
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Beatriz Martins | + 3290 word(s) | 3290 | 2021-05-07 09:52:00 | | | |
| 2 | Peter Tang | Meta information modification | 3290 | 2021-06-24 05:45:36 | | |
Video Upload Options
Methylmercury (MeHg) toxicity is a major environmental concern. In the aquatic reservoir, MeHg bioaccumulates along the food chain until it is consumed by riverine populations. The central nervous system is particularly susceptible to the deleterious effects of MeHg, as evidenced by clinical symptoms and histopathological changes in poisoned humans. In vitro and in vivo studies have been crucial in deciphering the molecular mechanisms underlying MeHg-induced neurotoxicity. A collection of cellular and molecular alterations including cytokine release, oxidative stress, mitochondrial dysfunction, Ca2+ and glutamate dyshomeostasis, and cell death mechanisms are important consequences of brain cells exposure to MeHg.
1. Introduction
Mercury (Hg) is a toxic metal with recognized adverse health impacts [1][2][3]. It is ubiquitously distributed across the globe and considered to be one of the major environmental pollutants, widely used by humans for centuries in several activities such as agriculture, industry, and medicine [4][5][6][7][8].
The US Government Agency for Toxic Substances and Disease Registry considers Hg to be the third most toxic substance on the planet [9]. In the same line, the World Health Organization considers Hg as one of the ten chemicals of major public health concern [10]. Due to its nature, it can be reduced, but never completely removed or destroyed [4][10].
Hg may exist in a variety of forms including elemental Hg (Hg0), inorganic, and organic compounds [4][8][9][10]. Elemental Hg is liquid at room temperature and is easily converted to vapor and emitted into the atmosphere [4][9]. Inorganic Hg occurs in two forms (mercurous, Hg+; mercuric, Hg2+) that are usually in the solid state as salts. Organic mercury is produced by the combination with carbon, generating MeHg and ethylmercury [4][7]. Dimethylmercury is among the most dangerous mercury compounds. This is a highly toxic and deadly compound if in direct contact with skin or even through latex gloves [9]. Even though all forms of mercury have toxicity capacity, alkylmercury compounds are of particular concern because of their easy penetration of biological membranes, effective bioaccumulation, and long-term removal from tissues [11].
2. Environmental Impact of Mercury
2.1. Mercury Cycle
Mercury is naturally present at low levels in the environment [12]. However, anthropogenic activities may release large amounts of Hg into the environment, leading to widespread pollution. The natural causes of Hg emissions and distribution comprise volcanic and geothermal activity, land use, biomass burning, and meteorological events [6]. On the other hand, coal combustion, fluorescent lighting, cement production, amalgam fillings, crematoria, paper production, gold mining, perfume and fur industries are some examples of anthropogenic sources that add up to the natural counterparts [2][6][9][13][14][15]. Despite the fact that Hg originates from both natural and human sources, reports attribute environmental mercury contamination mainly due to human actions [8].
Hg is found throughout the biosphere, arising in water, soil, air, and living beings [6]. The major earth reservoirs of Hg include the atmosphere, the terrestrial ecosystems, and the aquatic compartments (which include both freshwater and the oceans). Hg is actively exchanged between these pools, creating the so-called Mercury Cycle [16]. Environmental Hg net pools and interactions are interchangeable and highly modulated by human activities. The Mercury Cycle experiences disturbances derived from alterations in anthropogenic activities like soil use and climate changes which mobilize Hg that has been accumulated and naturally stabilized in each environmental reservoir [16].
Due to its volatile nature, under normal conditions, Hg vaporizes and spreads through atmospheric processes [9][17][18]. Since the industrial revolution, human activities substantially increased the emissions of atmospheric gaseous mercury, almost tripling the amounts of Hg in the atmosphere [8][9][18]. It is the consensus that human influences have drastically changed the global cycle of Hg [14]. These alterations have been accelerated with rapid urban human population growth and industrialization since the 1970s [16]. Hg emanated into the atmosphere has a long lifetime (ranging between 6 and 12 months), facilitating its global dissemination [14]. In this way, Hg is ultimately deposited onto land or into water, even in remote areas [6][18]. Once deposited, Hg can be methylated, generating MeHg with high toxicity properties [4][6][19].
In the terrestrial reservoir, the largest Hg pool is located in soil [18], with the potential to enter the food chain via vegetables and livestock [9]. Nevertheless, vegetation is a low-level source of Hg [18]. Mangrove ecosystems, which constitute a mixture of both terrestrial and aquatic interactions, greatly contribute to Hg storage and transport [20]. Anthropogenic actions have considerably enhanced the accumulation of Hg into this ecosystem. Additionally, the terrestrial compartment is a significant indirect source of atmospheric Hg to aquatic systems via soil drainage [18]. However, unlike air emissions releases to land do not immediately circulate on a global scale [14].
The largest bulk of Hg inputs to water compartments comes from atmospheric deposition [21]. Oceans constitute a considerable fraction of the global Hg reservoir [16]. Hg may be transported by river and ocean circulation and settle in sediments or may be dissolved to its gaseous form and evade back to the atmosphere [16][21]. Curiously, reports indicate that Hg concentrations are lower in waters with more salinity [1][22]. Higher levels of Hg have been detected in freshwater environments than coastline waters and the open ocean, which is consistent with the fact that Hg releases to water do not instantly spread worldwide [14][17][20]. Similarly to the terrestrial reservoir, MeHg production in aquatic ecosystems emerges mainly from bacterial methylation of Hg [16].
2.2. Methylmercury Biomagnification
Most attention regarding Hg pollution is focused on MeHg [18]. MeHg is not only the most common form of organic Hg found in nature but also the main source of organic Hg in ecosystems, with an ability to accumulate in the aquatic food chain [9][18]. Since MeHg is created in the environment, its dynamics may be different from those of inorganic Hg [14]. Wetlands are seen as places for MeHg production in sediments, which can be transported to coastal waters and may end up entering the food chain [20]. In fact, this environment has ideal conditions for Hg methylation which include elevated temperature, low pH levels, high carbon availability, and substrate to support bacterial methylation activity [16][20][23]. Additionally, sunlight radiation and industrial activities are also routes for MeHg formation [5][7].
Afterwards, MeHg bioaccumulates in the marine food-web [7]. It is important to notice that water chemistry is crucial to determine MeHg concentrations in the food-chain due to the regulation of MeHg uptake at the bottom of the food chain [24].
Starting with phytoplankton, there is already an accumulation of MeHg above the concentration of the surrounding waters by passively diffusing across the cell membrane [23][24][25][26]. Then, MeHg accumulates from invertebrates like zooplankton and clams to mid-trophic fishes and marine mammals such as seals, reaching the highest concentrations in the top predators, a process known as biomagnification [5][8][23]. Moreover, MeHg accumulated in organisms may be also produced by the hosts themselves in a process that involves both coenzymes and microbial activities in their body or gut [27]. The conversion of Hg to MeHg has also been detected in vivo in marine species [27]. The accumulation of MeHg in muscle tissues of predatory fish and shellfish constitutes an issue for humans that consume seafood, establishing this route as the main source of exposure to humans [7][21][28]. The exacerbated amounts of MeHg in higher trophic levels of the marine food web differ greatly from other metals whose concentrations either decline or stabilize with increasing levels in the aquatic food chain [24].
The fact that Hg concentration in vegetation is low makes terrestrial herbivores less exposed to this toxic metal. In fact, bioaccumulation of MeHg is less pronounced in terrestrial food chains than the aquatic equivalents. Because of this, the terrestrial pathway is not a relevant supplier of Hg to animals at higher levels of the food chain. Accordingly, piscivorous predators have higher MeHg loads than solely terrestrial predators [18].
2.3. The Minamata Disaster
The deadly consequences of organic mercury compounds have been demonstrated by mass-poisonings of human populations [29]. A severe MeHg intoxication occurred in Minamata and neighboring communities in Japan between the 1950s and 1960s [6][28][30]. This was the first and most famous event of severe MeHg poisoning caused by anthropogenic activities, later known as Minamata Disease [7][29][31]. The pollutant was produced from mercury as a by-product of acetaldehyde and vinyl compounds manufacturing by Chisso Co. Ltd. in Minamata City and discharged into the Minamata Bay [7][28][30]. This way, the population of the Minamata area that largely depended on fish and shellfish consumption from the Minamata Bay was contaminated with MeHg [7][28][31][32]. The Minamata disaster was a stereotypical case of biomagnification of MeHg through trophic levels that ended up in human exposure [29].
Affected individuals exhibited symptoms that indicate neurological alterations which include ataxia, visual field and hearing alterations, dysarthria, paresthesias in the distal parts of extremities, disequilibrium, gait impairment, tremors, muscle weakness, atypical eye movement, and seizures [28][29][30][31][33]. Occasionally, mental disorders and disturbances of smell and taste were also present [28]. Beyond injuries of the nervous system (the primary target of MeHg), other organs suffer minor damages, including kidneys and liver, pancreatic islet alterations, lymph node atrophy, and gastrointestinal tract inflammation [5][33]. Ultimately, MeHg led to the death of some poisoned locals [29].
Furthermore, MeHg is associated with fetotoxicity translating into miscarriage, stillbirth, low birth weights, and spontaneous abortions [9]. A significant number of fetuses was exposed to MeHg in utero during this period [31]. These children were born with conditions of cerebral palsy, intellectual disability, ataxia, and hypersalivation [28][31]. This is explained by the fact that mercury amounts in cord blood tend to be greater than in maternal blood since Hg is known to easily cross the placenta [9][34]. The fetal brain is more vulnerable to the noxious effects of MeHg, which translates into a disruption of the cerebral architecture and severe mental deficits, as previously mentioned [5]. Additionally, recent in vitro studies with human trophoblastic cells have been providing insights into pregnancy-related diseases caused by MeHg [35]. Another striking finding was the unexpectedly low numbers of males born in Minamata following the environmental disaster [7]. Over 50 cases of Fetal Minamata Disease were diagnosed, revealing a special sensitivity of fetal toxicity even in the absence of mother’s symptoms [7][8][28][29][31]. Fetal exposure to MeHg in Minamata has carried consequences until the present day, since older adult patients born in Minamata in the 1950s exhibit steeper declines in physical and cognitive functions than subjects of similar ages born in the same period but in non-affected areas [31].
Alongside clinical symptoms of MeHg intoxication in the population, locals reported strange phenomena namely agglomerations of fish on the surface of the Bay with outlandish swimming behaviors, sea birds incapable of flying properly and cats constantly drooling and running in circles [30]. According to the locals, the death of cats, dogs, and pigs was a recurrent event during this period, resembling a similar pattern to the increase of human patients [32].
A second episode of MeHg poisoning happened in 1965, derived from the same acetaldehyde production factory. As a measure to diminish the MeHg content in Minamata Bay, discharges were directed into the Agano River, which caused another outbreak that affected Niigata [5]. This incident is popularly known as the “Niigata Minamata Disease”. Chisso Co. Ltd. eventually ceased functions in May 1969 [7]. As this event was the first case of MeHg intoxication caused by environmental pollution, it took many years to establish the cause of the outbreak [7]. The recognition of mercury’s environmental impacts and acceptance of its global distribution needed decades of expert research [6]. Preservation and analysis of children’s umbilical cords tissue born in the region were crucial to assess MeHg exposure levels and estimate the time-course alterations in MeHg pollution [7].
2.4. Additional Impactful Events of MeHg Poisoning
A similar incident to the Minamata Disaster occurred in Iraq in the early 1970s [9]. Thousands of cases were admitted to hospitals with severe intoxication, and many passed away [7][36]. The consumption of bread made from wheat seed treated with MeHg-based fungicides was determined as the cause of this epidemic [5]. Several incidents of poisoning have been reported in Pakistan and Guatemala due to the ingestion of flour and wheat seed treated with MeHg compounds [36]. In the same decade, residents of Grassy Narrows, Ontario, Canada showed evidence of neurological alterations which met the criteria for MeHg poisoning, after mercury contamination of the local aquatic system [5].
Local Brazil populations are also exposed to small-scale gold mining activities and mangrove pollution [3][37][38]. The region of Tapajós is one of the largest gold production areas in Brazil, making it a site of chronic exposure to mercury compounds. Human communities living in this environment have been chronically exposed since the 1980s, especially those living in riverside areas. It is believed that working conditions and fish consumption are at the core of MeHg accumulation, raising levels of organic compounds to 70% of total Hg in the blood of mining workers [3][11]. Locals were described with alterations of motor and visual performances, as well as teratogenesis and carcinogenesis, though the latter were difficult to relate to MeHg due to their multifactorial origin [11]. Recently, also in Brazil, two significant environmental disasters occurred in two important ore tailings dams in Minas Gerais (Brumadinho and Mariana) [39]. Thus, the exposed population has become vulnerable to the deleterious low (chronic) level of MeHg, which can lead to low-intensity cognitive impairments, neurodegenerative diseases, and premature aging. This important public health issue should raise the awareness of the Brazilian governmental authorities about the consequences of human exposure to MeHg [39][40].
Other impactful historical events related to MeHg poisoning have been documented around the world, such as the United States, Somalia, Russia, China, England, or Zimbabwe [9][41].
2.5. The Minamata Convention
It is unclear how anthropogenic actions will affect the Hg cycle in the years to come. Forestry practices and wildfires may affect watershed Hg processes and MeHg bioaccumulation with a direct impact on terrestrial and aquatic ecosystems. Both oceans and freshwater compartments may be influenced by climate-induced alterations. The tendency for MeHg generation may be increased as a consequence of organic matter remineralization due to increasing seawater temperatures. Regarding the atmospheric reservoir, Hg concentrations are decreasing in Europe and North America whereas the opposite trend happens in Asia [16].
Reports from the United Nations stated that Hg emissions were increasing in developing countries due to coal-burning and gold mining activities [10]. Actions on Hg products and waste could have a beneficial impact in minimizing local exposure to Hg, mainly in developing countries and specific populations such as miners, fishermen, and their families [14]. Regulations and emission control technologies could be valuable to enhance the capabilities of countries to address this problem by implementing safe handling and disposal of Hg containing products [6][14]. Protection of the food-web from exposure to mercury compounds is an important mission for the protection of the population [6]. Other actions that may have a positive impact include the use of clean energy sources that do not rely on coal combustion and switching to non-mercury thermometers and sphygmomanometers in health care [6]. Global greenhouse pollution mitigation measures are believed to contribute to the reduction of anthropogenic Hg emissions [16].
Several international measures have been taken in recent decades to reduce mercury contamination of the environment and ultimately to prevent noxious consequences for ecosystems and human health [14]. The Minamata Convention is a global treaty that signs the commitment by governments of more than 140 nations to diminish Hg emissions and usage in order to reduce harm to both humans and the environment [10][14][42][43]. Nevertheless, it is not clear if the implementation of measures will effectively lead to ecological improvements [14]. Choices made by governments will certainly influence future actions to mitigate Hg emissions and control. However, predictions state that the global Hg cycle will continue to be vulnerable to emissions for decades [16].
3. Toxicokinetic Properties of MeHg
Toxicokinetics provides knowledge about how the body affects a toxicant through absorption, distribution, metabolism, and excretion in a time-dependent manner. In general, the toxicity of a xenobiotic depends on the chemical form, time, dose, and pathway of exposure as well as on individual susceptibility to compound hazards [44]. Because of the hazard of MeHg and its long half-life within the human body, the knowledge of its toxicokinetic behavior is important to address risk assessment [45]. From a chemical point of view, MeHg is characterized as a soft acid because the acceptor atom is of low positive charge, large size and has several easily excited outer electrons. This molecule has a high affinity for nucleophilic groups, such as -SeH and -SH groups presented on the structure of several biomolecules [46].
Consequently, the toxicokinetic behavior of MeHg is ultimately conditioned by the chemical profile of this organomercurial compound. The electrophilic nature of MeHg facilitates the reaction with several thiol groups leading to S-mercuriation of several proteins, allowing its transfer between nucleophilic molecules. These interactions modify the protein structure, oxidative state, and biological function [47].
Although all mercurial forms have known deleterious effects on human health at high doses, recent evidence indicates an association between chronic low doses of MeHg and cytotoxic effects, mainly in the central nervous system (CNS) [48][49][50]. Pathological examination of patients poisoned with MeHg showed that brain tissue loss is mainly found in occipito-temporal lobes, cortical areas, and cerebellum [51][52]. The damage of MeHg on the occipital lobe, including the primary visual area, or the primary auditory area in the temporal lobe is responsible for alterations in visual field and loss of auditory acuity, common symptoms after a mercuric-related disaster [53][54]. Visual disturbances might be correlated with MeHg binding to outer segments of photoreceptor cells [54]. Regarding the noxious effect on cerebellum, the most susceptible described region to MeHg toxicity, loss of cerebellar glutamatergic granule cells is potentially associated with ataxia, locomotor impairment and occasionally tonic seizures. A recent case report study described several glial reactivity and neuronal loss the occipital lobe and cerebellum in a postmortem toxicological analysis of a 40-year-old man who suffered a fatal intoxication caused by injection of a fluid containing organic mercury [55].
4. Cellular and Molecular Mechanisms Involved in MeHg Neurotoxicity
Understanding how MeHg leads to cytotoxicity is crucial to determine the process that underlies biological perturbations that lead up to the recognized signs of MeHg intoxication [8]. Despite the numerous studies, the mechanisms by which MeHg induces toxicity have not been fully disclosed.
MeHg-induced neurotoxic effects are intimately related with the capacity of this compound to readily disturb and cross BBB (Figure 1B) [56][57][58]. Growing in vitro and in vivo evidence indicates an association between MeHg exposure and BBB dysfunction, with immunoglobulin extravasation, decreased expression of endothelial cell antigen-1, and vascular endothelial growth factor (VEGF) upregulation [59][60]. In fact, VEGF and VEGF receptor-1/-2 expression is enhanced in endothelial cells upon MeHg exposure, which is relevant since VEGF is able to induce vessel hyperpermeability and subsequently vascular leakage [59][60]. Moreover, MeHg leads to delayed maturation of vessels which translates into a defective barrier property [56].
The developing nervous system seems to be sensitive to noxious effects of MeHg, but the adult system can also be affected, particularly following long-scale environmental accidents. Organotypic cultures of rat cerebellar cultures exposed to MeHg showed a delay in synaptogenesis, impairment in cell migration, and a disorganized cerebellar architecture [61][62][63]. Since prenatal exposure to MeHg is implicated in childhood cognitive deficit, especially in learning and memory, hippocampal neurogenesis has been pointed out as a likely vulnerable target of MeHg noxious effect. In fact, despite the non-existence of clear evidence in humans, in vivo experimental models reported pathophysiological alterations in hippocampus following both perinatal and post-natal MeHg exposure [64][65][66].
At the cellular level, MeHg has been correlated with changes in oxidative stress, increase in excitotoxicity, deoxyribonucleic acid (DNA) damage, alterations in neurogenesis, calcium (Ca2+) dyshomeostasis, exacerbation of neuroinflammation, and concomitantly cell death mechanisms (Figure 1) [9][12][56][67][68][69].
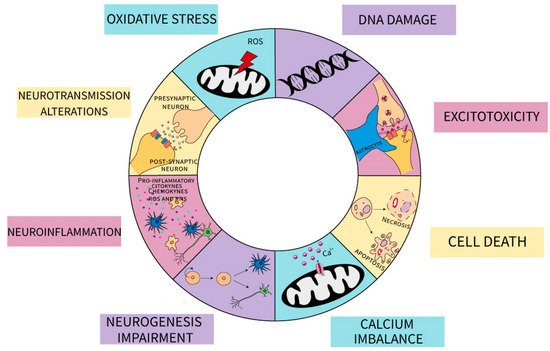
Figure 1. Schematic representation of the impact of MeHg on neuronal and glial cells. The different mechanisms are indicated: oxidative stress mediated by mitochondrial production of reactive oxygen species (ROS) and decrease in antioxidant defenses; DNA damage; excitotoxicity due to changes in both astrocytic and neuronal glutamate metabolism; cell death by apoptotic and necrotic pathways; Calcium imbalance characterize by an increase in [Ca2+] cytoplasmatic; neurogenesis impairment both in neuronal and glial-committed lineages; exacerbation in neuroinflammation by an increase in proinflammatory mediators released by both glial and neuronal cells as cytokines, chemokines, ROS and reactive nitrogen species (RNS); changes in synaptic neurotransmission.
References
- Cesario, R.; Mota, A.M.; Caetano, M.; Nogueira, M.; Canario, J. Mercury and methylmercury transport and fate in the water column of Tagus estuary (Portugal). Mar. Polut. Bull. 2018, 127, 235–250.
- Pacyna, E.G.P.; Pacyna, J.M.; Sundseth, K.; Munthe, J.; Kindbom, K.; Wilson, S.; Steenhuisen, F.; Maxson, P. Global emission of mercury to the atmosphere from anthropogenic sources in 2005 and projections to 2020. Atmos. Environ. 2010, 44, 2487–2499.
- Pinheiro, M.C.; Crespo-Lopez, M.E.; Vieira, J.L.; Oikawa, T.; Guimaraes, G.A.; Araujo, C.C.; Amoras, W.W.; Ribeiro, D.R.; Herculano, A.M.; do Nascimento, J.L.; et al. Mercury pollution and childhood in Amazon riverside villages. Environ. Int. 2007, 33, 56–61.
- Park, J.D.; Zheng, W. Human exposure and health effects of inorganic and elemental mercury. J. Prev. Med. Public Health 2012, 45, 344–352.
- Jackson, A.C. Chronic Neurological Disease Due to Methylmercury Poisoning. Can. J. Neurol. Sci. 2018, 45, 620–623.
- Kimakova, T.; Nasser, B.; Issa, M.; Uher, I. Mercury cycling in the terrestrial, aquatic and atmospheric environment of the Slovak Republic—An overview. Ann. Agric. Environ. Med. 2019, 26, 273–279.
- Sakamoto, M.; Tatsuta, N.; Izumo, K.; Phan, P.T.; Vu, L.D.; Yamamoto, M.; Nakamura, M.; Nakai, K.; Murata, K. Health Impacts and Biomarkers of Prenatal Exposure to Methylmercury: Lessons from Minamata, Japan. Toxics 2018, 6, 45.
- Yang, L.; Zhang, Y.; Wang, F.; Luo, Z.; Guo, S.; Strahle, U. Toxicity of mercury: Molecular evidence. Chemosphere 2020, 245, 125586.
- Rice, K.M.; Walker, E.M., Jr.; Wu, M.; Gillette, C.; Blough, E.R. Environmental mercury and its toxic effects. J. Prev. Med. Public Health 2014, 47, 74–83.
- O’Donoghue, J.L.; Watson, G.E.; Brewer, R.; Zareba, G.; Eto, K.; Takahashi, H.; Marumoto, M.; Love, T.; Harrington, D.; Myers, G.J. Neuropathology associated with exposure to different concentrations and species of mercury: A review of autopsy cases and the literature. Neurotoxicology 2020, 78, 88–98.
- Berzas Nevado, J.J.; Rodriguez Martin-Doimeadios, R.C.; Guzman Bernardo, F.J.; Jimenez Moreno, M.; Herculano, A.M.; do Nascimento, J.L.; Crespo-Lopez, M.E. Mercury in the Tapajos River basin, Brazilian Amazon: A review. Environ. Int. 2010, 36, 593–608.
- Farina, M.; Rocha, J.B.; Aschner, M. Mechanisms of methylmercury-induced neurotoxicity: Evidence from experimental studies. Life Sci. 2011, 89, 555–563.
- Sundseth, K.; Pacyna, J.M.; Pacyna, E.G.; Pirrone, N.; Thorne, R.J. Global Sources and Pathways of Mercury in the Context of Human Health. Int. J. Environ. Res. Public Health 2017, 14, 105.
- Selin, N.E. Global change and mercury cycling: Challenges for implementing a global mercury treaty. Environ. Toxicol. Chem. 2014, 33, 1202–1210.
- Moody, K.H.; Hasan, K.M.; Aljic, S.; Blakeman, V.M.; Hicks, L.P.; Loving, D.C.; Moore, M.E.; Hammett, B.S.; Silva-Gonzalez, M.; Seney, C.S.; et al. Mercury emissions from Peruvian gold shops: Potential ramifications for Minamata compliance in artisanal and small-scale gold mining communities. Environ. Res. 2020, 182, 109042.
- Obrist, D.; Kirk, J.L.; Zhang, L.; Sunderland, E.M.; Jiskra, M.; Selin, N.E. A review of global environmental mercury processes in response to human and natural perturbations: Changes of emissions, climate, and land use. Ambio 2018, 47, 116–140.
- Beckers, F.R.; Rinklebe, J. Cycling of mercury in the environment: Sources, fate, and human health implications: A review. Crit. Rev. Environ. Sci. Technol. 2017, 47, 693–794.
- Grigal, D.F. Mercury sequestration in forests and peatlands: A review. J. Environ. Qual. 2003, 32, 393–405.
- Queipo Abad, S.; Rodriguez-Gonzalez, P.; Davis, W.C.; Garcia Alonso, J.I. Development of a Common Procedure for the Determination of Methylmercury, Ethylmercury, and Inorganic Mercury in Human Whole Blood, Hair, and Urine by Triple Spike Species-Specific Isotope Dilution Mass Spectrometry. Anal. Chem. 2017, 89, 6731–6739.
- Lei, P.; Zhong, H.; Duan, D.; Pan, K. A review on mercury biogeochemistry in mangrove sediments: Hotspots of methylmercury production? Sci. Total Environ. 2019, 680, 140–150.
- Mason, R.P.; Choi, A.L.; Fitzgerald, W.F.; Hammerschmidt, C.R.; Lamborg, C.H.; Soerensen, A.L.; Sunderland, E.M. Mercury biogeochemical cycling in the ocean and policy implications. Environ. Res. 2012, 119, 101–117.
- Leermakers, M.G.S.; De Galan, S.; Brion, N.; Baeyens, W. Mercury in the Southern North Sea and Scheldt estuary. Mar. Chem. 2001, 75, 229–248.
- Harding, G.; Dalziel, J.; Vass, P. Bioaccumulation of methylmercury within the marine food web of the outer Bay of Fundy, Gulf of Maine. PLoS ONE 2018, 13, e0197220.
- Mason, R.P.; Reinfelder, J.R.; Morel, F.M.M. Bioaccumulation of mercury and methylmercury. Water Air Soil Pollut. 1995, 80, 915–921.
- Watras, C.J.; Back, R.C.; Halvorsen, S.; Hudson, R.J.; Morrison, K.A.; Wente, S.P. Bioaccumulation of mercury in pelagic freshwater food webs. Sci. Total Environ. 1998, 219, 183–208.
- Pickhardt, P.C.; Fisher, N.S. Accumulation of inorganic and methylmercury by freshwater phytoplankton in two contrasting water bodies. Environ. Sci. Technol. 2007, 41, 125–131.
- Xu, Z.; Fan, W.; Shi, Z.; Tan, C.; Cui, M.; Tang, S.; Qiu, G.; Feng, X. Mercury and methylmercury bioaccumulation in a contaminated bay. Mar. Polut. Bull. 2019, 143, 134–139.
- Eto, K. Minamata disease. Neuropathology 2000, 20, S14–S19.
- James, A.K.; Nehzati, S.; Dolgova, N.V.; Sokaras, D.; Kroll, T.; Eto, K.; O’Donoghue, J.L.; Watson, G.E.; Myers, G.J.; Krone, P.H.; et al. Rethinking the Minamata Tragedy: What Mercury Species Was Really Responsible? Environ. Sci. Technol. 2020, 54, 2726–2733.
- Yorifuji, T. Lessons From an Early-stage Epidemiological Study of Minamata Disease. J. Epidemiol. 2020, 30, 12–14.
- Yorifuji, T.; Takaoka, S.; Grandjean, P. Accelerated functional losses in ageing congenital Minamata disease patients. Neurotoxicol. Teratol. 2018, 69, 49–53.
- Kitamura, S.; Miyata, C.; Tomita, M.; Date, S.; Kojima, T.; Minamoto, H.; Kurimoto, S.; Noguchi, Y.; Nakagawa, R. A Central Nervous System Disease of Unknown Cause That Occurred in the Minamata Region: Results of an Epidemiological Study. J. Epidemiol. 2020, 30, 3–11.
- Landrigan, P.J.; Stegeman, J.J.; Fleming, L.E.; Allemand, D.; Anderson, D.M.; Backer, L.C.; Brucker-Davis, F.; Chevalier, N.; Corra, L.; Czerucka, D.; et al. Human Health and Ocean Pollution. Ann. Glob. Health 2020, 86, 1–64.
- Vahter, M.; Akesson, A.; Lind, B.; Bjors, U.; Schutz, A.; Berglund, M. Longitudinal study of methylmercury and inorganic mercury in blood and urine of pregnant and lactating women, as well as in umbilical cord blood. Environ. Res. 2000, 84, 186–194.
- Liao, Y.; Peng, S.; He, L.; Wang, Y.; Li, Y.; Ma, D.; Wang, Y.; Sun, L.; Zheng, H.; Yang, W.; et al. Methylmercury cytotoxicity and possible mechanisms in human trophoblastic HTR-8/SVneo cells. Ecotoxicol. Environ. Saf. 2021, 207, 111520.
- Bakir, F.; Damluji, S.F.; Amin-Zaki, L.; Murtadha, M.; Khalidi, A.; al-Rawi, N.Y.; Tikriti, S.; Dahahir, H.I.; Clarkson, T.W.; Smith, J.C.; et al. Methylmercury poisoning in Iraq. Science 1973, 181, 230–241.
- Malm, O. Gold mining as a source of mercury exposure in the Brazilian Amazon. Environ. Res. 1998, 77, 73–78.
- Kehrig, H.A.P.; Pinto, F.N.; Moreira, I.; Malm, O. Heavy metals and methylmercury in a tropical coastal estuary and a mangrove in Brazil. Org. Geochem. 2003, 34, 661–669.
- Raposo, R.S.; Pinto, D.V.; Moreira, R.; Dias, R.P.; Ribeiro, C.A.F.; Oriá, R.B.; Malva1, J.O. Methylmercury Impact on Adult Neurogenesis: Is the Worst Yet to Come from Recent Brazilian Environmental Disasters? Front. Aging Neurosci. 2020, 12.
- Crespo-Lopez, M.E.; Augusto-Oliveira, M.; Lopes-Araujo, A.; Santos-Sacramento, L.; Yuki Takeda, P.; Macchi, B.M.; do Nascimento, J.L.M.; Maia, C.S.F.; Lima, R.R.; Arrifano, G.P. Mercury: What can we learn from the Amazon? Environ. Int. 2020, 146, 106223.
- Mambrey, V.; Rakete, S.; Tobollik, M.; Shoko, D.; Moyo, D.; Schutzmeier, P.; Steckling-Muschack, N.; Muteti-Fana, S.; Bose-O’Reilly, S. Artisanal and small-scale gold mining: A cross-sectional assessment of occupational mercury exposure and exposure risk factors in Kadoma and Shurugwi, Zimbabwe. Environ. Res. 2020, 184, 109379.
- Basu, N. The Minamata Convention on Mercury and the role for the environmental sciences community. Environ. Toxicol. Chem. 2018, 37, 2951–2952.
- Bishop, K.; Shanley, J.B.; Riscassi, A.; de Wit, H.A.; Eklof, K.; Meng, B.; Mitchell, C.; Osterwalder, S.; Schuster, P.F.; Webster, J.; et al. Recent advances in understanding and measurement of mercury in the environment: Terrestrial Hg cycling. Sci. Total Environ. 2020, 721, 137647.
- Gupta, P.K. (Ed.) Chapter 9—Principles and basic concepts of toxicokinetics. In Fundamentals of Toxicology; Academic Press: Cambridge, MA, USA, 2016; pp. 87–107.
- Jo, S.; Woo, H.D.; Kwon, H.-J.; Oh, S.-Y.; Park, J.-D.; Hong, Y.-S.; Pyo, H.; Park, K.S.; Ha, M.; Kim, H.; et al. Estimation of the Biological Half-Life of Methylmercury Using a Population Toxicokinetic Model. Int. J. Environ. Res. Public Health 2015, 12, 9054–9067.
- Nogara, P.A.; Oliveira, C.S.; Schmitz, G.L.; Piquini, P.C.; Farina, M.; Aschner, M.; Rocha, J.B.T. Methylmercury’s chemistry: From the environment to the mammalian brain. Biochim. Biophys. Acta Gen. Subj. 2019, 1863, 129284.
- Farina, M.; Aschner, M. Glutathione antioxidant system and methylmercury-induced neurotoxicity: An intriguing interplay. Biochim. Biophys. Acta Gen. Subj. 2019, 1863, 129285.
- Montgomery, K.S.; Mackey, J.; Thuett, K.; Ginestra, S.; Bizon, J.L.; Abbott, L.C. Chronic, low-dose prenatal exposure to methylmercury impairs motor and mnemonic function in adult C57/B6 mice. Behav. Brain Res. 2008, 191, 55–61.
- Crespo-Lopez, M.E.; Costa-Malaquias, A.; Oliveira, E.H.C.; Miranda, M.S.; Arrifano, G.P.F.; Souza-Monteiro, J.R.; Sagica, F.E.-S.; Fontes-Junior, E.A.; Maia, C.S.F.; Macchi, B.M.; et al. Is Low Non-Lethal Concentration of Methylmercury Really Safe? A Report on Genotoxicity with Delayed Cell Proliferation. PLoS ONE 2016, 11, e0162822.
- Li, P.; Du, B.; Chan, H.M.; Feng, X.; Li, B. Mercury bioaccumulation and its toxic effects in rats fed with methylmercury polluted rice. Sci. Total Environ. 2018, 633, 93–99.
- Antunes Dos Santos, A.; Appel Hort, M.; Culbreth, M.; López-Granero, C.; Farina, M.; Rocha, J.B.; Aschner, M. Methylmercury and brain development: A review of recent literature. J. Trace Elem. Med. Biol. 2016, 38, 99–107.
- Björkman, L.; Lundekvam, B.F.; Laegreid, T.; Bertelsen, B.I.; Morild, I.; Lilleng, P.; Lind, B.; Palm, B.; Vahter, M. Mercury in human brain, blood, muscle and toenails in relation to exposure: An autopsy study. Environ. Health 2007, 6, 30.
- Mergler, D.; Anderson, H.A.; Chan, L.H.; Mahaffey, K.R.; Murray, M.; Sakamoto, M.; Stern, A.H. Methylmercury exposure and health effects in humans: A worldwide concern. Ambio 2007, 36, 3–11.
- Korbas, M.; Lai, B.; Vogt, S.; Gleber, S.C.; Karunakaran, C.; Pickering, I.J.; Krone, P.H.; George, G.N. Methylmercury targets photoreceptor outer segments. ACS Chem. Biol. 2013, 8, 2256–2263.
- Albers, A.; Gies, U.; Raatschen, H.J.; Klintschar, M. Another umbrella murder?—A rare case of Minamata disease. Forensic Sci. Med. Pathol. 2020, 16, 504–509.
- Bertossi, M.; Girolamo, F.; Errede, M.; Virgintino, D.; Elia, G.; Ambrosi, L.; Roncali, L. Effects of methylmercury on the microvasculature of the developing brain. Neurotoxicology 2004, 25, 849–857.
- Noguchi, Y.; Shinozaki, Y.; Fujishita, K.; Shibata, K.; Imura, Y.; Morizawa, Y.; Gachet, C.; Koizumi, S. Astrocytes protect neurons against methylmercury via ATP/P2Y(1) receptor-mediated pathways in astrocytes. PLoS ONE 2013, 8, e57898.
- Shinozaki, Y.; Nomura, M.; Iwatsuki, K.; Moriyama, Y.; Gachet, C.; Koizumi, S. Microglia trigger astrocyte-mediated neuroprotection via purinergic gliotransmission. Sci. Rep. 2014, 4, 4329.
- Takahashi, T.; Shimohata, T. Vascular Dysfunction Induced by Mercury Exposure. Int. J. Mol. Sci. 2019, 20, 2435.
- Takahashi, T.; Fujimura, M.; Koyama, M.; Kanazawa, M.; Usuki, F.; Nishizawa, M.; Shimohata, T. Methylmercury Causes Blood-Brain Barrier Damage in Rats via Upregulation of Vascular Endothelial Growth Factor Expression. PLoS ONE 2017, 12, e0170623.
- Bradford, A.B.; Mancini, J.D.; Atchison, W.D. Methylmercury-Dependent Increases in Fluo4 Fluorescence in Neonatal Rat Cerebellar Slices Depend on Granule Cell Migrational Stage and GABAA Receptor Modulation. J. Pharmacol. Exp. Ther. 2016, 356, 2–12.
- Mancini, J.D.; Autio, D.M.; Atchison, W.D. Continuous exposure to low concentrations of methylmercury impairs cerebellar granule cell migration in organotypic slice culture. Neurotoxicology 2009, 30, 203–208.
- Heimfarth, L.; Delgado, J.; Mingori, M.R.; Moresco, K.S.; Pureur, R.P.; Gelain, D.P.; Moreira, J.C.F. Delayed neurochemical effects of prenatal exposure to MeHg in the cerebellum of developing rats. Toxicol. Lett. 2018, 284, 161–169.
- Obiorah, M.; McCandlish, E.; Buckley, B.; DiCicco-Bloom, E. Hippocampal developmental vulnerability to methylmercury extends into prepubescence. Front. Neurosci. 2015, 9, 150.
- Falluel-Morel, A.; Sokolowski, K.; Sisti, H.M.; Zhou, X.; Shors, T.J.; Dicicco-Bloom, E. Developmental mercury exposure elicits acute hippocampal cell death, reductions in neurogenesis, and severe learning deficits during puberty. J. Neurochem. 2007, 103, 1968–1981.
- Aragão, W.A.B.; Teixeira, F.B.; Fagundes, N.C.F.; Fernandes, R.M.; Fernandes, L.M.P.; da Silva, M.C.F.; Amado, L.L.; Sagica, F.E.S.; Oliveira, E.H.C.; Crespo-Lopez, M.E.; et al. Hippocampal Dysfunction Provoked by Mercury Chloride Exposure: Evaluation of Cognitive Impairment, Oxidative Stress, Tissue Injury and Nature of Cell Death. Oxid. Med. Cell Longev. 2018, 2018, 7878050.
- Miura, K.; Imura, N. Mechanism of methylmercury cytotoxicity. Crit. Rev. Toxicol. 1987, 18, 161–188.
- Peraza, M.A.; Ayala-Fierro, F.; Barber, D.S.; Casarez, E.; Rael, L.T. Effects of micronutrients on metal toxicity. Environ. Health Perspect. 1998, 106, 203–216.
- Charleston, J.S.; Bolender, R.P.; Mottet, N.K.; Body, R.L.; Vahter, M.E.; Burbacher, T.M. Increases in the number of reactive glia in the visual cortex of Macaca fascicularis following subclinical long-term methyl mercury exposure. Toxicol. Appl. Pharmacol. 1994, 129, 196–206.




