Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Sándor Valkai | + 3227 word(s) | 3227 | 2021-06-15 08:38:43 | | | |
| 2 | Rita Xu | -31 word(s) | 3196 | 2021-06-25 11:04:12 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Valkai, S. Electrical Resistance Measurement. Encyclopedia. Available online: https://encyclopedia.pub/entry/11305 (accessed on 08 February 2026).
Valkai S. Electrical Resistance Measurement. Encyclopedia. Available at: https://encyclopedia.pub/entry/11305. Accessed February 08, 2026.
Valkai, Sándor. "Electrical Resistance Measurement" Encyclopedia, https://encyclopedia.pub/entry/11305 (accessed February 08, 2026).
Valkai, S. (2021, June 25). Electrical Resistance Measurement. In Encyclopedia. https://encyclopedia.pub/entry/11305
Valkai, Sándor. "Electrical Resistance Measurement." Encyclopedia. Web. 25 June, 2021.
Copy Citation
The blood–brain barrier (BBB) represents the tightest endothelial barrier within the cardiovascular system characterized by very low ionic permeability.
blood–brain barrier
cell culture insert
electrodes
endothelial cell
epithelial cell
impedance
lab-on-a-chip
1. Introduction
The blood–brain barrier (BBB) represents the tightest biological barrier within the cardiovascular system, which plays a crucial role in the protection of the central nervous system [1]. The interendothelial tight junctions of brain microvessels create the basis of this physical defense system which restricts the movement of ions through the paracellular space, thus contributing greatly to the development and maintenance of the ionic homeostasis in the brain [2]. This ionic tightness of the BBB can be characterized by the measurement of electrical resistance across the wall of brain microvessels [3] or culture models of the BBB using two-compartment setups [4][5]. The first measurements on the electrical resistance of brain surface microvessels were made in the early 1980s [3] and were soon followed by measurements on brain endothelial cell cultures [6], i.e., in vitro models of the BBB. In the following 40 years, great progress and an even greater diversification of the BBB culture models could be witnessed. In addition to primary brain endothelial cell- and cell-line-based systems, stem-cell-derived BBB models were introduced and have gained popularity rapidly, as reviewed recently [7]. The major technical advances in the measurement of transendothelial electrical resistance/resistivity (TEER) of BBB models include the introduction of cell culture inserts, electrodes, and instruments from commercial sources. While the culture inserts provide static conditions, the hollow-fiber cartridges [8] and, in the last 9 years, lab-on-a-chip microfluidic devices provide a dynamic environment for BBB models [9][10], providing more physiological conditions but also presenting new technical challenges.
TEER as a physical parameter is key to characterize the barrier tightness of the BBB in vitro models [4][5]. According to a large body of barrier tightness data measured on BBB culture models, both TEER and permeability for paracellular markers, biological factors influencing the tight junctions of cultured brain endothelial cells, are the most important determinants of ionic permeability across BBB models, as detailed in several works [4][7][11][12][13][14]. These biological factors include cell sources and types (primary or stem-cell-derived cells, immortalized cell lines, brain microvessels from different species), passage numbers, culture media and supplements, factors inducing BBB properties and/or elevating BBB tightness (second messengers, cytokines, mediators, receptor agonists or antagonists), culture types (mono- or different coculture settings), and culture conditions (static vs. dynamic setups with fluid flow generated shear stress). The present review does not detail these aspects of the barrier tightness of BBB culture models, which were well covered in other papers [4][7][11][12][13][14].
2. Electrical Resistance Measurements: Principles, Methods, and Influence of Physicochemical Parameters
2.1. Electric Circuit Analysis of Biological Barriers
The passive electric properties of biological barriers are determined by their mesoscopic organization including tightness of cell–cell connections, cell morphology, and membrane robustness. Hence, electric impedance measurements have been proven useful in 2D or 3D cell culture models of specific biological barriers including insert [5] or lab-on-a-chip models [15][16]. The architecture of cells—thin lipid membranes considered as fairly good insulators, surrounded by mobile ions of the electrolyte representing a relatively high conductivity at physiological salt concentrations—predestines the use of resistor–capacitor (RC) substitution-circuit models [5] to characterize the electric properties of biological barriers (Figure 1).
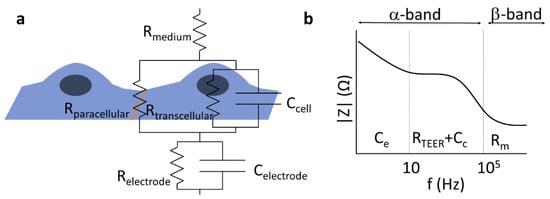
Figure 1. Components of the total electrical impedance measured across brain endothelial cells modeling the blood–brain barrier. (a) The equivalent circuit of a cell monolayer. The transendothelial resistance (RTEER) is primarily dominated by the paracellular resistance (Rparacellular). In the case of electrical impedance measurement, the transcellular pathway (Rtranscellular, Ccell) and the properties of the electrode (Relectrode, Celetrode) also affect the recorded signal. The orange dotted line depicts tight junctions between brain endothelial cells. (b) Schematic impedance spectrum of a cell monolayer. The α-band contains the electrode polarization, the resulting resistance of the layer (RTEER, determined by the para- and transcellular resistances: Rparacellular and Rtranscellular), and the capacitance of the cell layer (Cc), while the β-band is dominated by the resistance of the medium (Rm) and the resulting capacitance of the system. Modified from [5].
Depending on the frequency range analyzed, information can be gained about various features of the barriers, which may be associated to specific physiological functions. The TEER of BBB culture models is primarily determined by the tight interendothelial junctions [4]; however, in other biological barriers, active ion channels can contribute to the measured resistance [17]. Below, we give a short overview of the related principles and methods used to study the TEER of BBB in vivo and in vitro.
2.2. Electric Impedance Spectroscopy
Electric impedance spectroscopy (EIS) can be especially useful for leaky/low-resistance models of biological barriers where modeling of the recorded impedance spectra gives information about morphological changes of cells and the cellular organization of the tissue [5]. If an electric field is applied to the sample, the mobile ions inside and outside of the cells are displaced, typically alongside the highly charged membrane surfaces, resulting in a polarization of the cells, as well as the whole sample. Electric polarization is directly related to dielectric permittivity; hence, the method is also often cited as dielectric spectroscopy. Since charge displacements are inevitably accompanied by energy dissipation, the sample can be characterized by a complex dielectric permittivity, where the real part describes the polarization, while the imaginary part describes the dissipative properties. In electronic terms, the former corresponds to the capacitive properties, while the latter corresponds to resistive properties of the sample. The times necessary for the polarization of cells or components representing other hierarchical levels, such as cell associates or cell organelles and macromolecules, are characterized by time constants depending on the size of the given component. Conventionally, three ranges of the dissipation time constants are distinguished [18]: the α-band (between the mHz and kHz range) is associated with electrode polarization and counterion relaxations around tissues or cells, the β-band (between ~100 kHz and 100 MHz) is characteristic of transmembrane capacitive currents (Figure 1), and the γ-band (100 MHz–100 GHz) can be assigned to the polarization of macromolecules or the reorientation of water molecules. The contribution of the different factors to the overall impedance can be distinguished by the mathematical analysis of an extended frequency range. There is an interesting technique called two-path impedance spectroscopy [19], which allows the decomposition of RTEER into Rtranscellular and Rparacellular terms, under certain conditions. The method is based on an EIS measurement combined with a Ca++-dependent modulation of Rparacellular, as well as the determination of the permeability of a paracellular marker that is supposed to vary inversely to Rparacellular, without affecting Rtransellular.
There are several commercially available systems for EIS, including the ECIS (Electric Cell-Substrate Impedance Sensing; Applied BioPhysics) and xCELLigence (Agilent, USA) devices. The cells are grown on a solid support containing the integrated planar gold electrodes in both setups. The main difference between the two systems is that ECIS displays impedance spectra, while xCELLigence reports a dimensionless parameter called cell index, CI = Zn − Z0, where Zn is the impedance at time point n and Z0 is the impedance without cells. These methods allow the detection of small changes in morphology, strength of adhesion, and cell–cell interactions [20]. Another EIS based setup is the cellZscope system (NanoAnalytics, Germany), designed for measuring parameters of cell monolayers grown on culture inserts. In this setup, the cell monolayer covering the porous membrane of the insert is between a pair of electrodes [5]. The device automatically measures the impedance spectra and performs the mathematical analysis of the recorded signal. In addition to resistance, capacitance data are acquired. Increased cell layer capacitance due to changes in cell membrane properties can indicate cellular toxicity or dysfunction in culture models of the central nervous system barriers [21][22]. To characterize the tightness of culture models of biological barriers, however, low-frequency methods are the most widespread techniques [5].
2.3. Direct Current or Quasi-Direct Current Methods
The DC or quasi-DC methods are based on the measurement of ohmic resistance of the biological barriers, and they have been applied for measurements on microvessels in living tissues and brain endothelial monolayers in culture conditions. This transendo/epithelial electric resistance, measured in ohmic units, is also called “TEER”; however, in this paper, we denote it by RTEER, so as to distinguish it from the endothelial/epithelial surface area-corrected TEER values that represent a resistivity, measured in Ω·cm2 units.
In animal studies the electrical resistance of brain surface microvessels is determined similarly to the electrical resistance of the axonal membrane [23]. The four-point measurement system with capillary electrodes is inserted to the brain tissue and the microvessel (Figure 2a). The intracapillary potential (V(x)) is related to the potential at the tip of the current electrode (V(0)) via the following equation:
V(x) = V(0)e−x/λ
where x is the distance to the measuring voltage electrode, and λ is the length constant [3][23]. The resistance of the vessel can then be calculated from the length constant of the potential decay, the vessel radius, and the blood resistivity [3]. In culture studies, based on brain endothelial cell monolayers in mono- or co-culture conditions [4][7][12], the most straightforward approach is to place one electrode in the top fluid compartment and another one in the bottom compartment (Figure 2a,b). The two compartments are separated by the cell monolayer(s) grown on a porous membrane. The resistance is calculated on the basis of Ohm’s law by taking the ratio of voltage and current [16]. However, the impedance of the electrode–electrolyte interface usually seriously compromises the measurements at DC or low frequencies, when two electrodes are used. In order to overcome the problem of electrode polarization, the four-electrode method is applied for biological samples, where second-order conductors (electrolytes) are part of the system [24]. In this case, a pair of electrodes is used to inject constant current through the sample, and another pair of electrodes measures the generated voltage across the sample (Figure 2). Since, in this case, there is no current flowing through the voltage electrodes, they can accurately measure the voltage drop on the sample. The resulting resistance of the sample (RTEER + Rmedium + Relectrode) is calculated from the constant current of the current generator and the voltage measured by the voltage electrode pair, according to Ohm’s law. (Note that Relectrode can be considered negligible well above the decomposition potential of the solution.) In practice, low-frequency square waves are used instead of DC, in order to avoid gas evolution on the surface of the electrodes. The commercially available and widely used EVOM instrument (World Precision Instruments, USA) applies a 12.5 Hz square waveform. The range of the device is from 0–9999 Ω with a resolution of 1 Ω. This four-point measurement configuration is compatible with different kinds of electrode geometries as shown on Figure 2b,c,e.
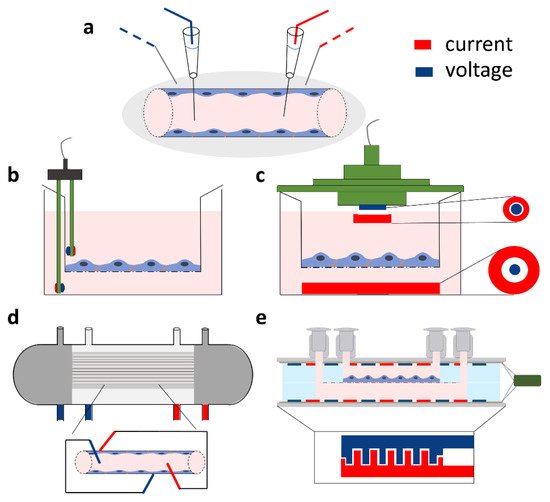
Figure 2. Direct current or quasi-direct current methods for in vivo and in vitro transcellular electrical resistance and impedance measurements. All types of setups include a four-point measurement method based on two current electrodes (red) and two voltage electrodes (blue). (a) Setup for electrical resistance registration of brain surface microvessels. (b) Chopstick and (c) chamber silver/silver chloride (Ag/AgCl) electrode arrangement for the measurement of transcellular resistance across cellular monolayers cultured on different sizes of cell culture inserts. (d) Impedance measurement setup on a hollow-fiber cartridge model. (e) Resistance measurement on a novel lab-on-a-chip model based on electrodes prepared with gold sputter coating (method described in [14]).
The most commonly used electrode type in models of the BBB using a culture insert setup is the so-called chopstick electrode which can be easily handled to quickly measure TEER in parallel samples (Figure 2b). The current passing electrodes are made from silver and the voltage measuring electrodes are silver/silver chloride (Ag/AgCl) electrodes. During measurement, one of the sticks is placed in the top and the other in the bottom compartment containing culture medium. The placement of the chopstick electrode needs extra attention as it can result in resistance measurement inaccuracies as compared to measurements with other devices, as discussed later. As an alternative to chopstick electrodes, chamber electrodes are available (Figure 2c). The culture insert can be placed in the chamber and covered with a cap. Both the chamber and the cap contain a pair of integrated electrodes: a centrally positioned Ag/AgCl for voltage measurement and an outer, concentrically placed silver current passing electrode. Compared to the chopstick electrode, the chamber has not only fixed positions which decrease the error of random electrode positioning, but also planar electrodes with large surfaces.
Another system that is used for in vitro modeling of the BBB is the hollow-fiber model (Figure 2d). The hollow-fiber cartridges contain a bundle of capillaries made from porous membranes which mimic the geometry of blood microvessels. By growing brain endothelial cells in the lumen of these capillaries and introducing flow of the culture medium, the system can be used as a dynamic model of the BBB [25]. The capillaries are embedded in a plastic housing, resulting in an outer compartment representing the extracapillary space. There are commercially available hollow-fiber models (Flocel, Cleveland, OH, USA) with integrated TEER measurement [8]. For these, four electrodes are usually installed, two at the beginning and end of the cartridges in the extracapillary space and two within the lumen of the capillaries (Figure 2d). The impedance is then measured and averaged; then, at a defined frequency, the TEER is determined.
In the last 9 years, several types of lab-on-a-chip (LOC) devices have been developed to model the BBB (for reviews, see [9][10]). Many of these devices allow the measurement of TEER with the integration of electrodes. The general structure of LOC devices is built up by two overlapping channels separated by a porous culture membrane, i.e., the layer structure and the topology of most chip devices do not differ from those of transwell inserts; hence, their equivalent circuit is the same. Of course, the values of the R and C components do depend on the actual geometry. The electrodes for the electrical measurements are placed in the top and bottom compartments (Figure 2e). The main differences between LOCs include the channel size, the size of the overlapping region/culture surface, the electrode geometry, and material [9]. The electrodes can be wires, plated or sputter coated metal layers, or indium tin oxide (ITO) glass. The wires are inserted in the channels; the material can be Ag/AgCl as the measuring electrodes and silver as the current electrode or platinum [15]. The advantage of the wire electrodes is that they do not need special laboratory equipment for their production. The drawback of the wire-type electrodes is that they, similarly to the chopstick case, create a nonuniform current profile; thus, they suit only smaller culture surfaces. In contrast, plated or sputter-coated metal electrodes in a four-electrode setting [14][26][27], especially in an interdigitating format (Figure 2e), provide a more homogenous sensitivity distribution [28] and, therefore, a more reliable TEER measurement.
2.4. Effects of Physical and Physicochemical Parameters on Electrical Resistance
While biological factors, such as brain endothelial cell type, passage number, coculture, culture medium composition, and barrier-inducing factors [29], greatly influence TEER values of BBB models, as reviewed earlier [4][12], the present overview focuses on the effects of physical and physicochemical parameters on TEER measurements.
When the electrical properties of brain endothelial cell monolayers or microvessels are measured, the properties of the periphery are also included in the results (Figure 1). The resistance of the medium (in vivo: blood, extracellular fluid; in vitro: culture medium), as well as the culture membrane of inserts, and the resistance and capacitance of the electrodes have to be excluded. Thus, these parameters have to be measured before the experiment to determine the blank resistance. In the case of culture models, inserts without cells should be used to measure this background resistance. It is very important to coat the membrane in a similar way as for cells, and at least a 30-min equilibration time is needed in complete culture medium before measurements. The blank value has to be subtracted from the measured resistance to receive the resistance of the microvessel or cell layer.
Temperature has a key importance in TEER measurement, since conductivity exhibits a linear relationship with the ion mobility (Nernst–Einstein equation). On the other hand, the ion mobility in an electrolyte exhibits an exponential type of relationship with the temperature [30]. This means that the TEER of BBB culture models is measured either inside the culture incubator or at room temperature. The advantage of chamber electrodes, cellZscope, and chip devices with continuous TEER monitoring is that they allow measurements in incubators. For hand-held chopstick electrodes, the measurement is made outside the culture incubators and often at room temperature. To avoid any temperature-based fluctuations, heating pads set to 37 °C are used for quick measurements, or a temperature equilibration period is used before monitoring TEER. The equilibration time depends on the volume of the fluid compartments and the material of the culture insert. In general, this time interval is between 5 and 40 min which should be determined for individual laboratory conditions. However, a longer equilibration period may compromise the barrier function of the endothelial cell layers due to temperature drop and also lead to a shift in the pH of the bicarbonate-based culture medium to more alkaline. Another possibility is the mathematical correction of the measured TEER values [31]. The correlation between temperature and TEER is measured, and an empirical formula is determined. This method saves the time of equilibration and eliminates temperature-caused fluctuation between separate experiments; however, the calibration has to be performed for each device and insert type, as well as formatted to make it compatible for a wider use. To avoid the problems related to temperature variations, a heating pad set to 37 °C can be placed under the culture plates or chamber electrodes.
The viscosity of the culture medium also influences the TEER of BBB models by decreasing the ion mobility in the system [30], thus increasing the TEER. In static BBB models, the widely used culture media show a viscosity close to that of isotonic saline (~1 mPa·s), and only its temperature dependence is expected to influence TEER to a moderate extent, as described above. In dynamic BBB models that mimic the dynamic viscosity of blood (3–4 mPa·s) by adding colloids (e.g., 3.5% dextran) to the culture medium [32], other effects may occur, mainly due to the increased shear stress and osmotic pressure exerted by the viscosity-increasing additives. Hydroxyethyl starches (HES) are used as blood volume expanders in patients with severe blood loss. The dynamic viscosity of a 6% HES solution (HES 130/0.4, Volulyte®, Fresenius Kabi, Germany) is 2.5 mPa·s [33]. In a recent study, the effect of HES 130/0.4 was tested on a BBB culture model, and a barrier-tightening effect was found according to marker molecule permeability and tight junction protein expression [34].
In the presence of 4% HES, the resistance of cell culture inserts (without cells) increased by 16%, while it was elevated by 24% in the case of the BBB model (Figure 3). Our data indicate that higher culture medium viscosity can directly increase TEER values of BBB models.
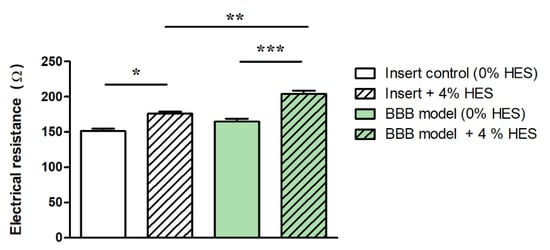
Figure 3. Electrical resistance of immortalized mouse cerebellar capillary endothelial cells (cerebEND) after hydroxyethylstarch (HES) treatment. Transparent columns: cell culture inserts without cells. Green columns: cell culture inserts with the cerebEND cell line modeling the blood–brain barrier (BBB) in vitro. The control groups contained 0% HES. Data are means ± SEM, n = 6–13, two-way ANOVA with Bonferroni post-test; * p < 0.05, *** p < 0.01, *** p < 0.001. Modified from [34].
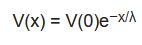
References
- Abbott, N.J.; Rönnbäck, L.; Hansson, E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat. Rev. Neurosci. 2006, 7, 41–53.
- Abbott, N.J.; Patabendige, A.A.; Dolman, D.E.; Yusof, S.R.; Begley, D.J. Structure and function of the blood-brain barrier. Neurobiol. Dis. 2010, 37, 13–25.
- Crone, C.; Olesen, S.P. Electrical resistance of brain microvascular endothelium. Brain Res. 1982, 241, 49–55.
- Deli, M.A.; Abrahám, C.S.; Kataoka, Y.; Niwa, M. Permeability studies on in vitro blood-brain barrier models: Physiology, pathology, and pharmacology. Cell. Mol. Neurobiol. 2005, 25, 59–127.
- Benson, K.; Cramer, S.; Galla, H.J. Impedance-based cell monitoring: Barrier properties and beyond. Fluids Barriers CNS 2013, 10, 5.
- Rutten, M.J.; Hoover, R.L.; Karnovsky, M.J. Electrical resistance and macromolecular permeability of brain endothelial monolayer cultures. Brain Res. 1987, 425, 301–310.
- Helms, H.C.; Abbott, N.J.; Burek, M.; Cecchelli, R.; Couraud, P.O.; Deli, M.A.; Förster, C.; Galla, H.J.; Romero, I.A.; Shusta, E.V.; et al. In vitro models of the blood-brain barrier: An overview of commonly used brain endothelial cell culture models and guidelines for their use. J. Cereb. Blood Flow Metab. 2016, 36, 862–890.
- Cucullo, L.; Hossain, M.; Tierney, W.; Janigro, D. A new dynamic in vitro modular capillaries-venules modular system: Cerebrovascular physiology in a box. BMC Neurosci. 2013, 14, 18.
- van der Helm, M.W.; van der Meer, A.D.; Eijkel, J.C.; van den Berg, A.; Segerink, L.I. Microfluidic organ-on-chip technology for blood-brain barrier research. Tissue Barriers 2016, 4, e1142493.
- Raimondi, I.; Izzo, L.; Tunesi, M.; Comar, M.; Albani, D.; Giordano, C. Organ-On-A-Chip in vitro Models of the Brain and the Blood-Brain Barrier and Their Value to Study the Microbiota-Gut-Brain Axis in Neurodegeneration. Front. Bioeng. Biotechnol. 2020, 7, 435.
- Reichel, A.; Begley, D.J.; Abbott, N.J. An overview of in vitro techniques for blood-brain barrier studies. Blood Brain Barrier 2003, 89, 307–324.
- Veszelka, S.; Kittel, Á.; Deli, M.A. Tools of modelling blood-brain barrier penetrability. In Solubility, Delivery and ADME Problems of Drugs and Drug-Candidates; Tihanyi, K., Vastag, M., Eds.; Bentham Science Publ. Ltd.: Washington, DC, USA, 2011; pp. 166–188.
- Veszelka, S.; Tóth, A.; Walter, F.R.; Tóth, A.E.; Gróf, I.; Mészáros, M.; Bocsik, A.; Hellinger, E.; Vastag, M.; Rákhely, G.; et al. Comparison of a Rat Primary Cell-Based Blood-Brain Barrier Model with Epithelial and Brain Endothelial Cell Lines: Gene Expression and Drug Transport. Front. Mol. Neurosci. 2018, 11, 166.
- Santa-Maria, A.R.; Heymans, M.; Walter, F.R.; Culot, M.; Gosselet, F.; Deli, M.A.; Neuhaus, W. Transport Studies Using Blood-Brain Barrier In Vitro Models: A Critical Review and Guidelines. Handb. Exp. Pharmacol. 2020.
- van der Helm, M.W.; Odijk, M.; Frimat, J.P.; van der Meer, A.D.; Eijkel, J.C.T.; van den Berg, A.; Segerink, L.I. Direct quantification of transendothelial electrical resistance in organs-on-chips. Biosens. Bioelectron. 2016, 85, 924–929.
- Odijk, M.; van der Meer, A.D.; Levner, D.; Kim, H.J.; van der Helm, M.W.; Segerink, L.I.; Frimat, J.P.; Hamilton, G.A.; Ingber, D.E.; van den Berg, A. Measuring direct current trans-epithelial electrical resistance in organ-on-a-chip microsystems. Lab Chip. 2015, 15, 745–752.
- Gróf, I.; Bocsik, A.; Harazin, A.; Santa-Maria, A.R.; Vizsnyiczai, G.; Barna, L.; Kiss, L.; Fűr, G.; Rakonczay, Z.J.; Ambrus, R.; et al. The Effect of Sodium Bicarbonate, a Beneficial Adjuvant Molecule in Cystic Fibrosis, on Bronchial Epithelial Cells Expressing a Wild-Type or Mutant CFTR Channel. Int. J. Mol. Sci. 2020, 21, 4024.
- Grimnes, S.; Martinsen, O.G. Bioimpedance and Bioelectricity Basics, 3rd ed.; Academic Press: Cambridge, MA, USA, 2015; pp. 1–411.
- Krug, S.M.; Fromm, M.; Günzel, D. Two-path impedance spectroscopy for measuring paracellular and transcellular epithelial resistance. Biophys. J. 2009, 97, 2202–2211.
- Solly, K.; Wang, X.; Xu, X.; Strulovici, B.; Zheng, W. Application of real-time cell electronic sensing (RT-CES) technology to cell-based assays. Assay Drug Dev. Technol. 2004, 2, 363–372.
- Müller, S.M.; Ebert, F.; Bornhorst, J.; Galla, H.J.; Francesconi, K.A.; Schwerdtle, T. Arsenic-containing hydrocarbons disrupt a model in vitro blood-cerebrospinal fluid barrier. J. Trace Elem. Med. Biol. 2018, 49, 171–177.
- Kuzmanov, I.; Herrmann, A.M.; Galla, H.J.; Meuth, S.G.; Wiendl, H.; Klotz, L. An In Vitro Model of the Blood-brain Barrier Using Impedance Spectroscopy: A Focus on T Cell-endothelial Cell Interaction. J. Vis. Exp. 2016, 118, 54592.
- Hodgkin, A.L. The ionic basis of electrical activity in nerve and muscle. Biol. Rev. 1951, 26, 339–409.
- Geddes, L.A. Who introduced the tetrapolar method for measuring resistance and impedance? IEEE Eng. Med. Biol. Mag. 1996, 15, 133–134.
- Neuhaus, W.; Lauer, R.; Oelzant, S.; Fringeli, U.P.; Ecker, G.F.; Noe, C.R. A novel flow based hollow-fiber blood-brain barrier in vitro model with immortalised cell line PBMEC/C1-2. J. Biotechnol. 2006, 125, 127–141.
- Walter, F.R.; Valkai, S.; Kincses, A.; Petneházi, A.; Czeller, T.; Veszelka, S.; Ormos, P.; Deli, M.A.; Dér, A. A versatile lab-on-a-chip tool for modeling biological barriers. Sens. Actuators B Chem. 2016, 222, 1209–1219.
- Kincses, A.; Santa-Maria, A.R.; Walter, F.R.; Dér, L.; Horányi, N.; Lipka, D.V.; Valkai, S.; Deli, M.A.; Dér, A. A chip device to determine surface charge properties of confluent cell monolayers by measuring streaming potential. Lab Chip 2020, 20, 3792–3805.
- Yeste, J.; Illa, X.; Gutiérrez, C.; Solé, M.; Guimerà, A.; Villa, R. Geometric correction factor for transepithelial electrical resistance measurements in transwell and microfluidic cell cultures. J. Phys. D Appl. Phys. 2016, 49, 375401.
- Deli, M.A.; Dehouck, M.P.; Abrahám, C.S.; Cecchelli, R.; Joó, F. Penetration of small molecular weight substances through cultured bovine brain capillary endothelial cell monolayers: The early effects of cyclic adenosine 3’,5’-monophosphate. Exp. Physiol. 1995, 80, 675–678.
- Crank, J. The Mathematics of Diffusion, 2nd ed.; Oxford University Press: Oxford, UK, 1975; pp. 352–374.
- Blume, L.-F.; Denker, M.; Gieseler, F.; Kunze, T. Temperature corrected transepithelial electrical resistance (TEER) measurement to quantify rapid changes in paracellular permeability. Die Pharm. 2010, 65, 19–24.
- Park, T.-E.; Mustafaoglu, N.; Herland, A.; Hasselkus, R.; Mannix, R.; FitzGerald, E.A.; Prantil-Baun, R.; Watters, A.; Henry, O.; Benz, M.; et al. Hypoxia-enhanced Blood-Brain Barrier Chip recapitulates human barrier function and shuttling of drugs and antibodies. Nat. Commun. 2019, 10, 2621.
- Walker, A.M.; Xiao, Y.; Johnston, C.R.; Rival, D.E. The viscous characterization of hydroxyethyl starch (HES) plasma volume expanders in a non-Newtonian blood analog. Biorheology 2013, 50, 177–190.
- Gerhartl, A.; Hahn, K.; Neuhoff, A.; Friedl, H.P.; Förster, C.Y.; Wunder, C.; Schick, M.; Burek, M.; Neuhaus, W. Hydroxyethylstarch (130/0.4) tightens the blood-brain barrier in vitro. Brain Res. 2020, 1727, 146560.
More
Information
Subjects:
Biophysics
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.6K
Revisions:
2 times
(View History)
Update Date:
25 Jun 2021
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




