
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Daniel Mdetele | + 2962 word(s) | 2962 | 2021-06-21 09:57:55 | | | |
| 2 | Lindsay Dong | Meta information modification | 2962 | 2021-06-22 03:10:47 | | |
Video Upload Options
Peste des petits ruminants (PPR) is an important transboundary animal disease of domestic small ruminants, camels, and wild artiodactyls. The disease has significant socio-economic impact on communities that depend on livestock for their livelihood and is a threat to endangered susceptible wild species.
1. Introduction
Peste des petits ruminants (PPR) is a highly contagious and economically important viral disease of domestic small ruminants [1]. It can also cause severe disease and mortality in some wild artiodactyl species and is a threat to biodiversity conservation [2][3][4], and can infect other atypical domestic species such as cattle, camels and pigs. It is caused by Small ruminant morbillivirus (commonly known as PPR virus) of the genus Morbillivirus and the family Paramyxoviridae, and has been classified into four genetically distinct lineages (I, II, III and IV) based on a partial sequence analysis of the fusion protein (F) and nucleoprotein (N) genes [5][6]. PPR virus (PPRV) was first identified in Côte d’Ivoire, West Africa, in 1942, and it is currently believed to be endemic across much of West, Central, North and East Africa, the Middle East and Central, South and East Asia [7]. Geographically, lineages I and II have been found predominantly in West and Central Africa, and lineage III has been found predominantly in East Africa and the Middle East [8]. Lineage IV is the main lineage found in Asia [9], both in wild and domestic small ruminants [10][11][12], and more recently it has been found in Africa [5][13][14].
Clinically, the disease in sheep and goats is characterised by a high fever, catarrhal ocular discharges, mucopurulent nasal discharges and erosive stomatitis in the early stages, followed by severe enteritis and bronchopneumonia [15].
PPRV is transmitted primarily through direct contact between infected and susceptible animals, therefore communal grazing areas and live animal markets are important places for the spread of the virus [8][16]. Large amounts of infective virus are excreted in secretions and discharges from the eyes, nose and mouth, as well as in faeces [17][18][19], and the primary route of infection is respiratory, by short-range aerosols generated by sneezing and coughing [20]. The virus is fragile, so transmission of the virus by fomites is unlikely [20]. Animals that are infected with PPRV start to develop antibodies from seven to ten days post-infection [17]. Those animals that recover from infection develop lifelong immunity that is fully protective against reinfection [20], and vaccination with live attenuated PPRV vaccine also provides lifelong protection [21][22]. The offspring of immune animals have protective maternal antibodies for up to three to four months of age [23][24][25].
The Global Strategy for the Control and Eradication of PPR (PPR GCES) was officially adopted in 2015 by the World Organization for Animal Health (OIE) and the Food and Agriculture Organization of the United Nations (FAO). The strategy describes the rationale for controlling and eradicating PPRV, the general principles, and the tools to be used [1]. A strategy for Africa has been developed by the African Union Inter-African Bureau for Animal Resources (AU-IBAR), and regional strategies that are aligned with the global strategy have been developed by the Inter-governmental Authority on Development (IGAD) for the Greater Horn region, and the Southern African Development Community (SADC) [26][27][28]. At the national level, Tanzania is in the process of developing a national PPRV control and eradication plan.
2. Peste des Petits Ruminants Occurrence and Spread in Tanzania
2.1. Evidence of PPRV in Tanzania Prior to Confirmation of Infection in 2008
Wambura (2000) reported that, prior to 1998, no PPR outbreaks had been reported, and no serological surveys had been carried out in Tanzania [29]. In 1998, a national cross-sectional serological survey for PPRV and rinderpest virus was conducted. A total of 3134 serum samples were collected from sheep and goats that were randomly selected from all 20 regions in all seven surveillance zones of mainland Tanzania (Figure 1). The samples were analysed for both rinderpest virus and PPRV antibodies using the rinderpest virus haemagglutinin (H) competitive enzyme-linked immunosorbent assay (c-ELISA) and the PPRV H c-ELISA [30], and all were negative in both tests, including 520 samples from Arusha region where the first confirmed outbreak of PPRV was subsequently detected in 2008 [29]. Rinderpest virus is a morbillivirus that is closely related to PPRV, for which cattle were the main host, but sheep and goats could be infected with rinderpest virus and develop antibodies. The global eradication of rinderpest was achieved in 2011. PPRV antibodies could cross-react with rinderpest virus c-ELISA, therefore it was necessary to run both assays in parallel to distinguish between PPRV and rinderpest virus antibodies.
2.2. The First Confirmed PPRV Disease Cases in Northern Tanzania, 2008
In summary, during 2008, PPR disease was confirmed in Ngorongoro district of northern Tanzania, and serological surveys in the Northern and Lake Zones demonstrated that PPRV had been circulating widely in northern Tanzania. It is likely that PPRV was introduced to northern Tanzania from infected areas of southern Kenya through the movement of live infected animals via trade or cross-border movements of flocks, and the delays in the diagnosis of PPRV and the implementation of control measures led to virus spread across the north of the country [31]. Vaccination campaigns during 2009–2012 reduced the number of outbreaks, but PPRV outbreaks continued to occur on an annual basis due to limited vaccination coverage.
2.3. Emergence of PPRV Disease in Southern Tanzania, 2009–2010
PPR disease in sheep and goats was first suspected in southern Tanzania in December 2009, based on interviews conducted with local animal health workers and veterinarians [32]. The first village to be affected was believed to be Likuna village in Newala district, Mtwara region, which borders with Mozambique (Figure 1). The source of infection was suspected to be from 70 goats that were purchased by a livestock trader from the Pugu livestock market, near Dar es Salaam.
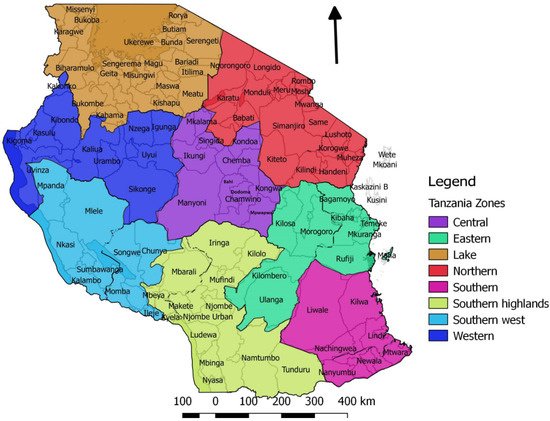
Figure 1. Map of Tanzania showing the seven surveillance zones and their respective districts. Each zone has a Veterinary Investigation Centre and a laboratory.
In October 2010, risk-based sero-surveillance was carried out in districts in southern Tanzania bordering Mozambique, Malawi, and Zambia. Out of the 720 sera collected, 36.9% were PPRV antibody positive [32]. Although positive serology in the absence of PPRV vaccination suggested that PPRV was circulating, PPRV was not confirmed as the cause of the disease in southern Tanzania until Muse et al. (2011) investigated clinical cases of suspected PPR in two villages in Tandahimba district in March 2011 [33]. PPRV ribonucleic acid (RNA) was detected in ocular and nasal swabs and tissues from post mortem examination by RT-PCR.
In summary, suspected PPR disease occurred in 2009 in southern Tanzania and was confirmed to be caused by PPRV in 2011. It is likely that the virus was introduced by animal trade from another part of Tanzania via a live animal market in Dar es Salaam and was subsequently spread through small ruminant trade and communal grazing. A delay in the confirmation of the diagnosis led to delays in the implementation of control measures which contributed to the persistence and spread of PPRV [32].
2.4. PPRV Disease in Eastern Tanzania, 2010
In April 2010, there was high mortality of sheep and goats from an unknown disease in Ulanga district in the southern part of Morogoro region in the Eastern Zone of Tanzania [32]. Investigations indicated that this could have been caused by PPRV, which could have been introduced to the area by the northward movement of migratory pastoralists from Mtwara and Lindi regions. Out of 200 serum samples collected, 76% were positive by PPRV H c-ELISA [32]. In June–July 2010, outbreaks of suspected PPR disease were reported in the Mvomero district in the north of Morogoro region [34]. An investigation was conducted and clinical cases in goats and sheep were found, with signs of nasal discharge, diarrhoea and oral ulcers, while enlarged and congested gastrointestinal and bronchial lymph nodes were observed during post mortem examinations. PPRV was confirmed in tissue samples by RT-PCR. In order to control the outbreaks in the Southern and Eastern Zones, the Directorate of Veterinary Services supported ring vaccination through the provision of PPRV vaccine to Mtwara and Ruvuma regions (252,000 doses), and to Ulanga in the Morogoro region (74,000 doses), which appeared to limit the spread of the disease [31].
2.5. PPRV Disease in Central Tanzania, 2014
In the Dodoma region of Central Tanzania, field and laboratory reports held at Dodoma Veterinary Investigation Centre provided evidence of PPRV circulation in this area from 2014. In August 2014, a disease outbreak was reported among small ruminants in Kongwa district, Dodoma Region, in which 19.5% of the affected animals died (Theodata, 2014 Field work report at Central Zone Investigation Centre, unpublished report). The animals were observed to have diarrhoea, profuse nasal discharge, dyspnoea and anorexia. Out of 30 serum samples collected, 53.3% were positive for PPRV by PPRV c-ELISA (the type of c-ELISA was not specified).
2.6. Evidence of PPRV Infection in Camels and Cattle
Serological evidence of PPRV infection in camels was found in a study carried out in northern Tanzania [35]. This study was carried out in eight districts of Arumeru, Longido, Monduli, Mwanga, Same, Hai, Simanjiro and Kilindi in four regions of northern Tanzania. During June and August 2010, a total of 193 serum samples were collected from 14 camel herds and tested for PPRV antibody by H c-ELISA. The overall seroprevalence was 2.6% (5/193) providing evidence of PPRV infection in camels, although no clinical disease had been observed.
2.7. Evidence of PPRV Infection in Wildlife
In June 2014, a study was carried out looking for evidence of a spillover of PPRV from domestic to wild ruminants in NCA [36]. Eleven sampling sites were purposively selected where resident wild ruminants were present in close proximity to domestic small ruminants, sharing grazing and water sources. Clinical cases of PPR disease were confirmed in sheep and goats in the area during the study period by a PPRV rapid diagnostic test (BDSL) and RT-PCR. Serum samples were collected from 46 wild animals. Overall, 63% of the animals sampled were positive by PPRV H c-ELISA (BDSL), and all herds and species had at least one sero-positive animal, except for Thomson’s gazelle for which only one animal was sampled: African buffalo (10 sampled from 2 herds, 50% positive); Grant’s gazelle (30 sampled from 8 herds, 67% positive); Thomson’s gazelle (1 sampled, 0% positive); wildebeest (Connochaetes taurinus) (2 sampled from one herd, 50% positive); impala (Aepyceros melampus) (3 sampled from one herd, 100% positive).
2.8. Molecular Biology of PPRV in Tanzania
As described earlier, tissue samples collected from goats with clinical signs of PPR from the Ngorongoro district during 2008 were PPRV RT-PCR positive [32]. A partial nucleoprotein gene sequence was obtained, and phylogenetic analysis showed that it was a lineage III virus, and that it clustered with PPRV detected in East Africa (Ethiopia in 1994, Sudan in 1972) as well as from the United Arab Emirates (1986) and Oman (1983).
During the outbreak investigations conducted by Namtimba in 2014–2015 in Morogoro district in Morogoro region, phylogenetic analysis of the nucleoprotein gene nucleotide sequences showed that the PPRV involved in the outbreak clustered with lineage III viruses and was closely related to those reported by Kgotlele et al., (2014) in Dakawa and Ngorongoro (Figure 2) [34][37].
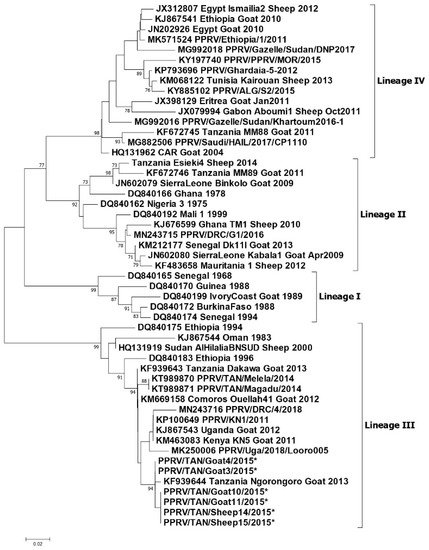
Figure 2. Phylogenetic tree reproduced from Jones et al. (2020) [38]. Neighbour-joining tree constructed on the basis of partial nucleoprotein gene sequences of the peste des petits ruminants virus (PPRV). The tree shows the relationships among African PPRV isolates. The scale bar indicates the nucleotide substitutions per site. The Kimura 2-parameter model with the percentage of replicate trees in which the associated taxa clustered together in the 1000 bootstrap replicates is shown next to the branches. The taxon name of the sequences retrieved from the GenBank contains the accession number followed by the name of the country and the year of isolation. The Tanzanian sequences appearing in this diagram and described in this paper are.
In summary, lineage III PPRVs that share a common ancestry with lineage III viruses from Kenya, Uganda and DRC have been identified in northern and eastern Tanzania between 2008 and 2015 (Figure 3). Lineage IV virus has been detected once from southern Tanzania, and closely related lineage II viruses have been detected in the north and the south. This diversity of viruses indicates that there have been multiple introductions of PPRV into Tanzania. PPRV lineage III viruses predominate in East Africa (Sudan, Ethiopia, Kenya, Uganda, Burundi and DRC), while lineage IV viruses have been detected in East (Uganda, South Sudan, Sudan and Ethiopia), Central and North Africa [39]. The detection of lineage II viruses could be due to the circulation of a virus that is a variant of the Nigeria 75/1 vaccine strain, or it is possible that the results are due to laboratory contamination [39]. It is therefore important to continue to obtain samples for genome sequencing from PPRV outbreaks in all parts of Tanzania to better represent the PPR viruses that are circulating, and to gain insights into how PPRV is spread within the country and across borders.
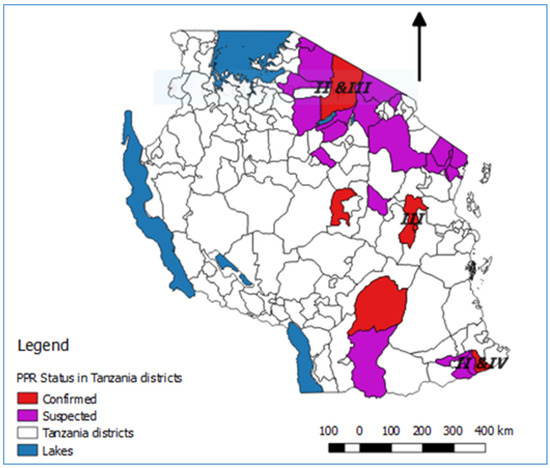
Figure 3. Map of Tanzania showing the districts where PPRV disease was laboratory confirmed or suspected during the period 2008 to 2018. The places where PPRV partial genome sequences were obtained are indicated by the relevant lineage numbers.
2.9. PPRV Serological Surveys
In summary, the serological surveys concluded that the occurrence of PPRV in the country shows ecological zone pre-disposition, with sero-prevalence being higher in semi-arid and coastal zones characterised by low relative humidity. They confirmed the presence of antibodies against PPRV in sheep and goats in regions of Tanzania that previously had little to no data on the disease, which indicated that PPRV had spread within Tanzania with the possibility of spread across the border to neighbouring countries.
2.10. PPRV Co-Infections with Other Pathogens
The confirmed PPRV outbreaks showed a diversity of clinical signs—some flocks primarily showed a respiratory syndrome, while others had a diarrhoea syndrome or a more classical PPR syndrome with nasal discharge, coughing and diarrhoea. In line with the clinical syndrome that they observed, the livestock keepers used different local names for the confirmed cases—local terms for respiratory disease, diarrhoea disease or rinderpest-like disease [38].
2.11. Challenges for the Control and Eradication of PPRV in Tanzania
In each area of Tanzania where PPRV emerged, infection spread widely before any control measures were carried out, and it is likely that PPRV is now endemic in many parts of Tanzania.
PPR disease shows a range of clinical signs that are similar to the signs of other diseases, such as contagious caprine pleuropneumonia, pasteurellosis, bluetongue, and foot-and-mouth disease. It was therefore not surprising that the livestock keepers and veterinarians had difficulty in differentiating the newly introduced PPR disease from other small ruminant diseases that were already present in their areas.
Most small ruminant production in Tanzania is under extensive management, either small-holder, pastoral or agro-pastoral production, which involves the movement of animals over shorter or longer distances on a daily or seasonal basis to access pasture, water and markets, and to avoid disease [40]. These movements lead to frequent contact between flocks, within and between districts, regions and countries, which facilitates the transmission of infectious pathogens such as PPRV and leads to the spread and maintenance of disease in Tanzania [41][35].
Tanzania has extensive land borders with eight countries in East, Central and Southern Africa, and there is unofficial movement of small ruminants across these borders for the purposes of accessing grazing or water sources, trade, or social reasons. The initial introduction of PPRV into Ngorongoro district has been attributed to the movement of pastoralist animals or the live animal trade with Kenya [32][42][31].
The live animal trade was mentioned as a possible source of introduction and spread of PPRV in Tanzania [42]. In particular, goats that were bought in a market near Dar es Salaam and transported to southern Tanzania were implicated as the cause of the PPR disease outbreak in the southern region [32][43].
The continued monitoring of wildlife for disease and evidence of infection will be important during the period of control or elimination of virus under the global eradication campaign for PPRV.
The OIE Performance of Veterinary Services (PVS) reports of 2009 and 2017 highlighted that the department of veterinary services in Tanzania is faced with a number of challenges that reduce its efficiency, from financial resources to the organizational structure [44][45]. This limits the capacity for the early detection, diagnosis, and rapid response to contain a transboundary disease such as PPR. The failure of the Veterinary Services to meet the OIE, FAO and World Trade Organization standards in terms of early reporting, identification (diagnosis), surveillance activities, livestock movement, border control, and timely management of disease outbreaks could have contributed to the initial spread of PPRV and the ongoing spread and maintenance of PPRV in different areas of Tanzania. Therefore, there is a need for a further strengthening of the veterinary services chain of command, capacity building of laboratories for efficient and timely disease diagnosis, and a strengthening of cross-border surveillance activities.
3. Conclusions
In Tanzania, PPRV was first confirmed and reported to the OIE in December 2008. Serological studies and reports indicate that PPRV may have entered the north of the country periodically before 2008 but this may be confounded by the presence of rinderpest virus up until 1999 or later in the border areas with Kenya and serum cross-reactivity for both viruses. It is likely that PPRV was introduced from neighbouring countries and then spread from the north to the south in Tanzania via the live animal trade and pastoralist movements. Although PPRV antibodies have been detected in sheep and goats in all zones, the highest prevalence of disease is in the Northern Zone where there is a large population of sheep and goats. PPRV antibodies have been detected in cattle, camels, and wildlife, and although no clinical cases have been reported in any of these species, there is an ongoing risk of spillover from sheep and goats at the wildlife–livestock interface and the risk of wildlife disease outbreaks is not negligible. The review shows that PPRV is now endemic in Tanzania, which is causing persistent economic losses in the livestock sector, disturbing livelihoods, and posing a potential threat to biodiversity conservation and the wildlife economy. This justifies investment in a rapid progression to the elimination of the virus from Tanzania in coordination with other countries in the region.
References
- FAO; OIE. Global strategy for the control and eradication of PPR. In Proceedings of the International Conference for the Control and Eradication of Peste des Petits Ruminants (PPR), Abidjan, Ivory Coast, 31 March–2 April 2015.
- Hoffmann, B.; Wiesner, H.; Maltzan, J.; Mustefa, R.; Eschbaumer, M.; Arif, F.A.; Beer, M. Fatalities in Wild Goats in Kurdistan Associated with Peste Des Petits Ruminants Virus. Transbound. Emerg. Dis. 2011, 59, 173–176.
- Munir, M. Role of Wild Small Ruminants in the Epidemiology of Peste des Petits Ruminants. Transbound. Emerg. Dis. 2013, 61, 411–424.
- Pruvot, M.; Fine, A.E.; Hollinger, C.; Strindberg, S.; Damdinjav, B.; Buuveibaatar, B.; Chimeddorj, B.; Bayandonoi, G.; Khishgee, B.; Sandag, B.; et al. Outbreak of peste des petits ruminants virus among critically endangered Mongolian saiga and other wild ungulates, Mongolia, 2016–2017. Emerg. Infect. Dis. 2020, 26, 51.
- Muniraju, M.; Munir, M.; Parthiban, A.R.; Banyard, A.C.; Bao, J.; Wang, Z.; Ayebazibwe, C.; Ayelet, G.; El Harrak, M.; Mahapatra, M.; et al. Molecular Evolution of Peste des Petits Ruminants Virus. Emerg. Infect. Dis. 2014, 20, 2023–2033.
- Libeau, G.; Diallo, A.; Parida, S. Evolutionary genetics underlying the spread of peste des petits ruminants virus. Anim. Front. 2014, 4, 14–20.
- Clarke, B.D.; Islam, M.R.; Abu Yusuf, M.; Mahapatra, M.; Parida, S. Molecular detection, isolation and characterization of Peste-des-petits ruminants virus from goat milk from outbreaks in Bangladesh and its implication for eradication strategy. Transbound. Emerg. Dis. 2018, 65, 1597–1604.
- Banyard, A.C.; Parida, S.; Batten, C.; Oura, C.; Kwiatek, O.; Libeau, G. Global distribution of peste des petits ruminants virus and prospects for improved diagnosis and control. J. Gen. Virol. 2010, 91, 2885–2897.
- Liu, F.; Li, J.; Li, L.; Liu, Y.; Wu, X.; Wang, Z. Peste des petits ruminants in China since its first outbreak in 2007: A 10-year review. Transbound. Emerg. Dis. 2018, 65, 638–648.
- Abu Elzein, E.M.E.; Housawi, F.M.T.; Bashareek, Y.; Gameel, A.A.; Al-Afaleq, A.I.; Anderson, E. Severe PPR Infection in gazelles kept under semi-free range conditions. J. Vet. Med. Ser. B Infect. Dis. Vet. Public Health 2004, 51, 68–71.
- Abubakar, M.; Munir, M. Peste des Petits Ruminants Virus: An Emerging Threat to Goat Farming in Pakistan. Transbound. Emerg. Dis. 2014, 61 (Suppl. 1), 7–10.
- Rahman, M.Z.; Haider, N.; Gurley, E.S.; Ahmed, S.; Osmani, M.G.; Hossain, M.B.; Islam, A.; Khan, S.A.; Hossain, M.E.; Epstein, J.H.; et al. Epidemiology and genetic characterization of Peste des petits ruminants virus in Bangladesh. Vet. Med. Sci. 2018, 4, 161–171.
- Kwiatek, O.; Ali, Y.H.; Saeed, I.; Khalafalla, A.I.; Mohamed, O.I.; Abu Obeida, A.; Abdelrahman, M.B.; Osman, H.M.; Taha, K.M.; Abbas, Z.; et al. Asian Lineage of Peste des Petits Ruminants Virus, Africa. Emerg. Infect. Dis. 2011, 17, 1223–1231.
- Misinzo, G.; Kgotlele, T.; Muse, E.A.; Van Doorsselaere, J.; Berg, M.; Munir, M. Peste des Petits Ruminants Virus Lineage II and IV From Goats in Southern Tanzania During an Outbreak in 2011. Br. J. Virol. 2015, 2, 1–4.
- Baron, M.D.; Parida, S.; Oura, C.A.L. Peste des petits ruminants: A suitable candidate for eradication? Vet. Rec. 2011, 169, 16–21.
- Madege. Peste des Petits Ruminants (PPR) and Contagious Pleuropneumonia (CBPP) Surveillance in Lake Zone, Presentation to the VACNADA Inception Workshop, Morogoro, 2011; Archived document at Zonal Veterinary Investigation Centre: Mwanza, Tanzania, 2011.
- Truong, T.; Boshra, H.; Embury-Hyatt, C.; Nfon, C.; Gerdts, V.; Tikoo, S.; Babiuk, L.A.; Kara, P.D.; Chetty, T.; Mather, A.; et al. Peste des Petits Ruminants Virus Tissue Tropism and Pathogenesis in Sheep and Goats following Experimental Infection. PLoS ONE 2014, 9, e87145.
- Baron, J.; Bin-Tarif, A.; Herbert, R.; Frost, L.; Taylor, G.; Baron, M.D. Early changes in cytokine expression in peste des petits ruminants disease. Vet. Res. 2014, 45, 22.
- Munir, M.; Zohari, S.; Berg, M.; Munir, M.; Zohari, S.; Berg, M. Replication and Virulence Determinants of Peste des Petits Ruminants Virus. In Molecular Biology and Pathogenesis of Peste des Petits Ruminants Virus; Springer: Berlin, Germany, 2013; pp. 23–32.
- Parida, S.; Muniraju, M.; Mahapatra, M.; Muthuchelvan, D.; Buczkowski, H.; Banyard, A. Peste des petits ruminants. Vet. Microbiol. 2015, 181, 90–106.
- Singh, R.K.; Rajak, K.K.; Muthuchelvan, D.; Banyard, A.C.; Parida, S. Chapter 11 Vaccines against Peste des Petits ruminants virus. In Peste des Petits Ruminants Virus; Munir, M., Ed.; Springer: Berlin/Heidelberg, Germany, 2015; pp. 51–67.
- Singh, R.P.; Bandyopadhyay, S.K. Peste des petits ruminants vaccine and vaccination in India: Sharing experience with disease endemic countries. Virus Dis. 2015, 26, 215–224.
- Awa, D.N.; Ngagnou, A.; Tefiang, E.; Yaya, D.; Njoya, A. Post vaccination and colostral peste des petits ruminants antibody dynamics in research flocks of Kirdi goats and Foulbe sheep of north Cameroon. Prev. Vet. Med. 2002, 55, 265–271.
- Balamurugan, V.; Sen, A.; Venkatesan, G.; Rajak, K.K.; Bhanuprakash, V.; Singh, R.K. Study on passive immunity: Time of vaccination in kids born to goats vaccinated against Peste des petits ruminants. Virol. Sin. 2012, 27, 228–233.
- Enchery, F.; Hamers, C.; Kwiatek, O.; Gaillardet, D.; Montange, C.; Brunel, H.; Goutebroze, S.; Philippe-Reversat, C.; Libeau, G.; Hudelet, P.; et al. Development of a PPRV challenge model in goats and its use to assess the efficacy of a PPR vaccine. Vaccine 2019, 37, 1667–1673.
- Britton, A.; Caron, A.; Bedane, B. Progress to Control and Eradication of Peste des Petits Ruminants in the Southern African Development Community Region. Front. Vet. Sci. 2019, 6, 6.
- African Union. The Pan African Strategy for Control and Eradication of Peste des Petits Ruminants; African Union—Interafrican Bureau for Animal Resources (AU-IBAR): Nairobi, Kenya, September 2015; pp. 1–3.
- ICPALD. The IGAD Regional Peste des Petits Ruminants (PPR) Control and Eradication Strategy Presented at the Global Conference In Abijan (31 March–2 April 2015). 2016. Available online: (accessed on 23 April 2019).
- Wambura, P.N. Serological evidence of the absence of peste des petits ruminants in Tanzania. Vet. Rec. 2000, 146, 473–474.
- Anderson, J.; McKay, J.A. The detection of antibodies against peste des petits ruminants virus in cattle, sheep and goats and the possible implications to rinderpest control programmes. Epidemiol. Infect. 1994, 112, 225–231.
- Diallo, A.; Okuthe, S.; Kamata, A. Rapid Assessment for the Prevention and Control of Peste des Petits Ruminants, United Republic of Tanzania, Mission Report September 2010; Crisis Management Centre Animal Health (CMC-AH), Food and Agriculture Organization of the United Nations (FAO) and World Organization for Animal Health (OIE), 2011; pp. 1–37, (Unpublished report).
- Kivaria, F.M.; Kwiatek, O.; Kapaga, A.M.; Swai, E.S.; Libeau, G.; Moshy, W.; Mbyuzi, A.O.; Gladson, J. The incursion, persistence and spread of peste des petits ruminants in Tanzania: Epidemiological patterns and predictions. Onderstepoort J. Vet. Res. 2013, 80, 10.
- Muse, E.A.; Matondo, R.B.; Karimuribo, E.D.; Misinzo, G.; Albano, M.O.; Gitao, G.C. Clinico-pathological findings of the 2011 outbreak of Peste des Petits (PPR) in Tandahimba district, southern Tanzania. Res. Opin. Anim. Vet. Sci. 2012, 2, 256–262.
- Kgotlele, T.; Kasanga, C.J.; Kusiluka, L.J.; Misinzo, G. Preliminary investigation on presence of peste des petits ruminants in Dakawa, Mvomero district, Morogoro region, Tanzania. Onderstepoort J. Vet. Res. 2014, 81, 3.
- Swai, E.; Moshy, W.; Mbise, E.; Kaaya, J.; Bwanga, S. Serological evidence of camels exposure to peste des petits ruminants virus in Tanzania. Res. Opin. Anim. Vet. Sci. 2011, 1, 325–329.
- Mahapatra, M.; Sayalel, K.; Muniraju, M.; Eblate, E.; Fyumagwa, R.; Shilinde, S.; MaulidMdaki, M.; Keyyu, J.; Parida, S.; Kock, R. Spillover of Peste des Petits Ruminants Virus from Domestic to Wild Ruminants in the Serengeti Ecosystem, Tanzania. Emerg. Infect. Dis. 2015, 21, 2230–2234.
- Namtimba, A.M. Seroprevalence and Genetic Characterisation of Peste Des Petit Ruminants Virus in Selected areas of Tanzania. MSc Thesis, Sokoine University of Agriculture, Morogoro, Tanzania, 2015.
- Jones, B.A.; Mahapatra, M.; Chubwa, C.; Clarke, B.; Batten, C.; Hicks, H.; Henstock, M.; Keyyu, J.; Kock, R.; Parida, S. Characterisation of Peste Des Petits Ruminants Disease in Pastoralist Flocks in Ngorongoro District of Northern Tanzania and Bluetongue Virus Co-Infection. Viruses 2020, 12, 389.
- Dundon, W.G.; Diallo, A.; Cattoli, G. Peste des petits ruminants in Africa: A review of currently available molecular epidemiological data, 2020. Arch. Virol. 2020, 165, 2147–2163.
- Kisoza, J. The role of local institutions in the management of agro-pastoral and pastoral systems: A case study of Mkata plains, Kilosa district and Ngorongoro Conservation Area, Ngorongoro district, Tanzania. MSc Thesis, The Sokoine University of Agriculture, Morogoro, Tanzania, 2007.
- Kivaria, F.M. Foot and mouth disease in Tanzania: An overview of its national status. Vet. Q. 2003, 25, 72–78.
- Swai, E.S.; Kapaga, A.; Kivaria, F.; Tinuga, D.; Joshua, G.; Sanka, P. Prevalence and distribution of Peste des petits ruminants virus antibodies in various districts of Tanzania. Vet. Res. Commun. 2009, 33, 927–936.
- Muse, E.A.; Karimuribo, E.D.; Gitao, G.C.; Misinzo, G.; Mellau, L.S.B.; Msoffe, P.L.M.; Swai, E.S.; Albano, M.O. Epidemiological investigation into the introduction and factors for spread of Peste des Petits Ruminants, southern Tanzania. Onderstepoort J. Vet. Res. 2012, 79, 6.
- Stermshorn, B.; Hargreaves, S.; Pacer, R.; Samake, F. PVS Gap Analysis: Preparation of a Plan to Strengthen the Veterinary Services of Tanzania. OIE Report—France, 2009. Available online: (accessed on 21 June 2020).
- Stermshorn, B.; Carron, M.; Munstermann, S.; Wakhusama, S. PVS Evaluation Follow-Up Mission Report. OIE Report France, 2016. Available online: (accessed on 21 June 2020).





