
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Helen H.W. Chen | + 2858 word(s) | 2858 | 2021-06-16 06:24:01 |
Video Upload Options
The platinum (Pt)-containing antitumor drugs including cisplatin (cis-diamminedichloroplatinum II, cDDP), carboplatin, and oxaliplatin, have been the mainstay of cancer chemotherapy. These drugs are effective in treating many human malignancies. The major cell-killing target of Pt drugs is DNA. Recent findings underscored the important roles of Pt drug transport system in cancer therapy. It was demonstrated that by reducing cellular Cu bioavailable levels by Cu chelators, hCtr1 is transcriptionally upregulated by transcription factor Sp1, which binds the promoters of Sp1 and hCtr1. In contrast, elevated Cu poisons Sp1, resulting in suppression of hCtr1 and Sp1, constituting the Cu-Sp1-hCtr1 mutually regulatory loop.
1. Introduction
2. The Transport Mechanisms of Pt Drugs
3. Connecting the Essential Trace Element Cu to Pt-Based Antitumor Drugs
3.1. Identification of the High-Affinity Copper Transporter (Ctr1) as cDDP Importer
3.2. Cu Chaperones in Intracellular cDDP Trafficking
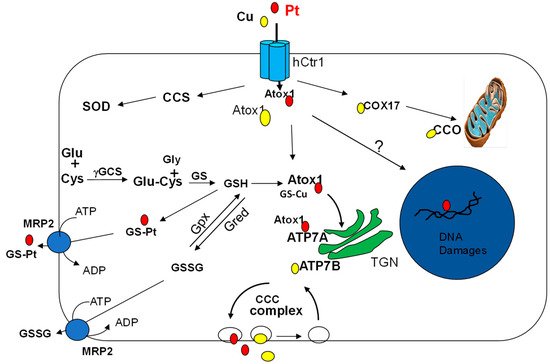
3.3. Cu-ATPases in Pt Drugs Efflux
3.4. Redox Regulation of the Cu Transport System in Pt Drug Pharmacology
4. Modulations of Copper Homeostasis and Pt Drug Cancer Chemotherapy
4.1. Roles of the Copper Transport System in Pt Drug Cancer Chemotherapy in Clinical Settings
4.1.1. hCtr1
4.1.2. Atox1, ATP7A and ATP7B
4.1.3. COMMD1
4.2. Enhanced cDDP Cell-Killing Activity through Upregulation of hCtr1 Expression
5. Overcoming cDDP Resistance by Copper Chelators
References
- Rottenberg, S.; Disler, C.; Perego, B. The rediscovery of platinum-based cancer therapy. Nat. Rev. Cancer 2021, 21, 37–50.
- Muggia, F.M.; Bonetti, A.; Hoeschele, J.D.; Rozencweig, M.; Howell, S.B. Platinum antitumor complexes: 50 years since Barnett Rosenberg′s discovery. J. Clin. Oncol. 2015, 33, 4219–4226.
- Chiruvella, V.; Annamaraju, P.; Guddati, P.A.K. Management of nephrotoxicity of chemotherapy and targeted agents: 2020. Am. J. Cancer. Res. 2020, 10, 4151–4164.
- Wertman, J.N.; Melong, N.; Stoyek, M.R.; Piccolo, O.; Langley, S.; Orr, B.; Steele, S.L.; Razaghi, B.; Berman, J.N. The identification of dual protective agents against cisplatin-induced oto- and nephrotoxicity using the zebrafish model. eLife 9 2020, 9.
- Rabik, C.A.; Dolan, M.E. Molecular mechanisms of resistance and toxicity associated with platinating agents. Cancer Treat. Rev. 2007, 33, 9–23.
- Wang, D.; Lippard, S.J. Cellular processing of platinum anticancer drugs. Nat. Rev. Drug Discov. 2005, 4, 307–320.
- Kim, E.S.; Lee, J.J.; He, G.; Chow, C.W.; Fujimoto, J.; Kalhor, N.; Swisher, S.G.; Wistuba, I.I.; Stewart, D.J.; Siddik, Z.H. Tissue platinum concentration and tumor response in non-small-cell lung cancer. J. Clin. Oncol. 2012, 30, 3345–3352.
- Gately, D.P.; Sharma, A.; Christen, R.D. Howell, S.B. Cisplatin and taxol activate different signal pathways regulating cellular injury-induced expression of GADD153. Br. J. Cancer 1996, 73, 18–23.
- Planells-Cases, R.; Lutter, D.; Guyader, C.; Gerhards, N.M.; Ullrich, F.; Elger, D.A.; Kucukosmanoglu, A.; Xu, G.; Voss, F.K.; Reincke, S.M.; et al. Subunit composition of VRAC channels determines substrate specificity and cellular resistance to Pt-based anti-cancer drugs. EMBO J. 2015, 34, 2993–3008.
- Konig, B.; Stauber, T. Biophysics and structure-function relationships of LRRC8-formed volume-regulated anion channels. Biophys. J. 2019, 116, 1185–1193.
- Sorensen, B.H.; Dam, C.S.; Sturup, S.; Lambert, I.H. Dual role of LRRC8A-containing transporters on cisplatin resistance in human ovarian cancer cells. J. Inorg. Biochem. 2016, 160, 287–295.
- Ruprecht, N.; Hofmann, L.; Hungerbuhler, M.N.; Kempf, C.; Heverhagen, J.T.; von Tengg-Kobligk, H. Generation of stable cisPt resistant lung adenocarcinoma cells. Pharmaceuticals 2020, 13, 109.
- Sprowl, J.A.; Ciarimboli, G.; Lancaster, C.S.; Giovinazzo, H.; Gibson, A.A.; Du, G.; Janke, L.J.; Cavaletti, G.; Shields, A.F.; Sparreboom, A. Oxaliplatin-induced neurotoxicity is dependent on the organic cation transporter OCT2. Proc. Natl. Acad. Sci. USA 2013, 110, 11199–11204.
- Gu, J.; Wang, L.; Li, T.; Tang, S.; Wang, Y.; Zhang, W.; Jiang, X. Role and mechanism of organic cation transporter 3 in oxaliplatin treatment of colon cancer in vitro and in vivo. Oncol. Rep. 2019, 42, 1355–1364.
- Jong, N.N.; Nakanishi, T.; Liu, J.J.; Tamai, I.; McKeage, M.J. Oxaliplatin transport mediated by organic cation/carnitine transporters OCTN1 and OCTN2 in overexpressing human embryonic kidney 293 cells and rat dorsal root ganglion neurons. J. Pharmacol. Exp. Ther. 2011, 338, 537–547.
- Kato, M.; Tsurudome, Y.; Kanemitsu, T.; Yasukochi, S.; Kanado, Y.; Ogino, T.; Matsunaga, N.; Koyanagi, S.; Ohdo, S. Diurnal expression of MRP4 in bone marrow cells underlies the dosing-time dependent changes in the oxaliplatin-induced myelotoxicity. Sci. Rep. 2020, 10, 13484.
- Samodelov, S.L.; Kullak-Ublick, G.A.; Gai, Z.; Visentin, M. Organic cation transporters in human physiology, pharmacology, and toxicology. Int. J. Mol. Sci. 2020, 21, 7890.
- Tatsumi, S.; Matsuoka, H.; Hashimoto, Y.; Hatta, K.; Maeda, K.; Kamoshida, S. Organic cation transporter 2 and tumor budding as independent prognostic factors in metastatic colorectal cancer patients treated with oxaliplatin-based chemotherapy. Int. J. Clin. Exp. Pathol. 2014, 7, 204–212.
- Ishida, S.; Lee, J.; Thiele, D.J.; Herskowitz, I. Uptake of the anticancer drug cisplatin mediated by the copper transporter Ctr1 in yeast and mammals. Proc. Natl. Acad. Sci. USA 2002, 99, 14298–14302.
- Georgatsou, E.; Mavrogiannis, L.A.; Fragiadakis, G.S.; Alexandraki, V. The yeast Fre1p/Fre2p cupric reductases facilitate copper uptake and are regulated by the copper-modulated Mac1p activator. J. Biol. Chem. 1997, 272, 13786–13792.
- Schwab, S.; Shearer, J.; Conklin, S.E.; Alies, B.; Haas, K.L. Sequence proximity between Cu(II) and Cu(I) binding sites of human copper transporter 1 model peptides defines reactivity with ascorbate and O2. J. Inorg. Biochem. 2016, 158, 70–76.
- Puig, S.; Lee, J.; Lau, M.; Thiele, D.J. Biochemical and genetic analyses of yeast and human high affinity copper transporters suggest a conserved mechanism for copper uptake. J. Biol. Chem. 2002, 227, 26021–26030.
- Liang, Z.D.; Stockton, D.; Savaraj, N.; Tien Kuo, M. Mechanistic comparison of human high-affinity copper transporter 1-mediated transport between copper ion and cisplatin. Mol. Pharmacol. 2009, 76, 843–853.
- Selvaraj, A.; Balamurugan, K.; Yepiskoposyan, H.; Zhou, H.; Egli, D.; Georgiev, O.; Thiele, D.J.; Schaffner, W. Metal-responsive transcription factor (MTF-1) handles both extremes, copper load and copper starvation, by activating different genes. Genes Dev. 2005, 19, 891–896.
- Ren, F.; Logeman, B.L.; Zhang, X.; Liu, Y.; Thiele, D.J.; Yuan, P. X-ray structures of the high-affinity copper transporter Ctr1. Nat. Commun. 2019, 10, 1386.
- Barresi, V.; Trovato-Salinaro, A.; Spampinato, G.; Musso, N.; Castorina, S.; Rizzarelli, E.; Condorelli, D.F. Transcriptome analysis of copper homeostasis genes reveals coordinated upregulation of SLC31A1, SCO1, and COX11 in colorectal cancer. FEBS Open Bio 2016, 6, 794–806.
- Perkal, O.; Qasem, Z.; Turgeman, M.; Schwartz, R.; Gevorkyan-Airapetov, L.; Pavlin, M.; Magistrato, A.; Major, D.T.; Ruthstein, S. Cu(I) Controls Conformational States in Human ATOX1 Metallochaperone: An EPR and Multiscale Simulation Study. J. Phys. Chem. B 2020, 124, 4399–4411.
- Wu, X.; Yuan, S.; Wang, E.; Tong, Y.; Ma, G.; Wei, K.; Liu, Y. Platinum transfer from hCTR1 to Atox1 is dependent on the type of platinum complex. Metallomics 2017, 9, 546–555.
- Kahra, D.; Kovermann, M.; Wittung-Stafshede, P. The C-terminus of human copper importer Ctr1 Acts as a binding site and transfers copper to Atox1. Biophys. J. 2016, 110, 95–102.
- Cox, D.W.; Moore, S.D. Copper transporting P-type ATPases and human disease. J. Bioenerg. Biomembr. 2002, 34, 333–338.
- Gudekar, N.; Shanbhag, V.; Wang, Y.; Ralle, M.; Weisman, G.A.; Petris, M.J. Metallothioneins regulate ATP7A trafficking and control cell viability during copper deficiency and excess. Sci. Rep. 2020, 10, 7856.
- Nardella, M.; Rosato, A.; Belviso, B.; Caliardro, R.; Natile, G.; Arnesano, F. Oxidation of human copper chaperone Atoxi and disusulfide bond cleavage by cisplatin and glutathione. Int. J. Mol. Sci. 2019, 20, 4390.
- Tapia, L.; Gonzalez-Aguero, M.; Cisternas, M.F.; Suazo, M.; Cambiazo, V.; Uauy, R.; Gonzalez, M. Metallothionein is crucial for safe intracellular copper storage and cell survival at normal and supra-physiological exposure levels. Biochem. J. 2004, 378, 617–624.
- Kozlowski, H.; Kolkowska, P.; Watly, J.; Krzywoszynska, K.; Potocki, S. General aspects of metal toxicity. Curr. Med. Chem. 2014, 21, 3721–3740.
- Margis, R.; Dunand, C.; Teixeira, F.K.; Margis-Pinheiro, M. Glutathione peroxidase family—an evolutionary overview. FEBS J. 2008, 275, 3959–3970.
- Ishida, S.; McCormick, F.; Smith-McCune, K.; Hanahan, D. Enhancing tumor-specific uptake of the anticancer drug cisplatin with a copper chelator. Cancer Cell 2010, 17, 574–583.
- Sun, S.; Cai, J.; Yang, Q.; Zhao, S.; Wang, Z. The association between copper transporters and the prognosis of cancer patients undergoing chemotherapy: A meta-analysis of literatures and datasets. Oncotarget 2017, 8, 16036–16051.
- Hanna, N.H.; Einhorn, L.H. Testicular cancer--discoveries and updates. N. Engl. J. Med. 2014, 371, 2005–2016.
- Ghaffari, R.; Di Bona, K.R.; Riley, C.L.; Richburg, J.H. Copper transporter 1 (CTR1) expression by mouse testicular germ cells, but not Sertoli cells, is essential for functional spermatogenesis. PLoS ONE 2019, 14, e0215522.
- Ghaffari, R.; Richburg, J.H. Mice with a Sertoli cell-specific knockout of the Ctr1 gene exhibit a reduced sensitivity to cisplatin-induced testicular germ cell apoptosis. Toxicol. Res. 2019, 8, 972–978.
- Hua, H.; Gunther, V.; Georgiev, O.; Schaffner, W. Distorted copper homeostasis with decreased sensitivity to cisplatin upon chaperone Atox1 deletion in Drosophila. Biometals 2011, 24, 445–453.
- Safaei, R.; Maktabi, M.H.; Blair, B.G.; Larson, C.A.; Howell, S.B. Effects of the loss of Atox1 on the cellular pharmacology of cisplatin. J. Inorg. Biochem. 2009, 103, 333–341.
- Petruzzelli, R.; Polishchuk, R.S. Activity and trafficking of copper-transporting ATPases in tumor development and defense against platinum-based drugs. Cells 2019, 8, 1080.
- Komatsu, M.; Sumizawa, T.; Mutoh, M.; Chen, Z.S.; Terada, K.; Furukawa, T.; Yang, X.L.; Gao, H.; Miura, N.; Sugiyama, T.; et al. Copper-transporting P-type adenosine triphosphatase (ATP7B) is associated with cisplatin resistance. Cancer Res. 2000, 60, 1312–1316.
- Leonhardt, K.; Gebhardt, R.; Mossner, J.; Lutsenko, S.; Huster, D. Functional interactions of Cu-ATPase ATP7B with cisplatin and the role of ATP7B in the resistance of cells to the drug. J. Biol. Chem. 2009, 284, 7793–7802.
- Nakagawa, T.; Abe, M.; Yamazaki, T.; Miyashita, H.; Niwa, H.; Kokubun, S.; Sato, Y. HEX acts as a negative regulator of angiogenesis by modulating the expression of angiogenesis-related gene in endothelial cells in vitro. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 231–237.
- Samimi, G.; Safaei, R.; Katano, K.; Holzer, A.K.; Rochdi, M.; Tomioka, M.; Goodman, M.; Howell, S.B. Increased expression of the copper efflux transporter ATP7A mediates resistance to cisplatin, carboplatin, and oxaliplatin in ovarian cancer cells. Clin. Cancer Res. 2004, 10, 4661–4669.
- Martinez-Balibrea, E.; Martinez-Cardus, A.; Musulen, E.; Gines, A.; Manzano, J.L.; Aranda, E.; Plasencia, C.; Neamati, N.; Abad, A. Increased levels of copper efflux transporter ATP7B are associated with poor outcome in colorectal cancer patients receiving oxaliplatin-based chemotherapy. Int. J. Cancer 2009, 124, 2905–2910.
- Nakayama, K.; Kanzaki, A.; Terada, K.; Mutoh, M.; Ogawa, K.; Sugiyama, T.; Takenoshita, S.; Itoh, K.; Yaegashi, N.; Miyazaki, K.; et al. Prognostic value of the Cu-transporting ATPase in ovarian carcinoma patients receiving cisplatin-based chemotherapy. Clin. Cancer Res. 2004, 10, 2804–2811.
- Weiskirchen, R.; Penning, L.C. COMMD1, a multi-potent intracellular protein involved in copper homeostasis, protein trafficking, inflammation, and cancer. J. Trace Elem. Med. Biol. 2021, 65, 126712.
- Fedoseienko, A.; Wieringa, H.W.; Wisman, G.B.; Duiker, E.; Reyners, A.K.; Hofker, M.H.; van der Zee, A.G.; van de Sluis, B.; van Vugt, M.A. Nuclear COMMD1 is associated with cisplatin sensitivity in ovarian cancer. PLoS ONE 2016, 11, e0165385.
- Eid, C.; Hemadi, M.; Ha-Duong, N.T.; El Hage Chahine, J.M. Iron uptake and transfer from ceruloplasmin to transferrin. Biochim. Biophys. Acta 2014, 1840, 1771–1781.
- Herman, S.; Lipinski, P.; Ogorek, M.; Starzynski, R.; Grzmil, P.; Bednarz, A.; Lenartowicz, M. Molecular regulation of copper homeostasis in the male gonad during the process of spermatogenesis. Int. J. Mol. Sci. 2020, 21, 9053.
- Dodani, S.C.; Firl, A.; Chan, J.; Nam, C.I.; Aron, A.T.; Onak, C.S.; Ramos-Torres, K.M.; Paek, J.; Webster, C.M.; Feller, M.B.; et al. Copper is an endogenous modulator of neural circuit spontaneous activity. Proc. Natl. Acad. Sci. USA 2014, 111, 16280–16285.
- Shi, H.; Jiang, Y.; Yang, Y.; Peng, Y.; Li, C. Copper metabolism in Saccharomyces cerevisiae: An update. Biometals 2020, 34, 3–14.
- Lai, Y.H.; Kuo, C.; Kuo, M.T.; Chen, H.H.W. Modulating chemosensitivity of tumors to platinum-based antitumor drugs by transcriptional regulation of copper homeostasis. Int. J. Mol. Sci. 2018, 19, 1486.
- Ooi, C.E.; Rabinovich, E.; Dancis, A.; Bonifacino, J.S.; Klausner, R.D. Copper-dependent degradation of the Saccharomyces cerevisiae plasma membrane copper transporter Ctr1p in the apparent absence of endocytosis. EMBO J. 1996, 15, 3515–3523.
- Safe, S.; Abbruzzese, J.; Abdelrahim, M.; Hedrick, E. Specificity protein transcription factors and cancer: Opportunities for drug development. Cancer Prev. Res. 2018, 11, 371–382.
- Helsel, M.E.; Franz, K.J. Pharmacological activity of metal binding agents that alter copper bioavailability. Dalton Trans. 2015, 44, 8760–8770.
- Baldari, S.; Di Rocco, G.; Toietta, G. Current biomedical use of copper chelation therapy. Int. J. Mol. Sci. 2020, 21, 1069.
- Cen, D.; Gonzalez, R.I.; Buckmeier, J.A.; Kahlon, R.S.; Tohidian, N.B.; Meyskens, F.L., Jr. Disulfiram induces apoptosis in human melanoma cells: A redox-related process. Mol. Cancer Ther. 2002, 1, 197–204.
- Chen, D.; Cui, Q.C.; Yang, H.; Dou, Q.P. Disulfiram, a clinically used anti-alcoholism drug and copper-binding agent, induces apoptotic cell death in breast cancer cultures and xenografts via inhibition of the proteasome activity. Cancer Res. 2006, 66, 10425–10433.
- Hasinoff, B.B.; Wu, X.; Yadav, A.A.; Patel, D.; Zhang, H.; Wang, D.S.; Chen, Z.S.; Yalowich, J.C. Cellular mechanisms of the cytotoxicity of the anticancer drug elesclomol and its complex with Cu(II). Biochem. Pharmacol. 2015, 93, 266–276.
- Guthrie, L.M.; Soma, S.; Yuan, S.; Silva, A.; Zulkifli, M.; Snavely, T.C.; Greene, H.F.; Nunez, E.; Lynch, B.; De Ville, C.; et al. Elesclomol alleviates Menkes pathology and mortality by escorting Cu to cuproenzymes in mice. Science 2020, 368, 620–625.
- Long, Y.; Tsai, W.B.; Chang, J.T.; Estecio, M.; Wangpaichitr, M.; Savaraj, N.; Feun, L.G.; Chen, H.H.; Kuo, M.T. Cisplatin-induced synthetic lethality to arginine-starvation therapy by transcriptional suppression of ASS1 is regulated by DEC1, HIF-1alpha, and c-Myc transcription network and is independent of ASS1 promoter DNA methylation. Oncotarget 2016, 7, 82658–82670.
- Liang, Z.D.; Long, Y.; Tsai, W.B.; Fu, S.; Kurzrock, R.; Gagea-Iurascu, M.; Zhang, F.; Chen, H.H.; Hennessy, B.T.; Mills, G.B.; et al. Mechanistic basis for overcoming platinum resistance using copper chelating agents. Mol. Cancer Ther. 2012, 11, 2483–2494.
- Kita, Y.; Hamada, A.; Saito, R.; Teramoto, Y.; Tanaka, R.; Takano, K.; Nakayama, K.; Murakami, K.; Matsumoto, K.; Akamatsu, S.; et al. Systematic chemical screening identifies disulfiram as a repurposed drug that enhances sensitivity to cisplatin in bladder cancer: A summary of preclinical studies. Br. J. Cancer 2019, 121, 1027–1038.
- Barca, A.; Ippati, S.; Urso, E.; Vetrugno, C.; Storelli, C.; Maffia, M.; Romano, A.; Verri, T. Carnosine modulates the Sp1-Slc31a1/Ctr1 copper-sensing system and influences copper homeostasis in murine CNS-derived cells. Am. J. Physiol. Cell Physiol. 2019, 316, C235–C245.
- Zhang, W.; Shi, H.; Chen, C.; Ren, K.; Xu, Y.; Liu, X.; He, L. Curcumin enhances cisplatin sensitivity of human NSCLC cell lines through influencing Cu-Sp1-CTR1 regulatory loop. Phytomedicine 2018, 48, 51–61.
- Fu, S.; Hou, M.M.; Wheler, J.; Hong, D.; Naing, A.; Tsimberidou, A.; Janku, F.; Zinner, R.; Piha-Paul, S.; Falchook, G.; et al. Exploratory study of carboplatin plus the copper-lowering agent trientine in patients with advanced malignancies. Investig. New Drugs 2014, 32, 465–472.
- Fu, S.; Naing, A.; Fu, C.; Kuo, M.T.; Kurzrock, R. Overcoming platinum resistance through the use of a copper-lowering agent. Mol. Cancer Ther. 2012, 11, 1221–1225.
- Huang, Y.F.; Kuo, M.T.; Liu, Y.S.; Cheng, Y.M.; Wu, P.Y.; Chou, C.Y. A dose escalation study of trientine plus carboplatin and pegylated liposomal doxorubicin in women with a first relapse of epithelial ovarian, tubal, and peritoneal cancer within 12 months after platinum-based chemotherapy. Front. Oncol. 2019, 9, 437.
- Leitao, M.M., Jr.; Hummer, A.; Dizon, D.S.; Aghajanian, C.; Hensley, M.; Sabbatini, P.; Venkatraman, E.; Spriggs, D.R. Platinum retreatment of platinum-resistant ovarian cancer after nonplatinum therapy. Gynecol. Oncol. 2003, 91, 123–129.
- Kavanagh, J.; Tresukosol, D.; Edwards, C.; Freedman, R.; Gonzalez de Leon, C.; Fishman, A.; Mante, R.; Hord, M.; Kudelka, A. Carboplatin reinduction after taxane in patients with platinum-refractory epithelial ovarian cancer. J. Clin. Oncol. 1995, 13, 1584–1588.




