
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Diogo Cruz | + 4296 word(s) | 4296 | 2020-06-08 08:05:39 | | | |
| 2 | Catherine Yang | + 15 word(s) | 4311 | 2020-06-18 08:51:44 | | | | |
| 3 | Catherine Yang | -103 word(s) | 4208 | 2020-10-27 10:11:58 | | |
Video Upload Options
Cyanobacteria have the potential to become an industrially sustainable source of functional biopolymers. Their exopolysaccharides (EPS) harbor chemical complexity, which predicts bioactive potential. Cyanobacterial EPS, from the bioprocess point of view, is seen as a by-product of biomass and/or metabolite production such as proteins and to a lesser extent lipid. The slimy texture of culture medium after EPS production hinders handling while structure elucidation and productivities gave a tough reputation to these classes of polysaccharides within the industrial sector; however, the uniqueness of the few resolved structures has opened markets to these polysaccharides. To develop an EPS cyanobacterial bioprocess (Cyano-EPS) three steps are highlighted: the selection of the cyanobacterial host; optimization of production parameters; downstream processing.
1. Strain Selection
The classical approach to bioprospecting an exemplar cyanobacterial EPS producer starts by looking at the growth rate and EPS content, to determine product titer and productivity. Cyanobacteria physiology is diverse and translates into a much wider strain-specific response to the stimulus applied during cultivation. Cultivation screenings can be a useful tool to compare the performance of different strains within a predefined environment. A compilation of cultivation screenings in cyanobacteria is presented in Table 1. Despite the diversity of habitats, we can observe that the same does not happen to the orders of strains studied. Most of the reports were done on Nostocales and Oscillatoriales orders, whereas the majority of tested organisms per study were from the Nostoc genus. In addition, the EPS fraction to be recovered varied among screenings to the methodologies applied.
Table 1. Compilation of screening conditions applied to Cyano-EPS based on their origin and order.
| Origin | Order (No. Strains) | Screening Conditions (Medium, Temperature, Light/Dark Cycle, Light Intensity, Air Supply/Mixing, Inoculum Conditions, Working Volume, Cultivation Days) |
EPS Target | Reference |
|---|---|---|---|---|
| Soil, Soil/water, Water, Plant symbiosis | Nostocales (40) | BG110, 30 °C, L/D (24/0 h), 100 μE, 5% (v/v) CO2-air, agitation, axenic, working volume: 0.4 L, 10–15 days | RPS | [1] |
| nd† | Nostocales, Chroococcales, Synechococcales (15) | BG110, 25 °C, L/D (16/10 h), 35 μE, L/D, axenic, working volume: 0.1 L, 44 days | RPS | [2] |
| Freshwater | Nostocales, Synechococcales, Oscillatoriales (25) | Z medium 2x concentrated, 20 °C, L/D (24/0 h) 15 W/m2, 12-36 months | RPS | [3] |
| Marine | Oscillatoriales (4) | modified f/2 plus sea mud extract, 29 °C, L/D (24/0 h), 2700 Lux, aeration, inoculum 8 × 105 –9 × 105 cells/mL, working volume: 0.05 L, 15 days | CPS | [4] |
| Indo-Burma hotspot | Nostocales, Oscillatoriales (40) | BG110/ BG11#, 28 °C, L/D (14/10h), 54–67 μE, mixing 2x day, 50 mg of wet pellet, working volume: 0.1 L, 30 days |
EPS | [5] |
| Freshwater (Indo-Burma hotspot) | Nostocales, Oscillatoriales (10) | BG110/ BG11#, 28 °C, L/D (14/10 h), 54–67 μE, mixing 2x day, 50 mg of wet pellet, working volume: 0.1 L, 30 days |
CPS, RPS |
[6] |
| Marine microbial mat French Polynesia | Oscillatoriales, Chroococcales, Synechococcales (6) | Conway (Fed-batch), 32 °C, L/D (12/12 h), 300 μE, 0.125 (v/v/min) pH 8.35 (CO2 on demand), 250 rpm, 10% inoculum non-axenic, working volume: 2 L, 25–35 days | CPS, RPS |
[7] |
| Soil contaminated, Gujarat, India nd† | Nostocales, Oscillatriales (4) | BG11 and ASN III, 27 °C, L/D (12/12 h), 3 kLux. Axenic inoculum (chlorophyll a concentration to ∼2.0 mg/L), working volume 0.6 L, 30 days | RPS | [8] |
| Eroded soils; wastewater treatment plant; sediments; Cabras Lagoon | Nostocales, Oscillatoriales, Synechocchales, (7) | BG110/BG11#, 18 °C, L/D (14:10 h), 18 μE, working volume 0.3 L, 25–30 days (until stationary phase) | RPS | [9] |
| Freshwater lakes, Turkey | Synechocchales (3) | BG11, 25 °C, L/D (12:12 h), 1200 μE, 100rpm, working volume: 0.1 L, 20 days | CPS | [10] |
| Soil, garden | Nostocales (3) | BG110 and BG11, 30 °C, continuous illumination, 70–160 μE, aeration pH control (7–8.5) with CO2-air, working volume: 0.25 L, inoculum: chlorophyll a concentration of 1.5 mg/mL, 8–15 days (until stationary phase) | RPS | [11] |
| Miscellaneous / Culture Collections; hard sands Pantelleria island, Italy; Antarctic lake, Antarctic | Nostocales, Oscillatoriales (16) | BG110/ BG11# or Allen and Arnon or alkaline medium, 25 °C or 11 °C (psychrophilic strains), 1.500 lux, aeration pH 7–8.5 (CO2 on demand), inoculum chlorophyll concentration 1.5 or 3 mg/mL, working volume: 1.5 L, 30 days | CPS, RPS |
[12] |
| Baltic Sea, Pacific Ocean, Atlantic Ocean, Mediterranean Sea, Red Sea | Synechocchales, Spirulinales, Pleurocapsales, Nostocales, Chroococcales (16) | PCR-11 medium, 20 °C, L/D (16:8 h), 150–300 μE, 120 rpm, working volume: 0.02 L, 30 days | RPS |
[13] |
Nd†—non-defined. BG110/BG11#—nitrate source was excluded (BG110) for all heterocystous strains or maintained (BG11) for non-heterocystous.
The cultivation screenings were characterized as being too long (30 days). This is explained by the cyanobacteria slow growth rate and also to EPS production associated with the stationary phase [14]. A dilution of N:P in BG11 to a factor of 7 was shown to anticipate the stationary phase in cultures of Microcystis aeruginosa and stationary phase characteristic EPS production was also observed under these conditions [15]. It is important to understand which is the cyanobacteria’s physiological state throughout time and especially to define the period of highest EPS production. Although it is more relevant at the optimization stage, it can be used as a cut-off parameter. Applying a shorter production time (15 days) on four Cyanothece sp. strains from seawater in China’s coast grown on modified f/2 with the addition of sea mud extract, aeration, and relatively strong inoculum for a small volume of cultivation was sufficient to highlight Cyanothece 113 producing abundant quantities of a CPS (α-d-1,6-glucan).
The cyanobacteria inoculum quality (age and concentration) determines the period of acclimation, thus a lag phase in response to the transition of conditions was applied. The number of cells inoculated should be based on the steady-state of each strain, however, this methodology requires the previous knowledge of each strain’s growth curve. The EPS screening studies have rarely provided information of inoculum, though some inoculated according to a determined concentration in terms of the number of cells, the mass of cells, chlorophyll a or even just a percentage. The culture medium provides the required nutrients for cyanobacterial growth, despite the existent formulations BG11 and BG110 (absence of nitrate source) were often applied. BG110 is used for nitrogen-fixing strains mostly from Nostocales order. Under diazotrophic conditions, growth is limited and the metabolism is re-directed towards nitrogen fixation with specialized cells and EPS production favored, namely CPS [1]. With respect to culture medium water sources, few studies have successfully applied seawater and other environmental resources to simulate habitats conditions and stimulate EPS production.
Light in the photoautotrophic metabolism is the energy provider for the cyanobacterial growth and it can be characterized in terms of light intensity (μE), light / dark cycles (L/Dh), and light quality. From Table 1, we can observe a heterogeneity of light conditions applied, while the majority opted for continuous illumination with low or high light intensity others opted for a balanced L/D cycle. No screening using other light sources such as natural light, different light qualities (monochrome) conditions were found. Temperature is a determinant factor for enzymatic activity and thus metabolism propeller. Cyanobacteria can be characterized by their adaptability to temperature as psychrophiles (below 15 °C), mesophiles, and thermophiles (above 40 °C). Very few studied strains belonging to the extremes of this classification, though they could have interesting industrial applications depending on the geographic location. Nonetheless the majority of studies applied constant temperature values within the mesophile range. In addition, no tests on temperature fluctuations were found. EPS production is known to affect the culture medium rheology; thus it is important to control the homogeneity of the cyanobacterial cultures in particular nutrients and pH. Culture mixing and/or aeration (air) were applied by the majority of the studies, though less often a CO2–air mixture was intentionally applied to control pH. The volume of cultivation used varied along with studies (0.01–2 L) whereas it reduces the comparison between results; however, one study at the microplate level revealed a novel way to screen EPS production in 880 microalgal strains by correlating culture medium viscosity with sugar content. An overproducing bacterial strain was found in non-axenic Mycrocistis aeruginosa f. flos-aquae culture [16].
2. Production and Optimization
Even though strain selection can anticipate the success of the whole bioprocess development the performance will always need to be ameliorated. At this step, it will be determined how much product can the cell produce in a determined volume per period of time, i.e., productivity. Consequently, the moment that the cell produces the highest amount of EPS and/or how long it takes to achieve will determine the strategy applied [17]. With respect to stress conditions applied to cyanobacterial strains nitrogen, salinity, and light are reported as the most frequent inducers of EPS production. Single parameter optimization has been extensively applied to optimize EPS production, see Table 2. As observed in strain selection, Nostocales and Oscillatoriales are among the most studied orders.
Table 2. Cyanobacterial EPS optimization studies on culture media and process parameters organized by order. Bold effects correspond to positive/or significant effect on EPS production.
| Strains | Optimization Factor | EPS Titer/Productivity /Yield |
||
|---|---|---|---|---|
| Culture Media | Process Parameters | Literature | ||
| Nostocales | ||||
| Anabaena augstmalis VRUC163 | nd† | Fed-batch; Film forming PBR | 14.73 mg/g (CPS) | [9] |
| Anabaena cylindrica 10 C | N source (NaNO3) Mixotrophy |
nd† | 2.36 mg/L (RPS) | [18] |
| Anabaena WSAF | N source (absence; NaNO3); P source (K2PHO4); | L/D cycle (continuous); Shear stress (aeration); | 1.86 mg/L/day (EPS) | [12] |
| Anabaena sp. ATCC 33047 | N source (N2, KNO3; NH4Cl); Salinity (NaCl/absence) | Temperature; Light intensity (medium); Shear stress (high aeration); Dilution rate (0.03 h−1) | 1100 mg/L/day (RPS + CPS) | [19] |
| Anabaena sp. BTA997 | nd† | Initial pH (8.5) | 1.7 g/L (RPS) | [6] |
| Anabaena turolosa | N source (absence; NaNO3); P source (K2PHO4); | L/D cycle (continuous); Shear stress (aeration); | 0.73 mg/L/day | [12] |
| Cyanospira capsulata | C flux metabolism (glyoxylate; nitrogen inhibitor) | nd† | 7.5 mg/L/day | [20] |
| Nostoc flagelliforme | N source (NaNO3); P source (K2PHO4); | Temperature (low); Light intensity (high); Initial pH (alkaline) | 14.29 mg/L/day (RPS) | [21] |
| Nostoc flagelliforme | nd† | Cultivation mode (Fed-batch); pH (8–9) | 8.86 mg/L/day (CPS) | [22] |
| Nostoc flagelliforme | nd† | light quality (monochromatic red, yellow, green, blue, purple) | 47.39 mg/g (RPS) | [23] |
| Nostoc flagelliforme | nd† | light quality (white fluorescent and monochromatic red, yellow, green, blue, purple); Red light intensity (medium) | 275 mg/g (CPS) | [24] |
| Nostoc flagelliforme | C source (absence; NaHCO3); N source (absence; NaNO3) | light quality (monochromatic red, blue) | nd† | [25] |
| Nostoc flagelliforme | N source (Urea, NaNO3, NH4Cl; Arginine) | Light intensity (low); Light quality (mixed wavelengths, red, blue, green); Wavelength shift | 5.42 mg/L/day (RPS) | [26] |
| Nostoc flagelliforme | C source (glucose); Salinity (NaCl) | nd† | 234.82 mg/g (CPS) | [27] |
| Nostoc sp. | nd† | Light intensity (high) | 134.26 mg/g DW (RPS) | [28] |
| Nostoc sp. BTA97 | N source (NaNO3, absence); | Initial pH (alkaline) | 53.3 mg/L/day (RPS + CPS) | [5] |
| Nostoc sp. PCC 7413 | N source (NaNO3; presence/absence) | Light intensity (low, high) | 150 mg L/day (RPS) | [11] |
| Scytonema tolypothrichoides | nd† | Temperature and light intensity crossed gradients | 310–360 mg/L (CPS) | [29] |
| Tolypothrix bouteillei | nd† | Temperature and light intensity crossed gradients | 186–216 mg/L (CPS) | [29] |
| Oscillatoriles | ||||
| Arthrospira platensis PCC 8005 | nd† | Light intensity (low) | nd† | [30] |
| Arthrospira platensis | C source (NaHCO3) | nd† | nd† | [31] |
| Arthrospira platensis “Compére 1968/3786” | nd† | Temperature; Light intensity | 11.76 mg/L/day (RPS) | [32] |
| Arthrospira platensis “Compére 1968/3786” | Photoautotrophic (light), Mixotrophic (light, glucose), Heterotrophic (glucose) | nd† | 26.4 mg/L/day (RPS) | [33] |
| Cyanothece epiphytica AUS-JR/DB/NT-021 | N source (absence; NaNO3); Salinity (NaCl); Micronutrients (MgSO4); Ozone | nd† | 9.66 mg/L/day (RPS + CPS) | [34] |
| Cyanothece sp. 113 | N source (absence; NaNO3); Salinity (NaCl); Micronutrients (MgSO4; NaH2PO4) | Aeration; Temperature; Light intensity; Time course | 1300 g/L/day (CPS) | [4][35] |
| Cyanothece sp. CCY 0110 | C source (glycerol); N source (absence/combined); Salinity (NaCl); Micronutrients (MgCl2) | Temperature; Light intensity; L/D cycle; shear stress (aeration) | 42.86 mg/L/day (RPS) | [36] |
| Microcoleus vaginatus | nd† | Light intensity; | 139 mg/g (RPS) | [37] |
| Synechococcales | ||||
| Limnothrix redekei PUPCCC 116 | N source (KNO3); Salinity (NaCl) | nd† | 14.48 mg/L/day | [38] |
| Oscillatoria formosa | N source (KNO3); Salinity (NaCl); Micronutrients (CaCl2) | Temperature (high); L/D cycles (14/10) | 9.88 mg/L/day (RPS) | [39] |
| Synechococcus sp. | N source (N2, nitrate, combined/absence) | Light intensity; L/D cycle | 330 mg/L/day | [40] |
| Synechocystis sp. BASO | Salinity (NaCl) | nd† | 500 mg/L (CPS) | [10] |
| Chroococcales | ||||
| Cyanobacterium aponinum | C source (5% CO2; NaHCO3) | Temperature (high); Light intensity (high) | 20 mg/L/day (RPS) | [41] |
| Spirulinales | ||||
| Spirulina sp. | N source (NaNO3); P source (K2PHO4); Salinity (NaCl) | Temperature | 1.83 mg/L/day (EPS) | [12] |
nd†—non-defined. Levels of process parameters are in agreement with the associated literature in question. In some cases, Nitrogen did not produce significant effects which can be justified by the fact the control had a defined nitrogen concentration, for instance in situations where BG11 was used as control.
Culture Medium
Culture medium optimization has a very important role in the economic feasibility of a bioprocess as well as to highlight and fine-tune the presence of certain nutrients, namely nitrogen [5][36][21][18][38][39][26][34][25][40][35], carbon [18][31][42][33], salinity [43][4][10][12][38][39][34][42][44][19] and micronutrients [4][12][36][21][39][34][35].
Process Conditions
Process conditions and respective control are important to assure the reproducibility and feasibility of the bioprocess. Nevertheless, these settings can possibly influence the desired outcome of the process.
Among them, pH [7][11][12][19][22], shear stress [43][5][7][12][36][21][38][39][40][19][45][41], temperature [36][21][35][19][41][29], Light/Dark (L/D) cycles [12][36][39][40][46] have been highly addressed.
Much work has been done on Nostoc flagelliforme [47][26][25][23][24][48][49][50][27]. This strain is an edible terrestrial cyanobacterium that is used as food in China for more than 2000 years. The EPS from N. flagelliforme are reported to be bioactive and possess interesting physico-chemical properties. Light quality experiments have shown that N. flagelliforme has a higher growth rate and EPS production under monochromatic light, namely red and blue light when compared to white light [23][24]. However, no relationship between monosaccharidic composition and the quality of light applied was found [24]. The same was found for another Nostoc sp. [28]. Culture medium optimization was found to enhance the antioxidant activity of CPS fraction [42]. Different light intensities of red light induced photoinhibition which possibly stimulated the protection of the cell by the production of EPS, namely CPS [24]. Optimization of carbon, nitrogen source, and light quality reinforced the fact that growth and EPS have their own requirements. In addition, these culture conditions also affected the EPS-associated enzymes, showing a correlation with the number of EPS produced [25]. More recently, a transcriptomic analysis revealed that light quality regulated EPS biosynthesis via the intracellular reactive oxygen species (ROS) level directly other than oxidative stress of N. flagelliforme [50].
Meta-bibliographic research was conducted to infer which were the cultivation factors that most influence the EPS and glycogen production in Arthropira platensis. The results showed a considerable heterogeneity of authors’ results explained by different operating conditions and extraction/purification methodologies applied. The authors found light intensity as the most preponderant factor in their desk-research and experimented with different light intensities (100, 400, 800, and 1200 μE). The ratio EPS/glycogen was found higher with lower light intensities. In addition, EPS monosaccharides composition was significantly different among different light intensities applied [30].
For those cases, where there was no correlation between growth and RPS production, a two-stage cultivation might be the most appropriate way to attain high biomass and then obtain a higher amount of the RPS at a second stage. This was already suggested in [9]. Playing with intensities of multiple wavelengths (red, blue, and green) and nitrogen source a strategy was defined and an increase in 66% and 217.3% in N. flagelliforme growth an RPS production was attained, respectively. The growth phase was set with white light for nine days while the EPS phase was followed by nine days of mixed wavelength (red/blue/green = 12:5:5) using urea as a nitrogen source [26]. The use of light as a way to control the production of cyanobacterial products has already been reported [51][52]. Moreover, lower light intensity was also utilized as a strategy to increase EPS production [26][53]. Arthrospira sp. was grown in two-stage cultivation wherein biomass production (Zarrouk medium, 30 °C, 80 µE) was favored at first. Secondly, the combined effect of light intensity and salinity (NaCl) was found antagonistic on EPS enhancement. Optimal conditions were found (10 µE and 39 g/L of NaCl) allowing EPS production to have a 1.67-fold increase when compared to optima growth conditions [53].
Applying outdoor cultivation systems can potentially reduce production costs by reducing light and temperature control [25][54][55][56]. Relevance was found in an outdoor pilot scale cultivation of Spirulina sp. LEB-18 on 250 L of open raceways in the summer of Salvador, Bahia, Brazil. The crude RPS demonstrated pseudoplastic behavior and high thermal stability. At day 30, the biomass reached a titer of 1.01 g/L while the crude EPS production was shown to attain 9.5 g/L, wherein the highest productivity happened on day 10 (0.6 g/L/day); however, it is known that outdoor conditions can affect productivities due to variable climate conditions and geographic location [57].
2.1. Modes of Cultivation and PBR Design
Cultivation modes are used as a strategy to increase biomass and EPS production [58]. The majority of Cyano-EPS are reported in batch mode followed by almost unexplored fed-batch [9][22] and continuous mode [19]. Anabaena sp. ATCC 33047 grown under different dilution rates was found optimal EPS/biomass ratio of higher than 1.5 and EPS productivity attaining 1.1 g/L/day at 0.03 hour−1. In addition, EPS and biomass production phases were shown to be disassociated [19]. Other authors have claimed the same disassociation of the kinetics [41][29][23].
2.2. Design of Experiments
Optimization refers to the discovery of the conditions of a system, a process, or a product to obtain the maximum benefit from it [61]. The classical methods involve the optimization of just one condition at a time, a methodology highly applied in Cyano-EPS (see Table 2). The optimization method must take into account all the interactions and the optimum conditions obtained are validated. The design of experiments is highly used in bioprocess optimization, though the examples in Cyano-EPS are less frequent [34][33][53][32].
3. Downstream Processes and Cyano-EPS Global Market
Interest for cyanobacteria EPS has increased significantly during the last two decades as they offer some structural originalities compared to those extracted from marine microalgae and to hydrocolloids from macroalgae and plants [14][8]. They have wide putative applications such as: food additives, soil (water holding capacity), wastewater treatments (removal of heavy metals), bioactive agents for nutraceutic, pharmaceutical, and cosmetic fields [62]. The development of strategies for their extraction and purification is strongly correlated with their cellular location. EPS should be employed only to reference polysaccharides excreted by microorganisms in their extracellular environment and not having covalent links with their cellular envelope. Considering the abundant literature focusing on polysaccharides from cyanobacteria they can be divided into three groups: biopolymers poorly associated with the cell surface and usually encompassed under the term of slime or released polysaccharides (RPS); polysaccharide structured as a sheath, which is a thin layer next to the outer cell membrane and containing fibers; capsular polysaccharides (CPS) intimately associated with the envelope cells [63]. The extraction and purification of these polysaccharides from cyanobacterial culture media is a major concern before the investigation of their structure and/or to find commercial applications. The main question before developing a strategy for their purification is what kind of EPS is the objective of the extraction: slime, sheath, or capsular polysaccharides? Several articles or reviews detail the processes available for the extraction of these three kinds of biopolymers, often calling them with different appellations, increasing the confusion between these different classes [14][64][1][63][65][66][67]. All the protocols are summarized in Figure 1. Firstly, the extracellular medium of cyanobacteria cultures is collected generally by centrifugation, but tangential microfiltration may be an interesting alternative as described by [68] for red microalgae or by [14]. Note that [67]suggested suspending the pellet in Milli-Q water and to incubate them at 4 °C during 12 h before to centrifuge them again to collect, by depletion, the maximum of slime. The cyanobacteria free supernatants or permeates are then treated by polar alcohols (methanol, ethanol, or isopropanol) or acetone to precipitate the EPS. The volume ratios are different depending on studies but two or three volumes of cold ethanol (or isopropanol) is probably a good compromise. Sometimes the supernatant is concentrated under vacuum at low temperatures (50–60 °C) to limit the volume of used alcohol or acetone [14][8]. This sole EPS precipitation is generally not enough to remove salts and/or low molecular weight metabolites from extracted polysaccharides as they co-precipitated during alcohol/acetone treatment. So, two protocols are generally used by authors to increase the purity level of polysaccharides, measured by Dubois assay [69]. The first one is a solubilization of dried polysaccharides in Milli-Q water before their precipitation using the same alcohol or acetone volume ratio. The second one is to dialyze them against water. Finally, the EPS are freeze-dried or dried under vacuum. The second extraction strategy focuses on polysaccharides designed as a sheath (or CPS) and concerns the main extraction protocols described in the literature. Numerous cyanobacteria such as Nostoc genus form macroscopic colonies embedded in extracellular polysaccharides protecting them from environment [70].
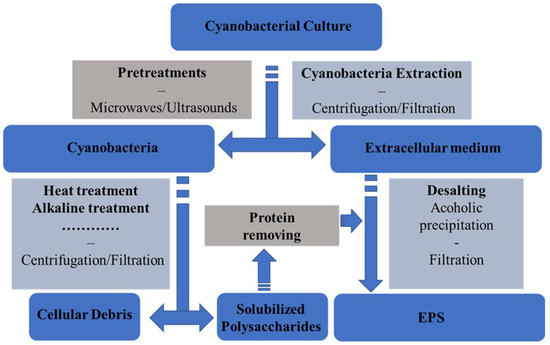
Figure 1. Process flow chart for the extraction of EPS from cyanobacteria.
Even if these polysaccharides do not have covalent linkages with the cyanobacteria cell wall, they interact strongly with it, which requires specific processes to dissociate them. Note that in numerous publications, authors include sheath and capsular polysaccharides as a unique fraction. These processes present some differences but can be resumed by collecting biomass using centrifugation and the extraction of EPS using hot distilled water (50–121 °C) in varying times (0.5–6 h). In other protocols, the extraction is achieved by differential sucrose gradient centrifugation of cyanobacterial biomass or after re-suspending it in a low ionic strength buffer at 100 °C [71][67][70]. After the extraction, the suspension is filtered through a filter paper, of 0.2 µm membrane or centrifuged to collect the permeate or the supernatant. The retentate or the pellets are extracted again several times using the same process to deplete the biomass (up to 5–6 times). Note that with this extraction numerous non-polysaccharidic compounds and notably proteins are co-extracted needing a purification supplementary step. Another way to start is by a 50 °C concentration under vacuum (not always necessary or done) followed by an optional protein extraction using the Sevag method or another. Then, an alcoholic (isopropanol or ethanol (2–3 v/v)) precipitation of polysaccharides is applied. Finally polysaccharides are freeze- or oven-dried [14][72][54][67]. Sometimes, the lipids and pigments of the biomass can be previously extracted using an organic solvent such as hexane, acetone, ethanol, or other [72][56]. This kind of process may be improved using microwave or ultrasound treatments as applied to Arthrospira biomass [73][74]. Finally, the last class of extracellular polysaccharides is that of capsular polysaccharide needing stronger conditions for their extraction. Often considered as EPS in literature, their extraction implies the use of alkaline treatments of biomass such as a reflux in NaOH 0.1 M for 5 h at 90 °C [75]. The polysaccharides are precipitated using two volumes of isopropanol and dissolved in Milli-Q water. A second precipitation with alcohol is performed to increase their purity level.
Cyanobacterial EPS have to compete with cheaper hydrocolloids from seaweeds, terrestrial plants, and non-photosynthetic bacteria, wherein some have authorizations for their use in food. What is the situation 21 years later? The number of publications focusing on the rheological and biological potential of these biopolymers increases dramatically [14][71][62][67][76] but is not correlated with the arrival on the market of EPS from cyanobacteria. Currently and to our knowledge, only four EPS from Cyanobacteria are commercially available and exploited in niche markets mainly in the cosmetic and nutraceutical area. They are Spirulan, Immulan, Nostoflan, and Emulcyan extracted from respectively Arthrospira platensis, Aphanotece halophytica, Nostoc flagelliforme, and Phormidium [71][77][78][79].
4. Considerations for Cyano-EPS Development
In light of bioprocess development, Cyano-EPS has still a long way to become a trustable supplier of polysaccharides for the hydrocolloids industry. For that, three key steps must interplay: strain selection, production process, and downstream processing. Increased cell robustness is a concept elaborated in [80], wherein the selection of the organism takes into account the relevant process parameters as well all the whole bioprocess stages until the final productperformance. According to [80] the stability of a bioprocess depends on the following factors: shear stress resistance; tolerance against temperature and/pH; low sensitivity to infections; robustness against contaminations; no sticking or biofouling. To date no comprehensive screening taking into account the sustainability of bioprocess was published for cyanobacterial EPS. Interesting examples are being applied to the microalgae field by: the selection of desert-adapted strains for commercial application and CO2 sequestration [81]; cold-adapted microalgae strains for the production of fatty acids and proteins [82]. Application of such strategies in Cyano-EPS might bring a new possibility, whereas it will be determinant the polymer functionality. Other possibilities include the use of genetic engineering technologies for the development of cyanobacterial tailor-made polymers [83][119]. For the production process, much can be done around optimization, especially by applying statistically-based experimental design methods. Culture medium components, pH, mass, and gas transference rate, light, and inoculum conditions are some of the factors to consider in Cyano-EPS optimization. For this, it is important to understand the kinetics of biomass and EPS production for the cultivation conditions applied and opt for the most convenient strategy. The use of omics as a support tool can along with cultivation unroll unknown factors [84]. One stage cultivation seems practical for cases in which growth and EPS are associated, whereas two-stage cultivation will fit for the decoupled cases and biomass can be produced followed by the EPS production phase. PBR design has still considerable space for improvement with special needs for the mass and gas transfer within the culture vessel due to culture medium viscosity increase.
The location of the EPS should be taken into account since it will strongly affect the downstream processing. RPS can be easily recovered from the culture medium allowing the possibility to valorize the biomass produced. Conversely, CPS attached to the cell surface involve more destructive methodologies that might restrict the valorization of the produced biomass. However, an alternative to this was found through the combination of methodologies for the extraction of CPS and pigments. This methodology was confirmed in four cyanobacterial strains and proposes the CPS extraction followed by freeze-drying of the biomass, extraction of phycobiliproteins, and extraction of chlorophyll a and carotenoids [85]. Water and nutrients sources must be highly considered since the beginning of the bioprospection process not only for their sustainability but also for their future impacts on the EPS purification.
References
- De Philippis, R.; Faraloni, C.; Margheri, M.C.; Sili, C.; Herdman, M.; Vincenzini, M. Morphological and biochemical characterization of the exocellular investments of polysaccharide-producing Nostoc strains from the pasteur culture collection. World J. Microbiol. Biotechnol. 2000, 16, 655–661.
- Singh, S.; Das, S. Screening, production, optimization and characterization of cyanobacterial Polysaccharide. World J. Microbiol. Biotechnol. 2011, 27, 1971–1980.
- Uhliariková, I.; Chválová, B.; Matulová, M.; Cepák, V.; Lukavský, J.; Capek, P. Extracellular biopolymers produced by freshwater cyanobacteria: A screening study. Chem. Pap. 2019, 73, 771–776.
- Zhang, Y.; Chi, Z.; Lu, W. Exopolysaccharide production by four cyanobacterial isolates and preliminary identification of these isolates. J. Ocean Univ. China 2007, 6, 147–152.
- Tiwari, O.N.; Khangembam, R.; Shamjetshabam, M.; Sharma, A.S.; Oinam, G.; Brand, J.J. Characterization and optimization of bioflocculant exopolysaccharide production by Cyanobacteria Nostoc sp. BTA97 and Anabaena sp. BTA990 in culture conditions. Appl. Biochem. Biotechnol. 2015, 176, 1950–1963.
- Khangembam, R.; Tiwari, O.; Kalita, M. Production of exopolysaccharides by the cyanobacterium Anabaena sp. BTA992 and application as bioflocculants. J. Appl. Biol. Biotechnol. 2016, 4, 8–11.
- Richert, L.; Golubic, S.; Le Guédès, R.; Ratiskol, J.; Payri, C.; Guezennec, J. Characterization of exopolysaccharides produced by Cyanobacteria isolated from Polynesian microbial mats. Curr. Microbiol. 2005, 51, 379–384. [CrossRef]
- Parikh, A.; Madamwar, D. Partial characterization of extracellular polysaccharides from Cyanobacteria. Bioresour. Technol. 2006, 97, 1822–1827.
- Di Pippo, F.; Ellwood, N.T.W.; Gismondi, A.; Bruno, L.; Rossi, F.; Magni, P.; De Philippis, R. Characterization of exopolysaccharides produced by seven biofilm-forming cyanobacterial strains for biotechnological applications. J. Appl. Phycol. 2013, 25, 1697–1708.
- Ozturk, S.; Aslim, B. Modification of exopolysaccharide composition and production by three cyanobacterial isolates under salt stress. Environ. Sci. Pollut. Res. 2010, 17, 595–602.
- Otero, A.; Vincenzini, M. Extracellular polysaccharide synthesis by Nostoc strains as affected by N source and light intensity. J. Biotechnol. 2003, 102, 143–152.
- Nicolaus, B.; Panico, A.; Lama, L.; Romano, I.; Manca, M.C.; De Giulio, A.; Gambacorta, A. Chemical composition and production of exopolysaccharides from representative members of heterocystous and non-heterocystous Cyanobacteria. Phytochemistry 1999, 52, 639–647.
- Gaignard, C.; Laroche, C.; Pierre, G.; Dubessay, P.; Delattre, C.; Gardarin, C.; Gourvil, P.; Probert, I.; Dubuffet, A.; Michaud, P. Screening of marine microalgae: Investigation of new exopolysaccharide producers. Algal Res. 2019, 44, 101711.
- Delattre, C.; Pierre, G.; Laroche, C.; Michaud, P. Production, extraction and characterization of microalgal and cyanobacterial exopolysaccharides. Biotechnol. Adv. 2016, 34, 1159–1179.
- Xu, H.; Cai, H.; Yu, G.; Jiang, H. Insights into extracellular polymeric substances of cyanobacterium Microcystis aeruginosa using fractionation procedure and parallel factor analysis. Water Res. 2013, 47, 2005–2014.
- Mancuso-Nichols,C.A.;Nairn,K.M.;Glattauer,V.;Blackburn,S.I.;Ramshaw,J.A.M.;Graham,L.D.Screening microalgal cultures in search of microbial exopolysaccharides with potential as adhesives. J. Adhes. 2009, 85, 97–125.
- Seviour, R.J.; McNeil, B.; Fazenda, M.L.; Harvey, L.M. Operating bioreactors for microbial exopolysaccharide production. Crit. Rev. Biotechnol. 2011, 31, 170–185.
- Lama, L.; Nicolaus, B.; Calandrelli, V.; Manca, M.C.; Romano, I.; Gambacorta, A. Effect of growth conditions onendo-andexopolymerbiosynthesisinAnabaenacylindrica10C.Phytochemistry1996,42,655–659.
- Moreno, J.; Vargas, M.A.; Olivares, H.; Rivas, J.; Guerrero, M.G. Exopolysaccharide production by the cyanobacterium Anabaenasp. ATCC 33047 in batch and continuous culture. J.Biotechnol. 1998, 60, 175–182.
- De Philippis, R.; Sili, C.; Vincenzini, M. Response of an exopolysaccharide-producing heterocystous cyanobacterium to changes in metabolic carbon flux. J. Appl. Phycol. 1996, 8, 275–281.
- Yu,H.;Jia,S.;Dai,Y.AccumulationofexopolysaccharidesinliquidsuspensioncultureofNostocflagelliforme cells. Appl. Biochem. Biotechnol. 2010, 160, 552–560.
- Tan, N.; Jia, S.R.; Han, P.P.; Guo, W.; Dai, Y.J. The open culture of Nostoc flagelliforme with a 25 L open pond. In Advanced Materials Research; Trans Tech Publications Ltd.: Bäch, Switzerland, 2012; Volume 554, pp. 1009–1012.
- Han, P.; Sun, Y.; Jia, S.; Zhong, C.; Tan, Z. Effects of light wavelengths on extracellular and capsular polysaccharide production by Nostoc flagelliforme. Carbohydr. Polym. 2014, 105, 145–151.
- Han, P.; Shen, S.; Wang, H.-Y.; Sun, Y.; Dai, Y.; Jia, S. Comparative metabolomic analysis of the effects of light quality on polysaccharide production of cyanobacterium Nostoc flagelliforme. Algal Res. 2015, 9, 143–150.
- Han, P.; Yao, S.; Guo, R.; Yan, R.; Wu, Y.; Shen, S.; Jia, S. Influence of culture conditions on extracellular polysaccharide production and the activities of enzymes involved in the polysaccharide synthesis of Nostoc flagelliforme. RSC Adv. 2017, 7, 45075–45084.
- Han, P.; Shen, S.; Wang, H.-Y.; Yao, S.; Tan, Z.; Zhong, C.; Jia, S. Applying the strategy of light environment control to improve the biomass and polysaccharide production of Nostoc flagelliforme. J. Appl. Phycol. 2017, 29, 55–65.
- Shen, S.-G.; Lin, Y.-H.; Zhao, D.-X.; Wu, Y.-K.; Yan, R.-R.; Zhao, H.-B.; Tan, Z.-L.; Jia, S.-R.; Han, P.-P. Comparisons of functional properties of polysaccharides from Nostoc flagelliforme under three culture conditions. Polymers 2019, 11, 263.
- Ge, H.; Xia, L.; Zhou, X.; Zhang, D.; Hu, C. Effects of light intensity on components and topographical structures of extracellular polysaccharides from the cyanobacteria Nostoc sp. J. Microbiol. 2014, 52, 179–183.
- Kvíderová, J.; Kumar, D.; Lukavský, J.; Kaštánek, P.; Adhikary, S.P. Estimation of growth and exopolysaccharide production by two soil Cyanobacteria, Scytonema tolypothrichoides and Tolypothrix bouteillei asdeterminedbycultivationinirradianceandtemperaturecrossedgradients. Eng. LifeSci. 2019,19,184–195.
- Phélippé, M.; Gonçalves, O.; Thouand, G.; Cogne, G.; Laroche, C. Characterization of the polysaccharides chemical diversity of the Cyanobacteria Arthrospira platensis. Algal Res. 2019, 38, 101426.
- Vergnes, J.-B.; Gernigon, V.; Guiraud, P.; Formosa-Dague, C. Bicarbonate concentration induces production of exopolysaccharides by Arthrospira platensis that mediate bioflocculation and enhance flotation harvesting efficiency. Acs Sustain. Chem. Eng. 2019, 7, 13796–13804.
- Trabelsi, L.; Ouada, H. B.; Bacha, H.; Ghoul, M. Combined effect of temperature and light intensity on growth and extracellular polymeric substance production by the cyanobacterium Arthrospira platensis. J.Appl. Phycol. 2009, 21, 405–412.
- Trabelsi, L.; Ouada, H.B.; Zili, F.; Mazhoud, N.; Ammar, J. Evaluation of Arthrospira platensis extracellular polymeric substances production in photoautotrophic, heterotrophic and mixotrophic conditions. Folia Microbiol. 2013, 58, 39–45.
- Borah, D.; Nainamalai, S.; Gopalakrishnan, S.; Rout, J.; Alharbi, N. S.; Alharbi, S. A.; Nooruddin, T. Biolubricant potential of exopolysaccharides from the cyanobacterium Cyanotheceepiphytica. Appl. Microbiol. Biotechnol. 2018, 102, 3635–3647.
- Su, C.; Chi, Z.; Lu, W. Optimization of medium and cultivation conditions for enhanced exopolysaccharide yield by marine Cyanothece sp. 113. Chin. J. Oceanol. Limnol. 2007, 25, 411–417.
- Mota, R.; Guimarães, R.; Büttel, Z.; Rossi, F.; Colica, G.; Silva, C. J.; Santos, C.; Gales, L.; Zille, A.; De Philippis, R. Production and characterization of extracellular carbohydrate polymer from Cyanothece sp. CCY 0110. Carbohydr. Polym. 2013, 92, 1408–1415.
- Ge, H.; Zhang, J.; Zhou, X.; Xia, L.; Hu, C. Effects of light intensity on components and topographical structures of extracellular polymeric substances from Microcoleus vaginatus (Cyanophyceae). Phycologia 2014, 53, 167–173.
- Khattar, J.I.S.; Singh, D.P.; Jindal, N.; Kaur, N.; Singh, Y.; Rahi, P.; Gulati, A. Isolation and characterization of exopolysaccharides produced by the cyanobacterium limnothrix redekei PUPCCC 116. Appl. Biochem. Biotechnol. 2010, 162, 1327–1338.
- Jindal, N.; Singh, D.P.; Khattar, J.I.S. Kinetics and physico-chemical characterization of exopolysaccharides produced by the cyanobacterium Oscillatoria formosa. World J. Microbiol. Biotechnol. 2011, 27, 2139–2146.
- Phlips, E.J.; Zeman, C.; Hansen, P. Growth, photosynthesis, nitrogen fixation and carbohydrate production by a unicellular cyanobacterium, Synechococcus sp.(Cyanophyta). J. Appl. Phycol. 1989, 1, 137–145.
- Gris, B.; Sforza, E.; Morosinotto, T.; Bertucco, A.; La Rocca, N. Influence of light and temperature on growth and high-value molecules productivity from Cyanobacterium aponinum. J. Appl. Phycol. 2017, 29, 1781–1790.
- Shen, S.; Jia, S.; Wu, Y.; Yan, R.; Lin, Y. H.; Zhao, D.; Han, P. Effect of culture conditions on the physicochemical properties and antioxidant activities of polysaccharides from Nostoc flagelliforme. Carbohydr. Polym. 2018,198, 426–433.
- Chi, Z.; Su, C. D.; Lu, W. D. A new exopolysaccharide produced by marine Cyanothece sp. 113. Bioresour.Technol. 2007, 98, 1329–1332.
- Bemal, S.; Anil, A.C. Effects of salinity on cellular growth and exopolysaccharide production of freshwater Synechococcus strain CCAP1405. J. Plankton Res. 2017, 40, 46–58.
- Trabelsi, L.; M’sakni, N.H.; Ouada, H.B.; Bacha, H.; Roudesli, S. Partial characterization of extracellular polysaccharides produced by cyanobacterium Arthrospira platensis. Biotechnol. BioprocessEng. 2009,14,27–31.
- Werner, A.; Broeckling, C.D.; Prasad, A.; Peebles, C.A.M. A comprehensive time-course metabolite profiling of the model cyanobacterium Synechocystis sp. PCC 6803 under diurnal light: Dark cycles. Plant J. 2019, 99, 379–388.
- Han, P.; Sun, Y.; Wu, X.; Yuan, Y.; Dai, Y.; Jia, S. Emulsifying, flocculating, and physicochemical properties of exopolysaccharide produced by cyanobacterium Nostoc flagelliforme. Appl. Biochem. Biotechnol. 2014, 172, 36–49.
- Han, P.; Yao, S.; Guo, R.; Shen, S.; Yan, R.; Tan, Z.; Jia, S. The relationship between monosaccharide composition of extracellular polysaccharide and activities of related enzymes in Nostoc flagelliforme under different culture conditions. Carbohydr. Polym. 2017, 174, 111–119.
- Han, P.; Guo, R.; Shen, S.; Yan, R.; Wu, Y.; Yao, S.; Wang, H.; Jia, S. Proteomic profiling of Nostoc flagelliforme reveals the common mechanism in promoting polysaccharide production by different light qualities. Biochem. Eng. J. 2018, 132, 68–78.
- Han, P.; Shen, S.; Guo, R.; Zhao, D.; Lin, Y.-H.; Jia, S.; Yan, R.; Wu, Y. ROS Is a Factor regulating the increased polysaccharide production by light quality in the edible cyanobacterium Nostocflagelliforme. J.Agric. Food Chem. 2019, 67, 2235–2244.
- Ho, S.-H.; Liao, J.-F.; Chen, C.-Y.; Chang, J.-S. Combining light strategies with recycled medium to enhance the economic feasibility of phycocyanin production with Spirulina platensis. Bioresour. Technol. 2018, 247, 669–675.
- Pagels, F.; Lopes, G.; Vasconcelos, V.; Guedes, A. C. White and red LEDs as two-phase batch for cyanobacterial pigments production. Bioresour. Technol. 2020, 123105.
- Chentir, I.; Hamdi, M.; Doumandji, A.; HadjSadok, A.; Ouada, H.B.; Nasri, M.; Jridi, M. Enhancement of extracellular polymeric substances (EPS) production in Spirulina (Arthrospirasp.) by two-step cultivation process and partial characterization of their polysaccharidic moiety. Int. J. Biol. Macromol. 2017, 105, 1412–1420.
- De Jesus, C.S.; de Jesus-Assis, D.; Rodriguez, M.B.; Menezes Filho, J.A.; Costa, J.A.V.; de Souza Ferreira, E.; Druzian, J.I. Pilot-scale isolation and characterization of extracellular polymeric substances (EPS) from cell-free medium of Spirulina sp. LEB-18 cultures under outdoor conditions. Int. J. Biol. Macromol. 2019, 124, 1106–1114.
- Abe, A.; Ohashi, E.; Ren, H.; Hayashi, T.; Endo, H. Isolation and characterization of a cold-induced nonculturable suppression mutant of Vibrio vulnificus. Microbiol. Res. 2007, 162, 130–138.
- Chen, H.-W.; Yang, T.-S.; Chen, M.-J.; Chang, Y.-C.; Lin, C.-Y.; Eugene, I.; Wang, C.; Ho, C.-L.; Huang, K.-M.; Yu, C.-C. Application of power plant flue gas in a photobioreactor to grow Spirulina algae, and a bioactivity analysis of the algal water-soluble polysaccharides. Bioresour. Technol. 2012, 120, 256–263.
- Tredici, M. R. Photobiology of microalgae mass cultures: Understanding the tools for the next green revolution. Biofuels 2010, 1, 143–162.
- Tiwari, O.N.; Chakraborty, S.; Devi, I.; Mondal, A.; Bhunia, B.; Boxiong, S. Bioprocess parameters of production of cyanobacterial exopolysaccharide: Biomass production and product recovery. In Handbook of Algal Technologies and Phytochemicals; CRC Press: Boca Raton, FL, USA, 2019; pp. 25–32.
- Pierre, G.; Delattre, C.; Dubessay, P.; Jubeau, S.; Vialleix, C.; Cadoret, J.-P.; Probert, I.; Michaud, P. What is in store for EPS Microalgae in the next decade? Molecules 2019, 24, 4296.
- Fischer, D.; Schlösser, U.G.; Pohl, P. Exopolysaccharide production by cyanobacteria grown in closed photobioreactors and immobilized using white cotton towelling. J.Appl. Phycol. 1997, 9, 205–213.
- Araujo, P.W.; Bereton, R.G. Experimental design II: Optimization. TrAC Trend. Anal. Chem. 1996, 15, 5–6.
- Wijffels, R.H.; Kruse, O.; Hellingwerf, K.J. Potential of industrial biotechnology with cyanobacteria and eukaryotic microalgae. Curr. Opin. Biotechnol. 2013, 24, 405–413.
- De Philippis, R.; Vincenzini, M. Outermost polysaccharidic investments of cyanobacteria: Nature,significance and possible applications. Recent Res. Dev. Microbiol. 2003, 7, 13–22.
- Helm, R. F.; Huang, Z.; Edwards, D.; Leeson, H.; Peery, W.; Potts, M. Structural characterization of the released polysaccharide of desiccation-tolerant Nostoc commune DRH-1. J. Bacteriol. 2000, 182, 974–982.
- Adhikary, S.P. Polysaccharides from mucilaginous envelope layers of Cyanobacteria and their ecological significance. J. Sci. Ind. Res. 1998, 57, 454–466.
- Bertocchi, C.; Navarini, L.; Cesàro, A.; Anastasio, M. Polysaccharides from Cyanobacteria. Carbohydr. Polym. 1990, 12, 127–153.
- Kumar, D.; Kastanek, P.; Adhikary, S.P. Exopolysaccharides from cyanobacteria and microalgae and their commercial application. Curr. Sci. 2018, 115, 234.
- Patel, A.K.; Laroche, C.; Marcati, A.; Ursu, A.V.; Jubeau, S.; Marchal, L.; Petit, E.; Djelveh, G.; Michaud, P. Separation and fractionation of exopolysaccharides from Porphyridium cruentum. Bioresour. Technol. 2013, 145, 345–350.
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.T.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356.
- De Philippis, R.; Vincenzini, M. Exocellular polysaccharides from Cyanobacteria and their possible applications. Fems Microbiol. Rev. 1998, 22, 151–175.
- Li, P.; Harding, S.E.; Liu, Z. Cyanobacterial exopolysaccharides: Their nature and potential biotechnological applications. Biotechnol. Genet. Eng. Rev. 2001, 18, 375–404.
- Wang, H.-B.; Wu, S.-J.; Liu, D. Preparation of polysaccharides from cyanobacteria Nostoc commune and their antioxidant activities. Carbohydr. Polym. 2014, 99, 553–555.
- Kurd, F.; Samavati, V. Water soluble polysaccharides from Spirulina platensis: Extraction and in vitro anti-cancer activity. Int. J. Biol. Macromol. 2015, 74, 498–506.
- DeSilva,A.S.;deMagalhaes,W.T.;Moreira,L.M.;Rocha,M.V.P.;Bastos,A.K.P.Microwave-assistedextraction of polysaccharides from Arthrospira (Spirulina) platensis using the concept of green chemistry. Algal Res. 2018, 35, 178–184.
- Rodriguez, S.; Torres, F.G.; López, D. Preparation and characterization of polysaccharide films from the cyanobacteria Nostoc commune. Polym. Renew. Resour. 2017, 8, 133–150.
- Pignolet, O.; Jubeau, S.; Vaca-Garcia, C.; Michaud, P. Highly valuable microalgae: Biochemical and topological aspects. J. Ind. Microbiol. Biotechnol. 2013, 40, 781–796.
- Fattom, A.; Shilo, M. Production of emulcyan by phormidium J-1: Its activity and function. FEMS Microbiol. Lett. 1985, 31, 3–9.
- Kanekiyo, K.; Hayashi, K.; Takenaka, H.; Lee, J.-B.; Hayashi, T. Anti-herpes simplex virus target of an acidic polysaccharide, nostoflan, from the edible blue-green alga Nostoc flagelliforme. Biol. Pharm. Bull. 2007, 30, 1573–1575.
- Patel, S.; Goyal, A. Current and prospective insights on food and pharmaceutical applications of spirulina. Curr. Trends Biotechnol. Pharm. 2013, 7, 681–695.
- Fresewinkel, M.; Rosello, R.; Wilhelm, C.; Kruse, O.; Hankamer, B.; Posten, C. Integration in microalgal bioprocess development: Design of efficient, sustainable, and economic processes. Eng. Life Sci. 2014, 14, 560–573.
- Schipper, K.; Al Muraikhi, M.; Alghasal, G.S.H.S.; Saadaoui, I.; Bounnit, T.; Rasheed, R.; Dalgamouni, T.; Al Jabri, H.M.S.J.; Wijffels, R.H.; Barbosa, M.J. Potential of novel desert microalgae and cyanobacteria for commercial applications and CO2 sequestration. J. Appl. Phycol. 2019, 1–13.
- Schulze, P.S.C.; Hulatt, C.J.; Morales-Sánchez, D.; Wijffels, R.H.; Kiron, V. Fatty acids and proteins from marine cold adapted microalgae for biotechnology. Algal Res. 2019, 42, 101604.
- Pereira, S.B.; Sousa, A.; Santos, M.; Araújo, M.; Serôdio, F.; Granja, P.; Tamagnini, P. Strategies to Obtain designer polymers based on cyanobacterial extracellular polymeric substances (EPS). Int. J.Mol. Sci. 2019, 20, 5693.
- Deschoenmaeker, F.; Bayon-Vicente, G.; Sachdeva, N.; Depraetere, O.; Pino, J.C.C.; Leroy, B.; Muylaert, K.; Wattiez, R. Impact of different nitrogen sources on the growth of Arthrospira sp. PCC 8005 under batch and continuous cultivation-A biochemical, transcriptomic and proteomic profile. Bioresour. Technol. 2017, 237, 78–88.
- Dorina, S.; Judith, S.; Björn, W.; Julia, S.; Andrea, S.; Muffler, K.; Roland, U. A new strategy for a combined isolation of EPS and pigments from Cyanobacteria. J. Appl. Phycol. 2020, 35, 1–12.




