurse Pract. 2007, 19, 368–377.
- Wilding, J.; Godec, T.; Khunti, K.; Pocock, S.; Fox, R.; Smeeth, L.; Clauson, P.; Fenici, P.; Hammar, N.; Medina, J. Changes in HbA1c and weight, and treatment persistence, over the 18 months following initiation of second-line therapy in patients with type 2 diabetes: Results from the United Kingdom Clinical Practice Research Datalink. BMC Med. 2018, 16, 1–12.
- Schernthaner, G.; Matthews, D.R.; Charbonnel, B.; Hanefeld, M.; Brunetti, P. Efficacy and safety of pioglitazone versus metformin in patients with type 2 diabetes mellitus: A double-blind, randomized trial. J. Clin. Endocrinol. Metab. 2004, 89, 6068–6076.
- Mahrouf, M.; Ouslimani, N.; Peynet, J.; Djelidi, R.; Couturier, M.; Therond, P.; Legrand, A.; Beaudeux, J.L. Metformin reduces angiotensin-mediated intracellular production of reactive oxygen species in endothelial cells through the inhibition of protein kinase C. Biochem. Pharm. 2006, 72, 176–183.
- Hattori, Y.; Suzuki, K.; Hattori, S.; Kasai, K. Metformin inhibits cytokine-induced nuclear factor κB activation via AMP-activated protein kinase activation in vascular endothelial cells. Hypertension 2006, 47, 1183–1188.
- Kim, Y.W.; Park, S.Y.; Kim, J.Y.; Huh, J.Y.; Jeon, W.S.; Yoon, C.J.; Yun, S.S.; Moon, K.H. Metformin restores the penile expression of nitric oxide synthase in high-fat-fed obese rats. J. Androl. 2007, 28, 555–560.
- Silva, F.H.; Alexandre, E.C.; Calmasini, F.B.; Calixto, M.C.; Antunes, E. Treatment with Metformin Improves Erectile Dysfunction in a Murine Model of Obesity Associated with Insulin Resistance. Urology 2015, 86, 423.e1–423.e6.
- Zhang, C.; Luo, D.; Li, T.; Yang, Q.; Xie, Y.; Chen, H.; Lv, L.; Yao, J.; Deng, C.; Liang, X.; et al. Transplantation of Human Urine-Derived Stem Cells Ameliorates Erectile Function and Cavernosal Endothelial Function by Promoting Autophagy of Corpus Cavernosal Endothelial Cells in Diabetic Erectile Dysfunction Rats. Stem Cells Int. 2019, 2019.
- Musicki, B.; Burnett, A.L. Constitutive NOS uncoupling and NADPH oxidase upregulation in the penis of type 2 diabetic men with erectile dysfunction. Andrology 2017, 5, 294–298.
- Labazi, H.; Wynne, B.M.; Tostes, R.; Webb, R.C. Metformin treatment improves erectile function in an angiotensin II model of erectile dysfunction. J. Sex. Med. 2013, 10, 2154–2164.
- Lanaspa, M.A.; Cicerchi, C.; Garcia, G.; Li, N.; Roncal-Jimenez, C.A.; Rivard, C.J.; Hunter, B.; Andrés-Hernando, A.; Ishimoto, T.; Sánchez-Lozada, L.G.; et al. Counteracting Roles of AMP Deaminase and AMP Kinase in the Development of Fatty Liver. PLoS ONE 2012, 7, e48801.
- Ouyang, J.; Parakhia, R.A.; Ochs, R.S. Metformin activates AMP kinase through inhibition of AMP deaminase. J. Biol. Chem. 2011, 286, 1–11.
- Phatarpekar, V.P.; Wen, J.; Xia, Y. Role of adenosine signaling in penile erection and erectile disorders. J. Sex. Med. 2010, 7, 3553–3564.
- Vignozzi, L.; Filippi, S.; Comeglio, P.; Cellai, I.; Morelli, A.; Rastrelli, G.; Maneschi, E.; Mannucci, E.; Maggi, M. Metformin in vitro and in vivo increases adenosine signaling in rabbit corpora cavernosa. J. Sex. Med. 2014, 11, 1694–1708.
- Rey-Valzacchi, G.N.J.; Costanzo, P.R.; Finger, L.A.; Layus, A.O.; Gueglio, G.M.; Litwak, L.N.E.; Knoblovits, P. Addition of metformin to sildenafil treatment for erectile dysfunction in eugonadal nondiabetic men with insulin resistance. A prospective, randomized, double-blind pilot study. J. Androl. 2012, 33, 608–614.
- Yan, W.J.; Mu, Y.; Yu, N.; Yi, T.L.; Zhang, Y.; Pang, X.L.; Cheng, D.; Yang, J. Protective effects of metformin on reproductive function in obese male rats induced by high-fat diet. J. Assist. Reprod. Genet. 2015, 32, 1097–1104.
- Morgante, G.; Tosti, C.; Orvieto, R.; Musacchio, M.C.; Piomboni, P.; De Leo, V. Metformin improves semen characteristics of oligo-terato-asthenozoospermic men with metabolic syndrome. Fertil. Steril. 2011, 95, 2150–2152.
- Furman, B.L. Acarbose. In xPharm: The Comprehensive Pharmacology Reference; Elsevier Inc.: Amsterdam, The Netherlands, 2007; pp. 1–3. ISBN 9780080552323.
- Sun, W.; Zeng, C.; Liao, L.; Chen, J.; Wang, Y. Comparison of acarbose and metformin therapy in newly diagnosed type 2 diabetic patients with overweight and/or obesity. Curr. Med. Res. Opin. 2016, 32, 1389–1396.
- Wang, J.-S.; Lin, S.-D.; Lee, W.-J.; Su, S.-L.; Lee, I.-T.; Tu, S.-T.; Tseng, Y.-H.; Lin, S.-Y.; Sheu, W.H.-H. Effects of Acarbose Versus Glibenclamide on Glycemic Excursion and Oxidative Stress in Type 2 Diabetic Patients Inadequately Controlled by Metformin: A 24-Week, Randomized, Open-Label, Parallel-Group Comparison. Clin. Ther. 2011, 33, 1932–1942.
- Chiasson, J.-L.; Josse, R.G.; Gomis, R.; Hanefeld, M.; Karasik, A.; Laakso, M. Acarbose for prevention of type 2 diabetes mellitus: The STOP-NIDDM randomised trial. Lancet 2002, 359, 2072–2077.
- Chiasson, J.-L.; Josse, R.G.; Gomis, R.; Hanefeld, M.; Karasik, A.; Laakso, M.; STOP-NIDDM Trial Research Group. Acarbose treatment and the risk of cardiovascular disease and hypertension in patients with impaired glucose tolerance: The STOP-NIDDM trial. JAMA 2003, 290, 486–494.
- Kato, T.; Inoue, T.; Node, K. Postprandial endothelial dysfunction in subjects with new-onset type 2 diabetes: An acarbose and nateglinide comparative study. Cardiovasc. Diabetol. 2010, 9.
- Oboh, G.; Ogunbadejo, M.D.; Ogunsuyi, O.B.; Oyeleye, S.I. Can gallic acid potentiate the antihyperglycemic effect of acarbose and metformin? Evidence from streptozotocin-induced diabetic rat model. Arch. Physiol. Biochem. 2020, 1–9.
- Oyeleye, S.I.; Ojo, O.R.; Oboh, G. Moringa oleifera leaf and seed inclusive diets influenced the restoration of biochemicals associated with erectile dysfunction in the penile tissue of STZ-induced diabetic male rats treated with/without Acarbose drug. J. Food Biochem. 2020, 45.
- Derosa, G.; Mereu, R.; D’Angelo, A.; Salvadeo, S.A.; Ferrari, I.; Fogari, E.; Gravina, A.; Palumbo, I.; Maffioli, P.; Randazzo, S.; et al. Effect of pioglitazone and acarbose on endothelial inflammation biomarkers during oral glucose tolerance test in diabetic patients treated with sulphonylureas and metformin. J. Clin. Pharm. 2010, 35, 565–579.
- Li, X.X.; Ling, S.K.; Hu, M.Y.; Ma, Y.; Li, Y.; Huang, P.L. Protective effects of acarbose against vascular endothelial dysfunction through inhibiting Nox4/NLRP3 inflammasome pathway in diabetic rats. Free Radic. Biol. Med. 2019, 145, 175–186.
- Bi, Y.; Zhang, B.; Xu, W.; Yang, H.; Feng, W.; Li, C.; Tong, G.; Li, M.; Wang, X.; Shen, S.; et al. Effects of exenatide, insulin, and pioglitazone on liver fat content and body fat distributions in drug-naive subjects with type 2 diabetes. Acta Diabetol. 2014, 51, 865–873.
- Kaku, K.; Itayasu, T.; Hiroi, S.; Hirayama, M.; Seino, Y. Efficacy and safety of alogliptin added to pioglitazone in Japanese patients with type 2 diabetes: A randomized, double-blind, placebo-controlled trial with an open-label long-term extension study. Diabetes Obes. Metab. 2011, 13, 1028–1035.
- Van Can, J.; Sloth, B.; Jensen, C.B.; Flint, A.; Blaak, E.E.; Saris, W.H.M. Effects of the once-daily GLP-1 analog liraglutide on gastric emptying, glycemic parameters, appetite and energy metabolism in obese, non-diabetic adults. Int. J. Obes. 2014, 38, 784–793.
- Basolo, A.; Burkholder, J.; Osgood, K.; Graham, A.; Bundrick, S.; Frankl, J.; Piaggi, P.; Thearle, M.S.; Krakoff, J. Exenatide has a pronounced effect on energy intake but not energy expenditure in non-diabetic subjects with obesity: A randomized, double-blind, placebo-controlled trial. Metabolism 2018, 85, 116–125.
- Kovanecz, I.; Ferrini, M.G.; Vernet, D.; Nolazco, G.; Rajfer, J.; Gonzalez-Cadavid, N.F. Pioglitazone prevents corporal veno-occlusive dysfunction in a rat model of type 2 diabetes mellitus. BJU Int. 2006, 98, 116–124.
- Kovanecz, I.; Ferrini, M.G.; Vernet, D.; Nolazco, G.; Rajfer, J.; Gonzalez-Cadavid, N.F. Ageing-related corpora veno-occlusive dysfunction in the rat is ameliorated by pioglitazone. BJU Int. 2007, 100, 867–874.
- Aliperti, L.A.; Lasker, G.F.; Hagan, S.S.; Hellstrom, J.A.; Gokce, A.; Trost, L.W.; Kadowitz, P.J.; Sikka, S.C.; Hellstrom, W.J.G. Efficacy of pioglitazone on erectile function recovery in a rat model of cavernous nerve injury. Urology 2014, 84, 1122–1127.
- Heppner, T.J.; Bonev, A.D.; Eckman, D.M.; Gomez, M.F.; Petkov, G.V.; Nelson, M.T. Novel PPARγ agonists GI 262570, GW 7845, GW 1929, and pioglitazone decrease calcium channel function and myogenic tone in rat mesenteric arteries. Pharmacology 2005, 73, 15–22.
- Goya, K.; Sumitani, S.; Otsuki, M.; Xu, X.; Yamamoto, H.; Kurebayashi, S.; Saito, H.; Kouhara, H.; Kasayama, S. The thiazolidinedione drug troglitazone up-regulates nitric oxide synthase expression in vascular endothelial cells. J. Diabetes Complicat. 2006, 20, 336–342.
- Katz, E.G.; Moustafa, A.A.; Heidenberg, D.; Haney, N.; Peak, T.; Lasker, G.F.; Knoedler, M.; Rittenberg, D.; Rezk, B.M.; Abd Elmageed, Z.Y.; et al. Pioglitazone Enhances Survival and Regeneration of Pelvic Ganglion Neurons after Cavernosal Nerve Injury. Urology 2016, 89, 76–82.
- Gholamine, B.; Motevallian, M.; Shafiei, M.; Mahmoudian, M. Effects of pioglitazone on erectile dysfunction in sildenafil poor-responders: A randomized, controlled study. J. Pharm. Pharm. Sci. 2008, 11, 22.
- Peuler, J.D.; Phelps, L.E. Sildenafil does not enhance but rather attenuates vasorelaxant effects of antidiabetic agents. J. Smooth Muscle Res. 2015, 51, 22–36.
- Ishibashi, Y.; Matsui, T.; Takeuchi, M.; Yamagishi, S.I. Glucagon-like peptide-1 (GLP-1) inhibits advanced glycation end product (AGE)-induced up-regulation of VCAM-1 mRNA levels in endothelial cells by suppressing AGE receptor (RAGE) expression. Biochem. Biophys. Res. Commun. 2010, 391, 1405–1408.
- Nyström, T.; Gutniak, M.K.; Zhang, Q.; Zhang, F.; Holst, J.J.; Ahrén, B.; Sjöholm, Å. Effects of glucagon-like peptide-1 on endothelial function in type 2 diabetes patients with stable coronary artery disease. Am. J. Physiol. Endocrinol. Metab. 2004, 287.
- Erdogdu, Ö.; Nathanson, D.; Sjöholm, Å.; Nyström, T.; Zhang, Q. Exendin-4 stimulates proliferation of human coronary artery endothelial cells through eNOS-, PKA- and PI3K/Akt-dependent pathways and requires GLP-1 receptor. Mol. Cell. Endocrinol. 2010, 325, 26–35.
- Gaspari, T.; Liu, B.H.; Welungoda, I.; Hu, Y.; Widdop, R.E.; Knudsen, L.B.; Simpson, R.W.; Dear, A.E. A GLP-1 receptor agonist liraglutide inhibits endothelial cell dysfunction and vascular adhesion molecule expression in an ApoE-/- mouse model. Diabetes Vasc. Dis. Res. 2011, 8, 117–124.
- Cheng, C.K.; Luo, J.Y.; Lau, C.W.; Cho, W.C.; Ng, S.C.F.; Ma, R.C.W.; Tian, X.Y.; Huang, Y. A GLP-1 analog lowers ER stress and enhances protein folding to ameliorate homocysteine-induced endothelial dysfunction. Acta Pharm. Sin. 2021, 1–12.
- Koska, J.; Sands, M.; Burciu, C.; D’Souza, K.M.; Raravikar, K.; Liu, J.; Truran, S.; Franco, D.A.; Schwartz, E.A.; Schwenke, D.C.; et al. Exenatide protects against glucoseand lipid-induced endothelial dysfunction: Evidence for direct vasodilation effect of glp-1 receptor agonists in humans. Diabetes 2015, 64, 2624–2635.
- Cai, X.; She, M.; Xu, M.; Chen, H.; Li, J.; Chen, X.; Zheng, D.; Liu, J.; Chen, S.; Zhu, J.; et al. GLP-1 treatment protects endothelial cells from oxidative stress-induced autophagy and endothelial dysfunction. Int. J. Biol. Sci. 2018, 14, 1696–1708.
- Altabas, V.; Altabas, K. DPP-4 inhibition improves a sexual condition? Med. Hypotheses 2015, 85, 124–126.
- Yuan, P.; Ma, D.; Gao, X.; Wang, J.; Li, R.; Liu, Z.; Wang, T.; Wang, S.; Liu, J.; Liu, X. Liraglutide Ameliorates Erectile Dysfunction via Regulating Oxidative Stress, the RhoA/ROCK Pathway and Autophagy in Diabetes Mellitus. Front. Pharm. 2020, 11.
- Maclusky, N.J.; Cook, S.; Scrocchi, L.; Shin, J.; Kim, J.; Vaccarino, F.; Asa, S.L.; Drucker, D.J. Neuroendocrine function and response to stress in mice with complete disruption of glucagon-like peptide-1 receptor signaling. Endocrinology 2000, 141, 752–762.
- Zhang, E.; Xu, F.; Liang, H.; Yan, J.; Xu, H.; Li, Z.; Wen, X.; Weng, J. GLP-1 Receptor Agonist Exenatide Attenuates the Detrimental Effects of Obesity on Inflammatory Profile in Testis and Sperm Quality in Mice. Am. J. Reprod. Immunol. 2015, 74, 457–466.
- Giagulli, V.A.; Carbone, M.D.; Ramunni, M.I.; Licchelli, B.; De Pergola, G.; Sabbà, C.; Guastamacchia, E.; Triggiani, V. Adding liraglutide to lifestyle changes, metformin and testosterone therapy boosts erectile function in diabetic obese men with overt hypogonadism. Andrology 2015, 3, 1094–1103.
- Shao, N.; Yu, X.Y.; Yu, Y.M.; Li, B.W.; Pan, J.; Wu, W.H.; Zhang, H.J.; Ma, X.F.; Hao, M.; Kuang, H.Y. Short-term combined treatment with exenatide and metformin is superior to glimepiride combined metformin in improvement of serum testosterone levels in type 2 diabetic patients with obesity. Andrologia 2018, 50.
- Fontoura, P.; Cardoso, M.C.D.A.; Erthal-Martins, M.C.; Werneck, C.; Sartorio, C.; Ramos, C.F. The effects of liraglutide on male fertility: A case report. Reprod. Biomed. Online 2014, 29, 644–646.
- Sopko, N.A.; Hannan, J.L.; Bivalacqua, T.J. Understanding and targeting the Rho kinase pathway in erectile dysfunction. Nat. Rev. Urol. 2014, 11, 622–628.
- Dalaklioglu, S.; Tasatargil, A.; Kuscu, N.; Celik, S.; Celik-Ozenci, C.; Ozdem, S.; Barutcigil, A.; Kucukcetin, I. Protective effect of exendin-4 treatment on erectile dysfunction induced by chronic methylglyoxal administration in rats. Peptides 2018, 106, 1–8.
- Huang, C.-Y.; Shih, C.-M.; Tsao, N.-W.; Lin, Y.-W.; Huang, P.-H.; Wu, S.-C.; Lee, A.-W.; Kao, Y.-T.; Chang, N.-C.; Nakagami, H.; et al. Dipeptidyl peptidase-4 inhibitor improves neovascularization by increasing circulating endothelial progenitor cells. Br. J. Pharm. 2012, 167, 1506–1519.
- Fadini, G.P.; Avogaro, A. Dipeptidyl peptidase-4 inhibition and vascular repair by mobilization of endogenous stem cells in diabetes and beyond. Atherosclerosis 2013, 229, 23–29.
- Amadesi, S.; Reni, C.; Katare, R.; Meloni, M.; Oikawa, A.; Beltrami, A.P.; Avolio, E.; Cesselli, D.; Fortunato, O.; Spinetti, G.; et al. Role for substance P-based nociceptive signaling in progenitor cell activation and angiogenesis during ischemia in mice and in human subjects. Circulation 2012, 125, 1774–1786.
- Castellani, M.L.; Galzio, R.J.; Felaco, P.; Tripodi, D.; Toniato, E.; De Lutiis, M.A.; Conti, F.; Fulcheri, M.; Conti, C.; Theoharides, T.C.; et al. VEGF, substance P and stress, new aspects: A revisited study. J. Biol. Regul. Homeost. Agents 2010, 24, 229–237.
- Harmar, A.J.; Fahrenkrug, J.; Gozes, I.; Laburthe, M.; May, V.; Pisegna, J.R.; Vaudry, D.; Vaudry, H.; Waschek, J.A.; Said, S.I. Pharmacology and functions of receptors for vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide: IUPHAR Review 1. Br. J. Pharm. 2012, 166, 4–17.
- Traish, A.M.; Galoosian, A. Androgens Modulate Endothelial Function and Endothelial Progenitor Cells in Erectile Physiology. Korean J. Urol. 2013, 54, 721.
- Aini, K.; Fukuda, D.; Tanaka, K.; Higashikuni, Y.; Hirata, Y.; Yagi, S.; Kusunose, K.; Yamada, H.; Soeki, T.; Sata, M. Vildagliptin, a DPP-4 Inhibitor, Attenuates Endothelial Dysfunction and Atherogenesis in Nondiabetic Apolipoprotein E-Deficient Mice. Int. Heart J. 2019, 60, 1421–1429.
- Hibi, H.; Ohori, T.; Yamada, Y. DPP-IV inhibitor may affect spermatogenesis. Diabetes Res. Clin. Pract. 2011, 93.
- Verma, S.; McMurray, J.J.V. SGLT2 inhibitors and mechanisms of cardiovascular benefit: A state-of-the-art review. Diabetologia 2018, 61, 2108–2117.
- Lee, M.M.Y.; Petrie, M.C.; McMurray, J.J.V.; Sattar, N. How Do SGLT2 (Sodium-Glucose Cotransporter 2) inhibitors and GLP-1 (glucagon-like peptide-1) receptor agonists reduce cardiovascular outcomes?: Completed and ongoing mechanistic trials. Arter. Thromb. Vasc. Biol. 2020, 40, 506–522.
- Morieri, M.L.; Consoli, A.; Sesti, G.; Purrello, F.; Avogaro, A.; Fadini, G.P. Comparative effectiveness of dapagliflozin vs DPP-4 inhibitors on a composite endpoint of HbA1c, body weight and blood pressure reduction in the real world. Diabetes. Metab. Res. Rev. 2021, 37.
- Cowie, M.R.; Fisher, M. SGLT2 inhibitors: Mechanisms of cardiovascular benefit beyond glycaemic control. Nat. Rev. Cardiol. 2020, 17, 761–772.
- Jayawardene, D.; Ward, G.M.; O’Neal, D.N.; Theverkalam, G.; MacIsaac, A.I.; MacIsaac, R.J. New treatments for type 2 diabetes: Cardiovascular protection beyond glucose lowering? Hear. Lung Circ. 2014, 23, 997–1008.
- Kluger, A.Y.; Tecson, K.M.; Lee, A.Y.; Lerma, E.V.; Rangaswami, J.; Lepor, N.E.; Cobble, M.E.; McCullough, P.A. Class effects of SGLT2 inhibitors on cardiorenal outcomes. Cardiovasc. Diabetol. 2019, 18, 99.
- Park, S.H.; Farooq, M.A.; Gaertner, S.; Bruckert, C.; Qureshi, A.W.; Lee, H.H.; Benrahla, D.; Pollet, B.; Stephan, D.; Ohlmann, P.; et al. Empagliflozin improved systolic blood pressure, endothelial dysfunction and heart remodeling in the metabolic syndrome ZSF1 rat. Cardiovasc. Diabetol. 2020, 19.
- Ganbaatar, B.; Fukuda, D.; Shinohara, M.; Yagi, S.; Kusunose, K.; Yamada, H.; Soeki, T.; Hirata, K.I.; Sata, M. Empagliflozin ameliorates endothelial dysfunction and suppresses atherogenesis in diabetic apolipoprotein E-deficient mice. Eur. J. Pharm. 2020, 875.
- Lee, D.M.; Battson, M.L.; Jarrell, D.K.; Hou, S.; Ecton, K.E.; Weir, T.L.; Gentile, C.L. SGLT2 inhibition via dapagliflozin improves generalized vascular dysfunction and alters the gut microbiota in type 2 diabetic mice. Cardiovasc. Diabetol. 2018, 17.
- Solini, A.; Giannini, L.; Seghieri, M.; Vitolo, E.; Taddei, S.; Ghiadoni, L.; Bruno, R.M. Dapagliflozin acutely improves endothelial dysfunction, reduces aortic stiffness and renal resistive index in type 2 diabetic patients: A pilot study. Cardiovasc. Diabetol. 2017, 16.
- Uthman, L.; Homayr, A.; Juni, R.P.; Spin, E.L.; Kerindongo, R.; Boomsma, M.; Hollmanna, M.W.; Preckel, B.; Koolwijk, P.; Van Hinsbergh, V.W.M.; et al. Empagliflozin and dapagliflozin reduce ROS generation and restore no bioavailability in tumor necrosis factor α-stimulated human coronary arterial endothelial cells. Cell. Physiol. Biochem. 2019, 53, 865–886.
- Assaly, R.; Gorny, D.; Compagnie, S.; Mayoux, E.; Bernabe, J.; Alexandre, L.; Giuliano, F.; Behr-Roussel, D. The Favorable Effect of Empagliflozin on Erectile Function in an Experimental Model of Type 2 Diabetes. J. Sex. Med. 2018, 15, 1224–1234.




