
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Pietro Bertoglio | + 2405 word(s) | 2405 | 2021-06-11 05:51:04 | | | |
| 2 | Lily Guo | Meta information modification | 2405 | 2021-06-25 10:36:38 | | |
Video Upload Options
Mesothelioma is an aggressive disease arising from parietal pleura. Surgery is a valuable option in the frame of a multimodality treatment. Several surgical approaches have been standardized with the aim of a macroscopic complete resection; these often require homolateral diaphragm and pericardial resection and reconstruction. Extrapleural pneumonectomy (EPP) and extended pleurectomy decortication (EPD) have been recognized as radical surgical procedures. Nevertheless, both operations are technically challenging and associated with a significant rate of peri-operative morbidity and non-negligible mortality. The diaphragmatic and pericardial reconstruction technique is mandatory to avoid respiratory impairment and to reduce post-operative complications like gastric and cardiac herniation. Moreover, in the case of localized chest wall recurrence, surgery might be considered a valuable therapeutical option for highly selected and fit patients. All the technical aspects of the resection and reconstruction of the diaphragm, pericardium, and chest wall are described as well as the possible use of new minimally invasive techniques. In addition, the choice of different prosthetic materials, considering the most recent innovations in the field, are discussed.
1. Introduction
2. Surgical Procedures for MPM Resection
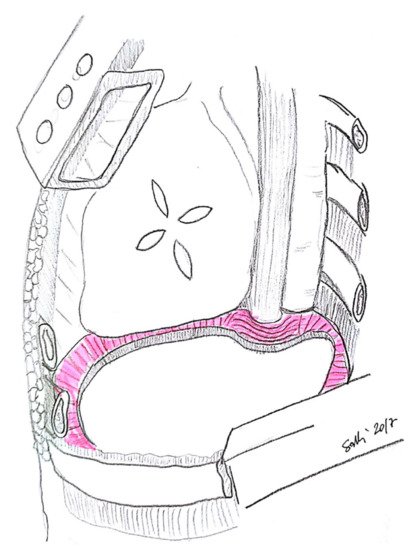
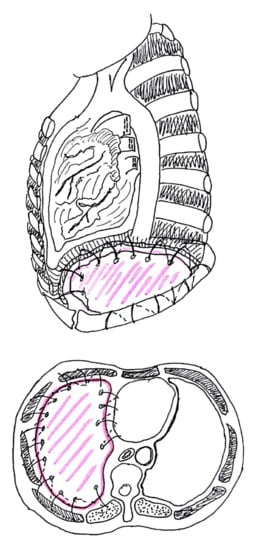
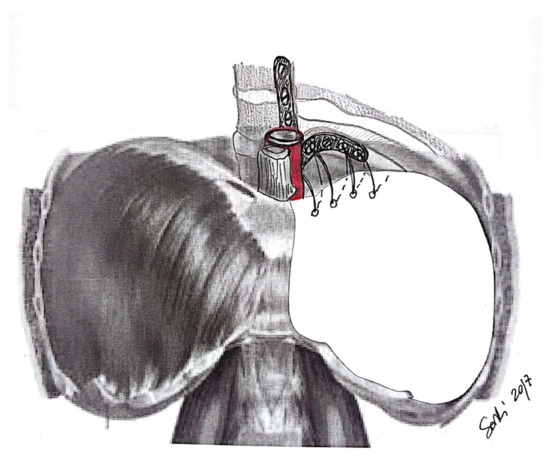
3. Diaphragm
3.1. Diaphragm Management
3.2. Types of Prosthesis
3.3. Technical Aspects
4. Pericardium
4.1. Pericardium Management
4.2. Types of Prosthesis
4.3. Technical Aspects
5. Chest Wall
5.1. Chest Wall Management
5.2. Types of Prosthesis
5.3. Technical Aspects
References
- Bertoglio, P.; Waller, D.A. The role of thoracic surgery in the management of mesothelioma: An expert opinion on the limited evidence. Expert Rev. Respir. Med. 2016, 10, 663–672.
- Vogelzang, N.J.; Rusthoven, J.J.; Symanowski, J.; Denham, C.; Kaukel, E.; Ruffie, P.; Gotzemeier, U.; Boyer, M.; Emri, S.; Paoletti, P.; et al. Phase III study of pemetrexed in combination with cisplatin versus cisplatin alone in patients with malignant pleural mesothelioma. J. Clin. Oncol. 2003, 21, 2636–2644.
- Mutti, L.; Peikert, T.; Robinson, B.W.; Scherpereel, A.; Tsao, A.S.; de Perrot, M.; Woodard, G.A.; Jablons, D.M.; Wiens, J.; Hirsch, F.R.; et al. Scientific Advances and New Frontiers in Mesothelioma Therapeutics. J. Thorac. Oncol. 2018, 13, 1269–1283.
- Bertoglio, P.; Aprile, V.; Ambrogi, M.C.; Mussi, A.; Lucchi, M. The role of intracavitary therapies in the treatment of malignant pleural mesothelioma. J. Thorac. Dis. 2018, 10 (Suppl. 2), S293–S297.
- Ambrogi, M.C.; Bertoglio, P.; Aprile, V.; Chella, A.; Korasidis, S.; Fontanini, G.; Fanucchi, O.; Lucchi, M.; Mussi, A. Diaphragm and lung-preserving surgery with hyperthermic chemotherapy for malignant pleural mesothelioma: A 10-year experience. J. Thorac. Cardiovasc. Surg. 2018, 155, 1857–1866.
- Bertoglio, P.; Ambrogi, M.C.; Chella, A.; Aprile, V.; Dini, P.; Korasidis, S.; Fanucchi, O.; Mussi, A. Is less also better? A single-institution experience on treatment of early stage Malignant Pleural Mesothelioma. Eur. J. Surg. Oncol. 2017, 43, 1365–1371.
- Casiraghi, M.; Maisonneuve, P.; Brambilla, D.; Solli, P.; Galetta, D.; Petrella, F.; Piperno, G.; De Marinis, F.; Spaggiari, L. Induction chemotherapy, extrapleural pneumonectomy and adjuvant radiotherapy for malignant pleural mesothelioma. Eur. J. Cardiothorac. Surg. 2017, 52, 975–981.
- Sugarbaker, D.J. Macroscopic complete resection: The goal of primary surgery in multimodality therapy for pleural mesothelioma. J. Thorac. Oncol. 2006, 1, 175–176.
- Rice, D.; Rusch, V.; Pass, H.; Asamura, H.; Nakano, T.; Edwards, J.; Giroux, D.; Hasegawa, S.; Kernstine, K.H.; Waller, D.; et al. Recommendations for uniform definitions of surgical techniques for malignant pleural mesothelioma: A consensus report of the international association for the study of lung cancer international staging committee and the international mesothelioma interest group. J. Thorac. Oncol. 2011, 6, 1304–1312.
- Bellini, A.; Dell’Amore, A.; Terzi, S.; Zambello, G.; Zuin, A.; Pasello, G.; Calabrese, F.; Schiavon, M.; Rea, F. Relapse Patterns and Tailored Treatment Strategies for Malignant Pleural Mesothelioma Recurrence after Multimodality Therapy. J. Clin. Med. 2021, 10, 1134.
- Treasure, T.; Lang-Lazdunski, L.; Waller, D.; Bliss, J.M.; Tan, C.; Entwisle, J.; Snee, M.; O’brien, M.; Thomas, G.; Peto, J.; et al. Extra-pleural pneumonectomy versus no extra-pleural pneumonectomy for patients with malignant pleural mesothelioma: Clinical outcomes of the Mesothelioma and Radical Surgery (MARS) randomised feasibility study. Lancet Oncol. 2011, 12, 763–772.
- Weder, W.; Stahel, R.A.; Baas, P.; Dafni, U.; de Perrot, M.; McCaughan, B.C.; Nakano, T.; Pass, H.I.; Robinson, B.W.; van Zandwijk, N.; et al. The MARS feasibility trial: Conclusions not supported by data. Lancet Oncol. 2011, 12, 1093–1094.
- Sharkey, A.J.; Bilancia, R.; Tenconi, S.; Nakas, A.; Waller, D.A. The management of the diaphragm during radical surgery for malignant pleural mesothelioma. Eur. J. Cardiothorac. Surg. 2016, 50, 311–316.
- Talon, I.; Schneider, A.; Ball, V.; Hemmerlé, J. Functionalization of PTFE Materials Using a Combination of Polydopamine and Platelet-Rich Fibrin. J. Surg. Res. 2020, 251, 254–261.
- Solli, P.; Brandolini, J.; Pardolesi, A.; Nardini, M.; Lacava, N.; Parri, S.F.; Kawamukai, K.; Bonfanti, B.; Bertolaccini, L. Diaphragmatic and pericardial reconstruction after surgery for malignant pleural mesothelioma. J. Thorac. Dis. 2018, 10 (Suppl. 2), S298–S303.
- Rolli, L.; Leuzzi, G.; Girotti, P.; Duranti, L.; Pastorino, U. Permeable Nonabsorbable Mesh for Total Diaphragmatic Replacement in Extended Pneumonectomy. Ann. Thorac. Surg. 2017, 104, e105–e107.
- Sugarbaker, D.J.; Jaklitsch, M.T.; Bueno, R.; Richards, W.; Lukanich, J.; Mentzer, S.J.; Colson, Y.; Linden, P.; Chang, M.; Zellos, L.S.; et al. Prevention, early detection, and management of complications after 328 consecutive extrapleural pneumonectomies. J. Thorac. Cardiovasc. Surg. 2004, 128, 138–146.
- Al-Nouri, O.; Hartman, B.; Freedman, R.; Thomas, C.; Esposito, T. Diaphragmatic rupture: Is management with biological mesh feasible? Int. J. Surg. Case Rep. 2012, 3, 349–353.
- Bedini, A.V.; Andreani, S.M.; Muscolino, G. Latissimus dorsi reverse flap to substitute the diaphragm after extrapleural pneumonectomy. Ann. Thorac. Surg. 2000, 69, 986–988.
- Schiavon, M.; De Filippis, A.; Marulli, G.; Rea, F. A new technique of diaphragmatic patch fixation in extrapleural pneumonectomy. Eur. J. Cardiothorac. Surg. 2010, 38, 798–800.
- Chen, R.F.; Lai, C.P. Constrictive pericarditis associated with Marlex mesh. Two case reports. Tex. Heart Inst. J. 2001, 28, 63–64.
- Urschel, J.D.; Takita, H. Pericardial closure after intrapericardial pneumonectomy. Ann. Thorac. Surg. 1999, 67, 295–296.
- Bertoglio, P.; Fanucchi, O.; Ricciardi, S.; Chella, A.; Lucchi, M.; Mussi, A. Chest wall resection for mesothelioma recurrence after surgery. Asian Cardiovasc. Thorac. Ann. 2016, 24, 893–895.
- Elsayed, H.H.; Hassaballa, A.S.; Ahmed, T.A.; Sharkawy, H.Y. Recurrence of mesothelioma after a macroscopic complete resection procedure: Is a second radical surgery justified? Interact. Cardiovasc. Thorac. Surg. 2021, 32, 761–763.
- Burt, B.M.; Ali, S.O.; DaSilva, M.C.; Yeap, B.Y.; Richards, W.G.; Baldini, E.H.; Sugarbaker, D.J. Clinical indications and results after chest wall resection for recurrent mesothelioma. J. Thorac. Cardiovasc. Surg. 2013, 146, 1373–1379.
- Politi, L.; Borzellino, G. Second surgery for recurrence of malignant pleural mesothelioma after extrapleural pneumonectomy. Ann. Thorac. Surg. 2010, 89, 207–210.
- Khullar, O.V.; Fernandez, F.G. Prosthetic Reconstruction of the Chest Wall. Thorac. Surg. Clin. 2017, 27, 201–208.
- Weyant, M.J.; Bains, M.S.; Venkatraman, E.; Downey, R.J.; Park, B.J.; Flores, R.M.; Rizk, N.; Rusch, V.W. Results of chest wall resection and reconstruction with and without rigid prosthesis. Ann. Thorac. Surg. 2006, 81, 279–285.
- Berthet, J.P.; Caro, A.G.; Solovei, L.; Gilbert, M.; Bommart, S.; Gaudard, P.; Canaud, L.; Alric, P.; Marty-Ané, C.H. Titanium Implant Failure After Chest Wall Osteosynthesis. Ann. Thorac. Surg. 2015, 99, 1945–1952.
- Sanna, S.; Brandolini, J.; Pardolesi, A.; Argnani, D.; Mengozzi, M.; Dell’Amore, A.; Solli, P. Materials and techniques in chest wall reconstruction: A review. J. Vis. Surg. 2017, 3, 95.




