Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Nicolás Loyacono | + 2336 word(s) | 2336 | 2021-06-07 09:59:36 | | | |
| 2 | Rita Xu | Meta information modification | 2336 | 2021-06-22 04:02:15 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Loyacono, N. AIM Classification System. Encyclopedia. Available online: https://encyclopedia.pub/entry/11107 (accessed on 07 February 2026).
Loyacono N. AIM Classification System. Encyclopedia. Available at: https://encyclopedia.pub/entry/11107. Accessed February 07, 2026.
Loyacono, Nicolás. "AIM Classification System" Encyclopedia, https://encyclopedia.pub/entry/11107 (accessed February 07, 2026).
Loyacono, N. (2021, June 22). AIM Classification System. In Encyclopedia. https://encyclopedia.pub/entry/11107
Loyacono, Nicolás. "AIM Classification System." Encyclopedia. Web. 22 June, 2021.
Copy Citation
The rationale of an Advanced Integrative Model and an Advanced Integrative Approach is presented. In the context of Allopathic Medicine, this model introduces the evaluation, clinical exploration, diagnosis, and treatment of concomitant medical problems to the diagnosis of Autism Spectrum Disorder.
integrative
model
ASD
concomitant
condition
disorder
1. Introduction
1.1. One Finding, One Treatment—The Old Era of Simplification as the Goal
Most recent manuscripts introduce Autism Spectrum Disorder or ASD as “Autism spectrum disorder defines a broad group of NDDs characterized by (i) young age of onset, (ii) impairment in communication and social abilities, (iii) restricted interests and repetitive behaviors, and (iv) symptoms that affect patients’ function in various areas of their life”. Many of today’s manuscripts about Autism Spectrum Disorder (ASD) begin with the phrase: “The complex pathophysiology of autism spectrum disorder encompasses interactions between genetic and environmental factors” or similar [1]. The diagnosis of ASD is given considering the Diagnostic and Statistical Manual of Mental Disorders (DSM) in 2021, with its fifth version or DSM-5. A recent review summarized the history of the DSM from Kanner to DSM-5 [2]. In precision medicine, the gap between bodily behaviors and genomics is being addressed, including the study of gene expression on tissues beyond the brain, in organs for vital functions. This approach is proposed to reframe Psychiatry [3].
Recent manuscripts reviewed the so-called comorbidities in ASD [4][5]. Comorbidities may be psychiatric [6], neurological [7], or related to medical conditions beyond the brain in the field of Pediatrics or General Medicine [8]. Recent literature has demonstrated that people with ASD diagnosis may have multiple comorbidities in different combinations and severity [9] and even temporal, transient hyper-multimorbidity. Multimorbidity is present when multiple medical issues (called comorbidities) are diagnosed in the same person. The present manuscript will call the medical issues that are frequently present in people with ASD as “concomitant medical problems to diagnosis” (of ASD) or CMPD. These CMPD are outside the brain or related to the brain (neurological and psychiatric CMPD of ASD). Previous attempts proposed potentially different roles for CMPD [10].
1.2. Trans-Discipline for the Analysis of the So Called “Comorbidities”
Complexity science forces us to see the dynamic properties of systems and the varying properties that are related to social roots [11][12]. ASD may be considered a complex diagnosis that resists the finding of new approaches via traditional models. It would be better tackled through interdisciplinary, systems-level approaches, considering implementation science [13][14].
Somatic health is a key point to move forward [15]. Several important reports have alerted about the need for the serious consideration of the CMPD of ASD [3][4][16][17][18] with transdisciplinary and interdisciplinary collaboration in the context of the multimorbidity [19]. As reference [17] cites, ASD is defined behaviorally. It includes the consideration of impairments in social behavior, stereotypic movements, and communication issues with impact on social skills, called “core symptoms of ASD”. All these symptoms significantly impair the quality of life of people diagnosed with ASD [20][21].
Medical conditions such as gut dysbiosis [22], non-celiac gluten sensitivity [23], cerebral folate deficiency [24], food allergies and intolerances [25], gastrointestinal [26], metabolic [27] and biochemical issues [28], immune dysfunction [29], autoimmune problems [30], mitochondrial dysfunction [31], barrier permeabilities [32], oxidative stress [33], endocrine issues [34] and more are not explored (sometimes for years) in ASD patients. The most advanced approaches have shifted the focus of the “causation search” original framework to the study of the (epi) genetic susceptibility for ASD, from the brain to the whole body [1][35], and to the importance of humanism in medicine [36] as well as to the study of genes expressed in tissues outside of the brain [37]. As Constantino recently reported, the so-called “co-morbidities” of ASD are inappropriately named if they actually contribute to (or exacerbate) the severity of autism itself [38]. Multimorbidity affects the generation of evidence [39] and a new Evidence Pyramid in Evidence Based Medicine was recently proposed [40]. Multimorbidity and hyper-multimorbidity should be taken into account in the case of ASD. The field of ASD needs personalized medicine as the norm.
2. When the Conclusion Should Not Be the Presumption
Looking at the published research in ASD, there is plenty of information about neurological [41], psychiatric [42] and biological (outside the brain) CMPD of ASD [3]. These are almost always called comorbidities. However, comorbidities mean that medical conditions present are not related to a main diagnosis, in this case ASD. The design of the research studies in ASD is performed considering OFAT (one factor at a time) instead of the context of multimorbidity. ASD is a model psychiatric disorder following the DSM-5 for the analysis of multimorbidity and personalized medicine.
In this case, multimorbidity is present in the brain (psychiatric and neurological issues) and outside the brain (biological problems in body systems outside the brain) with behavioral, emotional, motor, sensorial and communicational symptoms. The physicians related to these areas are from Psychiatry, Neurology and Pediatrics. The Pediatrician detects ASD and refers to other areas. However, in the Advanced Integrative Model the Pediatrician (or General Practitioner) by training, experience, and competence, is of paramount importance in the Advanced Integrative Approach.
2.1. The Response to Treatment of CMPD in ASD
When a family receives a diagnosis, CMPD are considered comorbid, not related to ASD and of little or no impact on core symptoms or trajectory of ASD. The recommended practice, if it includes exploration of CMPD, is only the limited exploration of gastrointestinal issues, beyond the neurological or psychiatric co-occurring medical problems. In a recent manuscript the recommendations were educational practices, developmental therapies, and behavioral interventions, but CMPD (in particular the out-of-the-brain biological issues) were not properly considered in the state-of-the art knowledge [43]. It has not been considered, historically, that the results of all the behavioral, relational, developmental or psychoeducative methods to approach ASD are strongly related to the biological status of the person with the ASD diagnosis.
The question is how could the treatment(s) of CMPD affect the ASD symptoms?
There are several possible outcomes to the treatments of CMPD of ASD outside the brain.
There are people diagnosed with ASD (mainly children) whose core ASD symptoms disappear after the adequate treatment of CMPD outside the brain. ASD symptoms seem to be only symptoms of a few CMPD outside the brain with a causal relation [44].
There are people diagnosed with ASD (all ages) whose core ASD symptoms ameliorate after the adequate treatment of CMPD outside the brain. Many times, several CMPD need to be considered and properly treated to show an impact in the core symptoms. ASD symptoms appear to be related to CMPD in ASD [45].
There are people diagnosed with ASD (all ages) whose core ASD symptoms do not change after the adequate treatment of CMPD outside the brain, even when several CMPD are considered and properly treated. ASD symptoms are not related to CMPD in ASD. In this case, they could be called “comorbid”.
The individual response to the most rigorous, controlled, and serious allopathic treatments of CMPD in ASD, taking into account multimorbidity and complexity, gives clues to their roles. Therefore, the role of CMPD in ASD would be shown or concluded after and not before the treatment of them.
As Dr. Frye’s group has reported, in ASD many neurological issues have links to biological problems not related to the brain [46]. Dr. Frye has published several important manuscripts about CMPD in ASD and from the design the work is presented differently than other manuscripts. The titles of these manuscripts generally are “XXX as treatment of YYY in ASD”. This kind of approach to the problem takes into account, since the design, the multiple CMPD in ASD. Historically the presentation of the treatment of CMPD was “XXX as treatment of ASD”. Many recent manuscripts detect, count, and report the so called “comorbidities” instead of considering new models for the role of these CMPD [4][47][48]. Not all children with gut dysbiosis have ASD, not all children with mitochondrial dysfunction or with some immune deficiency have ASD. There should be another component to take into account and address this complexity and this other component is the brain status in ASD.
2.2. The Controversy about Whether a Static or Dynamic Encephalopathy Contributes to ASD
Encephalopathy is a term used here for a diffuse disorder (or disease) that alters brain function or structure. An encephalopathy is dynamic when it responds to treatments of CMPD outside the brain. An encephalopathy is static when it does not change; it does not respond to treatments of CMPD. A central point is if the encephalopathy in ASD is static or dynamic and how. The dynamic encephalopathy in ASD is considered at first to be chronic and difficult to change, once present. The dynamism of the encephalopathy would also be related to the plastic nature of the brain and the number, combination, and severity of the CMPD. The development of the encephalopathy and the path to chronicity of it is then considered a process, not a genes-mediated fact for all people diagnosed with ASD. This process may begin prenatally (as vulnerability and/or through a genetic mutation/s or polymorphism/s and combinations of them with environmental impact) and/or postnatally. The mechanisms for the encephalopathy to develop involve the genetic susceptibility to CMPD in the brain and outside the brain and the individual response to in-series and in-parallel exposures in the second decade of the XXI century. From processed food to antibiotics, from contaminated water and air to mitochondrial impact, from dysbiosis to whole-body dysfunction and more the many pathways to gut barrier permeability and brain–blood barrier permeability in vulnerable people are explained looking at the model of ASD as symptoms of a dynamic (but chronic) encephalopathy.
Since the presentation of the genetic model (GM) with the manuscript of Folstein and Rutter [49], 44 years has shown the exploration of the genetic basis of ASD. Meanwhile, the prevalence has grown up to 1 in 54 from the CDC data [50], that is more related to 1 in 36 [51] and near 1 in 20 males in children up to 17 years or even higher [52]. The main point of the GM is the consideration of the root of ASD as a static encephalopathy of prenatal origin [53]. In the neurodiversity model, ASD is a way of being [54]. These two points of view do not explain many findings, do not give tools or resources to professionals, non-professionals and families to address the individual complex medical, non-medical, and educational needs of many children, teens and adults diagnosed with ASD. There are several recent reports about these unmet needs [55][56][57].
The Advanced Integrative Model (AIM) is a new model of ASD. In this model the CMPD outside the brain should be properly diagnosed and treated. These CMPD may be related to a chronic encephalopathy through the barrier’s permeability. Gut and brain blood barrier permeabilities are important to understand in this proposal. The gut dysbiosis involves pathogenic bacteria, parasites, and fungus that may translocate and/or produce metabolites and correlates with inflammation in the presence of a permeable gut barrier. This abnormal situation produces an immune response. The immune system components and metabolites from gut dysbiosis reach the bloodstream due to the permeable gut barrier and finally the brain due to brain blood barrier permeability [15]. The idea of a chronic, dynamic encephalopathy as a model of ASD was presented by Dr. Herbert in 2005 [58] but unfortunately was not explored adequately up until the last 10 years and much more in the last 5 years.
In the framework of a model based on the explanation of ASD as a static encephalopathy of prenatal origin, the plausibility of a role of postnatal development disturbance is not taken into account and dismissed. CMPD have been labeled as “comorbid”: medical issues that have no link to ASD. Coincidence or simply better health has been the explanation for the reported improvements after treatments of CMPD, which are sometimes very dramatic. Regression (loss of speech and/or abilities and/or skills) continues to happen today without explanation in these models. No other proposals have been presented, even when no genetic link in brain to regression can be clearly shown in ASD [59]. Today, regression has been reported to be present in prospective studies in up to 88% of people with ASD [60], although the consensus in retrospective studies is lower and nearer 30% [61]. Regression is understood in AIM as the final point of a pre-encephalopathy process. The pathway to chronicity is considered to be an individual process and not a single event [62] and the final point could be considered to be the regression. The chronic status of the encephalopathy would be related to chronic pathophysiological processes in the brain in ASD (see reference [51] for further explanation).
3. AIM Classification System
Core symptoms of ASD include impairments in social interaction and communication, and restricted and repetitive behaviors. There are no known efficacious treatments for the core social symptoms, although effects on repetitive behaviors have been reported [63].
The main groups in ASD following the AIM would now be:
Main Group 1—Core ASD symptoms disappear after the adequate treatment of CMPD outside the brain. In this case the encephalopathy is dynamic and completely reversible with loss of the ASD diagnosis.
Main Group 2—Core ASD symptoms ameliorate after the adequate treatment of CMPD outside the brain. In this case the encephalopathy is dynamic but chronic, partially reversible, with improvements in ASD symptoms from mild to huge, even without loss of the ASD diagnosis.
Main Group 3—Core ASD symptoms do not change after the adequate treatment of CMPD outside the brain, even when several CMPD are considered and properly treated. Some people diagnosed with ASD without intellectual disability (ID) and Asperger’s syndrome following DSM-IV would be a subgroup where ASD is related to a condition as a way of being. Other subgroups would have strong links to genetics, with ID besides the ASD diagnosis, and the ASD symptoms would be related to a static encephalopathy in the subgroup called “syndromic autism” [64]. These subgroups are very different.
In these three main groups, many different subgroups may be defined, considering ID or not, speech problems, sex, age and more. Figure 1 shows the comparison between the Genetic Model (GM) and the Advanced Integrative Model (AIM) in their answer to the first of the three important questions this manuscript presents: What does ASD mean?
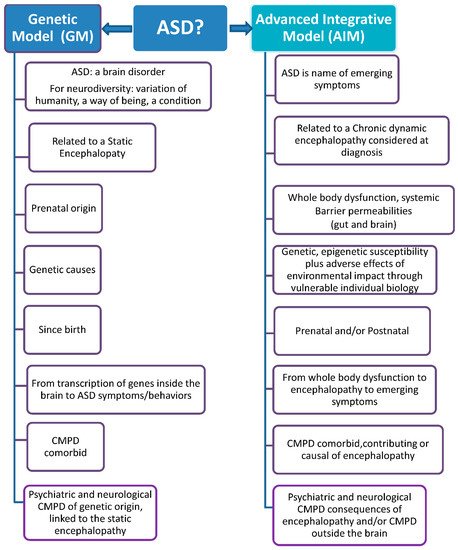
Figure 1. Genetic Model versus Advanced Integrative Model.
References
- Cheroni, C.; Caporale, N.; Testa, G. Autism spectrum disorder at the crossroad between genes and environment: Contributions, convergences, and interactions in ASD developmental pathophysiology. Mol. Autism 2020, 11, 1–18.
- Rosen, N.E.; Lord, C.; Volkmar, F.R. The Diagnosis of Autism: From Kanner to DSM-III to DSM-5 and Beyond. J. Autism Dev. Disord. 2021.
- Torres, E.B. Reframing Psychiatry for Precision Medicine. J. Pers. Med. 2020, 10, 144.
- Sala, R.; Amet, L.; Blagojevic-Stokic, N.; Shattock, P.; Whiteley, P. Bridging the Gap Between Physical Health and Autism Spectrum Disorder. Neuropsychiatr. Dis. Treat. 2020, 16, 1605–1618.
- Muskens, J.B.; Velders, F.P.; Staal, W.G. Medical comorbidities in children and adolescents with autism spectrum disorders and attention deficit hyperactivity disorders: A systematic review. Eur. Child. Adolesc. 2017, 26, 1093–1103.
- Lai, M.; Kassee, C.; Besney, R.; Bonato, S.; Hull, L.; Mandy, W.; Szatmari, P.; Ameis, S.H. Prevalence of co-occurring mental health diagnoses in the autism population: A systematic review and meta-analysis. Lancet Psychiatry 2019, 6, 819–829.
- Pan, P.Y.; Bölte, S.; Kaur, P.; Jamil, S.; Jonsson, U. Neurological disorders in autism: A systematic review and, meta-analysis. Autism 2020.
- Dizitzer, Y.; Meiri, G.; Flusser, H.; Michaelovski, A.; Dinstein, I.; Menashe, I. Comorbidity and health services’ usage in children with autism spectrum disorder: A nested case-control study. Epidemiol. Psychiatr. Sci. 2020, 29, e95.
- Cawthorpe, D. A 16-Year Cohort Analysis of Autism Spectrum Disorder-Associated Morbidity in a Pediatric Population. FrontPsychiatry 2018, 9, 635.
- Tye, C.; Runicles, A.K.; Whitehouse, A.J.O.; Alvares, G.A. Characterizing the Interplay Between Autism Spectrum Disorder and Comorbid Medical Conditions: An Integrative Review. Front. Psychiatry 2019, 9, 751.
- Miles, A. Complexity in medicine and healthcare: People and systems, theory and practice. J. Eval. Clin. Pract. 2009, 15, 409–410.
- Braithwaite, J.; Churruca, K.; Long, J.C.; Ellis, L.A.; Herkes, J. When complexity science meets implementation science: A theoretical and empirical analysis of systems change. BMC Med. 2018, 16, 63.
- Nilsen, P. Making sense of implementation theories, models and frameworks. Implement. Sci. 2015, 10, 53.
- Witteman, H.O.; Stahl, J.E. Facilitating interdisciplinary collaboration to tackle complex problems in health care: Report from an exploratory workshop. Health Syst. 2013, 2, 162–170.
- Pei-Yin, P.; Tammimies, G.; Bölte, S. The Association Between Somatic Health, Autism Spectrum Disorder, and Autistic. Traits Behav. Genet. 2020, 50, 233–246.
- Panisi, C.; Guerini, F.R.; Abruzzo, P.M.; Balzola, F.; Biava, P.M.; Bolotta, A.; Brunero, M.; Burgio, E.; Chiara, A.; Clerici, M.; et al. Autism Spectrum Disorder from the Womb to Adulthood: Suggestions for a Paradigm Shift. J. Pers. Med. 2021, 11, 70.
- Vargason, T.; Frye, R.E.; McGuinness, D.L.; Hahn, J. Clustering of co-occurring conditions in autism spectrum disorder during early childhood: A retrospective analysis of medical claims data. Autism Res. 2019, 12, 1272–1285.
- Randolph-Gips, M.; Srinivasan, P. Modeling autism: A systems biology approach. J. Clin. Bioinform. 2012, 2, 17.
- Rohleder, N. Translating biobehavioral research advances into improvements in health care-a “network of networks” approach to multimorbidity. J. Eval. Clin. Pract. 2017, 23, 230–232.
- Happè, F.; Frith, U. Annual research review: Towards a developmental neuroscience of atypical social cognition. J. Child. Psychol. Psych. 2014, 3, 553–577.
- Pino, M.C.; Mariano, M.; Peretti, S.; D’Amico, S.; Masedu, F.; Valenti, M.; Mazza, M. When do children with autism develop adequate social behaviour? Cross-sectional analysis of developmental trajectories. Eur. J. Develop. Psychol. 2018, 17, 71–87.
- Zou, R.; Xu, F.; Wang, F.; Duan, M.; Guo, M.; Zhang, Q.; Zhao, H.; Zheng, H. Changes in the Gut Microbiota of Children with Autism Spectrum Disorder. Autism Res. 2020, 13, 1614–1625.
- Catassi, C.; Bai, J.C.; Bonaz, B.; Bouma, G.; Calabrò, A.; Carroccio, A.; Castillejo, G.; Ciacci, C.; Cristofori, F.; Dolinsek, J.; et al. Non-Celiac Gluten sensitivity: The new frontier of gluten related disorders. Nutrients 2013, 5, 3839–3853.
- Quadros, E.V.; Sequeira, J.M.; Brown, T.; Mevs, C.; Marchi, E.; Flory, M.; Jenkins Velinov, M.T.; Cohen, I.L. Folate receptor autoantibodies are prevalent in children diagnosed with autism spectrum disorder, their normal siblings and parents. Autism Res. 2018, 11, 707–712.
- Xu, G.; Snetselaar, L.G.; Jing, J.; Liu, B.; Strathearn, L.; Bao, W. Association of Food Allergy and Other Allergic Conditions with Autism Spectrum Disorder in Children. JAMA Netw. Open 2018, 1, e180279.
- Chakraborty, P.; Carpenter, K.L.; Major, S.; Deaver, M.; Vermeer, S.; Herold, B.; Franz, L.; Howard, J.; Dawson, G. Gastrointestinal problems are associated with increased repetitive behaviors but not social communication difficulties in young children with autism spectrum disorders. Autism 2020.
- Frye, R.E.; Rossignol, D.A.; Scahill, L.; McDougle, C.J.; Huberman, H.; Quadros, E.V. Treatment of Folate Metabolism Abnormalities in Autism Spectrum Disorder. Semin. Pediatr. Neurol. 2020, 35, 100835.
- Delhey, L.M.; Tippett, M.; Rose, S.; Bennuri, S.C. Comparison of Treatment for Metabolic Disorders Associated with Autism: Reanalysis of Three Clinical Trials. Front. Neurosci. 2018, 12, 19.
- Pangrazzi, L.; Balasco, L.; Bozzi, Y. Oxidative Stress and Immune System Dysfunction in Autism Spectrum Disorders. Int. J. Mol. Sci. 2020, 21, 3293.
- Hughes, H.K.; Ko, E.M.; Rose, D.; Ashwood, P. Immune Dysfunction and Autoimmunity as Pathological Mechanisms in Autism Spectrum Disorders. Front. Cell Neurosci. 2018, 12, 405.
- Frye, R.E. Mitochondrial Dysfunction in Autism Spectrum Disorder: Unique Abnormalities and Targeted Treatments. Semin. Pediatr. Neurol. 2020, 35, 100829.
- Fiorentino, M.; Sapone, A.; Senger, S.; Camhi, S.S.; Kadzielski, S.M.; Buie, T.M.; Kelly, D.L.; Cascella, N.; Fasano, A. Blood-brain barrier and intestinal epithelial barrier alterations in autism spectrum disorders. Mol. Autism 2016, 7, 49.
- Hu, T.; Dong, Y.; He, C.; Zhao, M.; He, Q. The Gut Microbiota and Oxidative Stress in Autism Spectrum Disorders (ASD). Oxid. Med. Cell. Longev. 2020, 2020, 8396708.
- Wilson, H.A.; Creighton, C.; Scharfman, H.; Choleris, E.; MacLusky, N.J. Endocrine Insights into the Pathophysiology of Autism Spectrum Disorder. Neuroscientist 2020.
- Nudel, R.; Appadurai, V.; Schork, A.J.; Buil, A.; Bybjerg-Grauholm, J.; Børglum, A.D.; Daly, M.J.; Mors, O.; Hougaard, D.M.; Mortensen, P.B.; et al. A large population-based investigation into the genetics of susceptibility to gastrointestinal infections and the link between gastrointestinal infections and mental illness. Hum. Genet. 2020, 139, 593–604.
- Loyacono, N.; Ferreira, M.L.; Iermoli, R. Humanism in medicine: The critical role of pediatricians in autism spectrum disorder. Arch. Argent. Pediatr. 2019, 117, 195–197.
- Plummer, J.T.; Gordon, A.J.; Levitt, P. The Genetic Intersection of Neurodevelopmental Disorders and Shared Medical Comorbidities—Relations that Translate from Bench to Bedside. Front. Psychiatry 2016, 7, 142.
- Constantino, J.N. Deconstructing autism: From unitary syndrome to contributory developmental endophenotypes. Int. Rev. Psychiatry 2018, 30, 18–24.
- Weiss, C.O.; Varadhan, R.; Puhan, M.A.; Vickers, A.; Bandeen-Roche, K.; Boyd, C.M.; Kent, D.M. Multimorbidity and evidence generation. J. Gen. Intern. Med. 2014, 29, 653–660.
- Murad, M.H.; Asi, N.; Alsawas, M.; Alahdab, F. New evidence pyramid. BMJ Evid. Based Med. 2016, 21, 125–127.
- Jeste, S.S. The neurology of autism spectrum disorders. Curr. Opin. Neurol. 2011, 24, 132–139.
- Hossain, M.; Khan, N.; Sultana, A.; Ma, P.; McKyer, E.L.J.; Ahmed, H.U.; Purohit, N. Prevalence of comorbid psychiatric disorders among people with autism spectrum disorder: An umbrella review of systematic reviews and meta-analyses. Psychiatry Res. 2020, 287, 112922.
- Hyman, S.L.; Levy, S.E.; Myers, S. Identification, Evaluation, and Management of Children with Autism Spectrum Disorder. Pediatrics 2020, 145, e20193447.
- Genuis, S.J.; Bouchard, T.P. Celiac disease presenting as autism. J. Child. Neurol. 2010, 25, 114–119.
- Adams, J.B.; Audhya, T.; Geis, E.; Gehn, E.; Fimbres, V.; Pollard, E.L.; Mitchell, J.; Ingram, J.; Hellmers, R.; Laake, D.; et al. Comprehensive Nutritional and Dietary Intervention for Autism Spectrum Disorder-A Randomized, Controlled 12-Month Trial. Nutrients 2018, 10, 369.
- Frye, R.E. Metabolic and mitochondrial disorders associated with epilepsy in children with autism spectrum disorder. Epilepsy Behav. 2015, 47, 147–157.
- Li, X.; Liu, G.; Chen, W.; Bi, Z.; Liang, H. Network analysis of autistic disease comorbidities in Chinese children based on ICD-10 codes. BMC Med. Inform. Decis. Mak. 2020, 20, 268.
- Brooks, J.D.; Bronskill, S.E.; Fu, L.; Saxena, F.E.; Arneja, J.; Pinzaru, V.B.; Anagnostou, E.; Nylen, K.; McLaughlin, J.; Tu, K. Identifying Children and Youth with Autism Spectrum Disorder in Electronic Medical Records: Examining Health System Utilization and Comorbidities. Autism Res. 2020.
- Folstein, S.; Rutter, M. Infantile autism: A genetic study of 21 twin pairs. J. Child. Psychol. Psychiatry 1977, 18, 297–321.
- Maenner, M.J.; Shaw, K.A.; Baio, J.; Washington, A.; Patrick, M.; DiRienzo, M.; Christensen, D.L.; Wiggins, L.D.; Pettygrove, S.; Andrews, J.G.; et al. Prevalence of Autism Spectrum Disorder Among Children Aged 8 Years—Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2016. MMWR Surveill. Summ. 2020, 69, 1–12.
- Zablotsky, B.; Black, L.I.; Blumberg, S.J. Estimated Prevalence of Children with Diagnosed Developmental Disabilities in the United States, 2014–2016; NCHS Data Brief, No 291; National Center for Health Statistics: Hyattsville, MD, USA, 2017.
- Chiarotti, F.; Venerosi, A. Epidemiology of Autism Spectrum Disorders: A Review of Worldwide Prevalence Estimates Since 2014. Brain Sci. 2020, 10, 274–284.
- Amaral, D.G.; Anderson, G.M.; Bailey, A.; Bernier, R.; Bishop, S.; Blatt, G.; Canal-Bedia, R.; Charman, T.; Dawson, G.; de Vries, P.J.; et al. Gaps in Current Autism Research: The Thoughts of the Autism Research Editorial Board and Associate Editors. Autism Res. 2019, 12, 700–714.
- Masataka, N. Implications of the idea of neurodiversity for understanding the origins of developmental disorders. Phys. Life Rev. 2017, 20, 85–108.
- Wills, J.; Evans, S. Health and Service Provision for People with Autism Spectrum Disorders: A Survey of Parents in the United Kingdom, 2014; Queen Mary University of London: London, UK, 2016.
- Report from Autistica UK Personal Tragedies, Public Crisis, 2016, 12 Pages. Available online: (accessed on 14 January 2020).
- Drexler University Life Course Outcomes. Available online: (accessed on 2 June 2021).
- Herbert, M. Autism a brain disorder or a disorder that affects the brain? Clin. Neurol. 2005, 6, 354–379.
- Tammimies, K. Genetic mechanisms of regression in autism spectrum disorder. Neurosci. Biobehav. Rev. 2019, 102, 208–220.
- Ozonoff, S.; Gangi, D.; Hanzel, E.P.; Hill, A.; Hill, M.M.; Miller, M.; Schwichtenberg, A.J.; Steinfeld, M.B.; Parikh, C.; Iosif, A.M. Onset patterns in autism: Variation across informants, methods, and timing. Autism Res. 2018, 11, 788–797.
- Tan, C.; Frewer, V.; Cox, G.; Williams, K.; Ure, A. Prevalence and Age of Onset of Regression in Children with Autism Spectrum Disorder: A Systematic Review and Meta-analytical Update. Autism Res. 2021.
- Tanner, A.; Dounavi, K. The Emergence of Autism Symptoms Prior to 18 Months of Age: A Systematic Literature Review. J. Autism Dev. Disord. 2020.
- Farmer, C.; Thurm, A.; Grant, P. Pharmacotherapy for the Core Symptoms in Autistic Disorder: Current Status of the Research. Drugs 2013, 73, 303–314.
- Fernandez, B.A.; Scherer, S.W. Syndromic autism spectrum disorders: Moving from a clinically defined to a molecularly defined approach. Dialogues Clin. Neurosci. 2017, 19, 353–371.
More
Information
Subjects:
Psychology, Psychoanalysis
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.1K
Revisions:
2 times
(View History)
Update Date:
22 Jun 2021
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




