
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Susana Braga | + 2125 word(s) | 2125 | 2021-06-10 11:04:28 |
Video Upload Options
Ginger in its many forms, from juices of the fresh rhizome, to ginger powder and ginger essential oil, is growing in popularity for claimed universal health benefits. Nevertheless, and contrarily to the common notion of the public, ginger is not devoid of side effects, especially interactions with other drugs, and many of the claimed benefits remain to be substantiated.
1. Introduction
1.1. Ginger in History and in the Present Day
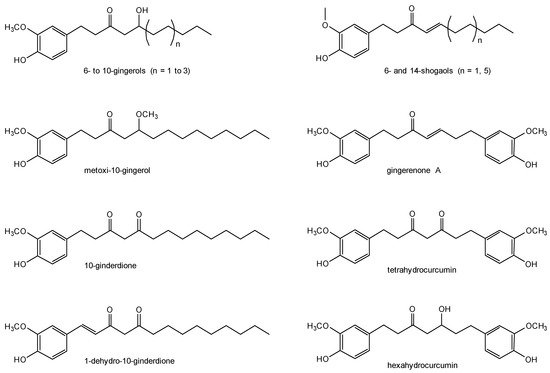
1.2. Ginger Chemistry
2. Pharmacology of Ginger
2.1. Pharmacokinetics from Oral Intake
|
Component |
Dose (mg) |
Cmax (μg/mL) |
AUC (μg·min·mL−1) |
tmax (min) |
t1/2β (min) |
|---|---|---|---|---|---|
|
6-gingerol, total |
1000 |
0.4 ± 0.2 |
12.6 ± 6.4 |
55.0 ± 7.7 |
— |
|
1500 |
1.69 ± 2.31 |
75.6 ± 110.3 |
60.0 ± 0.0 |
— |
|
|
2000 |
0.85 ± 0.43 |
65.6 ± 44.4 |
65.5 ± 22.6 |
110.0 ± 34.9 |
|
|
6-gingerol glucoronide |
1000 |
0.16 ± 0.15 |
— |
— |
— |
|
1500 |
0.62 ± 0.62 |
— |
— |
— |
|
|
2000 |
0.62 ± 0.56 |
— |
— |
— |
|
|
6-gingerol sulphate |
1000 |
0.02 ± 0.03 |
— |
— |
— |
|
1500 |
0.04 ± 0.04 |
— |
— |
— |
|
|
2000 |
0.33 ± 0.41 |
— |
— |
— |
|
|
8-gingerol, total |
1000 |
0.1 ± 0.1 |
2.1 ± 2.2 |
52.5 ± 8.7 |
— |
|
1500 |
0.1 ± 0.1 |
2.6 ± 2.0 |
60.0 ± 0.0 |
— |
|
|
2000 |
0.23 ± 0.16 |
18.1 ± 20.3 |
73.1 ± 29.4 |
113.5 ± 41.1 |
|
|
10-gingerol, total |
1000 |
0.1 ± 0.1 |
2.9 ± 3.2 |
60.0 ± 0.0 |
— |
|
1500 |
0.1 ± 0.02 |
7.7 ± 5.3 |
80.0 ± 34.6 |
— |
|
|
2000 |
0.53 ± 0.4 |
50.1 ± 49.3 |
75.0 ± 27.8 |
128.7 ± 38.8 |
|
|
6-shogaol, total |
1000 |
0.1 ± 0.1 |
0.8 ± 1.5 |
55.0 ± 8.7 |
— |
|
1500 |
0.4 ± 0.08 |
1.6 ± 2.8 |
60.0 ± 0.0 |
— |
|
|
2000 |
0.15 ± 0.12 |
10.9 ± 13.0 |
65.6 ± 22.6 |
120.4 ± 42.0 |
2.2. Safety and Interactions
2.3. Biological Activity
3. Encapsulation of Ginger
3.1. Dispersion and Micronisation
3.2. Liposomal Ginger
4. Novel Drug Delivery Technologies Based on Ginger
4.1. Ginger-Derived Nanoparticles (GDPs)
4.2. Ginger-Derived Nano-Vectors (GDNVs)
References
- Bode, A.M.; Dong, Z. The Amazing and Mighty Ginger. In Herbal Medicine: Biomolecular and Clinical Aspects, 2nd ed.; Benzie, I.F.F., Wachtel-Galor, S., Eds.; CRC Press/Taylor & Francis: Boca Raton, FL, USA, 2011.
- Ali, B.H.; Blunden, G.; Tanira, M.O.; Nemmar, A. Some physico-chemical, pharmacological and toxicological properties of ginger (Zingerber officinale)—A review. Food Chem. Toxicol. 2008, 46, 409–410.
- Tapsell, L.C.; Hemphill, I.; Cobiac, L.; Patch, C.S.; Sullivan, D.R.; Fenech, M.; Roodenrys, S.; Keogh, J.B.; Clifton, P.M.; Williams, P.G.; et al. Health benefits of herbs and spices: The past, the present, the future. Med. J. Aust. 2006, 185, S4–S24.
- WHO. WHO Monographs on Selected Medicinal Plants—Volume 1. WHO Essential Medicines and Health Products Information Portal; WHO: Geneva, Switzerland, 2018.
- US Food and Drug Administration. CFR—Code of Federal Regulations, Title 21—Food and Drugs, Chapter I—Food and Drug Administation, Subchapter B—Food for Human Consumption (continued), Part 182—Substances Generally Recognized as Safe, Subpart A—General Provisions: Sec. 182.20. Available online: (accessed on 28 January 2019).
- Transparency Market Research. Ginger Market (Form—Fresh, Dried, Pickled, Preserved, Crystallized, and Powdered; Distribution Channel—Modern Grocery Retail, Traditional Grocery Retail, and Non-Grocery Retail; Application—Culinary, Soups and Sauces, Snacks & Convenience Food, Bakery Products, Alcoholic Beverages, Non-Alcoholic Beverages, and Chocolate and Confectionery)—Global Industry Analysis, Size, Share, Growth, Trends and Forecast 2017—2022. Available online: (accessed on 28 January 2019).
- Pratap, S.R.; Gangadharappa, H.V.; Mruthunjaya, K. Ginger: A Potential Neutraceutical, An Updated Review. Int. J. Pharmacogn. Phytochem. Res. 2017, 9, 1227–1238.
- Schulick, P. Ginger: Common Spice and Wonder Drug, 3rd ed.; Hohm Press: Prescott, AZ, USA, 2012.
- Jolad, S.D.; Lantz, R.C.; Chen, G.J.; Bates, R.B.; Timmermann, B.N. Commercially processed dry ginger (Zingiber officinale): Composition and effects on LPS-stimulated PGE2 production. Phytochemistry 2005, 66, 1614–1635.
- Jiang, H.; Solyom, A.M.; Timmermann, B.N.; Gang, D.R. Characterization of gingerol-related compounds in ginger rhizome (Zingiber officinale Rosc.) by high-performance liquid chromatography/electrospray ionization mass spectrometry. Rapid Commun. Mass Spectrom. 2005, 19, 2957–2964.
- Kiuchi, F.; Shibuya, M.; Sankawa, U. Inhibitors of Prostaglandin Biosynthesis from Ginger. Chem. Pharm. Bull. 1982, 30, 754–757.
- Koh, E.M.; Kim, H.J.; Kim, S.; Choi, W.H.; Choi, Y.H.; Ryu, S.Y.; Kim, Y.S.; Koh, W.S.; Park, S.Y. Modulation of macrophage functions by compounds isolated from Zingiber officinale. Planta Med. 2009, 75, 148–151.
- Waldmann, S.; Almukainzi, M.; Bou-Chacra, N.A.; Amidon, G.L.; Lee, B.-J.; Feng, J.; Kanfer, I.; Zuo, J.Z.; Wei, H.; Bolger, M.B.; et al. Provisional Biopharmaceutical Classification of Some Common Herbs Used in Western Medicine. Mol. Pharm. 2012, 9, 815–822.
- Johji, Y.; Michihiko, M.; Rong, H.Q.; Hisashi, M.; Hajime, F. The Anti-Ulcer Effect in Rats of Ginger Constituents. J. Ethnopharmacol. 1988, 23, 299–304.
- Priyarani, M.; Padmakumari, K.P.; Sankariyutty, B.; Lijocherian, O.; Nisha, V.M.; Raghu, K.G. Inhibitory potential of ginger extracts against enzymes linked to type 2 diabetes, inflammation and induced oxidative stress. Int. J. Food Sci. Nutr. 2011, 62, 106–110.
- Yonei, Y.; Ohinata, H.; Yoshida, R.; Shimizu, Y.; Yokoyama, C. Extraction of Ginger Flavor with Liquid or Supercritical Carbon Dioxide. J. Supercrit. Fluids 1995, 8, 156–161.
- Salea, R.; Veriansyah, B.; Tjandrawinata, R.R. Optimization and Scale-up Process for Supercritical Fluids Extraction of Ginger Oil from Zingiber Officinale Var. Amarum. J. Supercrit. Fluids 2016, 120, 285–294.
- Švarc-Gajić, J.; Cvetanović, A.; Segura-Carretero, A.; Linares, I.B.; Mašković, P. Characterisation of Ginger Extracts Obtained by Subcritical Water. J. Supercrit. Fluids 2016, 123, 92–100.
- Solladié, G.; Ziani-Chérif, C. Total Synthesis of Natural Gingerols, the Three Active Principles of Ginger. J. Org. Chem. 1993, 58, 2181–2185.
- Nakazawa, T.; Ohsawa, K. Metabolism of [6]-gingerol in rats. Life Sci. 2002, 70, 2165–2175.
- Mukkavilli, R.; Yang, C.; Tanwar, R.S.; Ghareeb, A.; Luthra, L.; Aneja, R. Absorption, Metabolic Stability, and Pharmacokinetics of Ginger Phytochemicals. Molecules 2017, 22, 553.
- Zick, S.M.; Djuric, Z.; Ruffin, M.T.; Litzinger, A.J.; Normolle, D.P.; Alrawi, S.; Feng, M.R.; Brenner, D.E. Pharmacokinetics of 6-gingerol, 8-gingerol, 10-gingerol, and 6-shogaol and conjugate metabolites in healthy human subjects. Cancer Epidemiol. Biomark. Prev. 2008, 17, 1930–1936.
- Hoehle, S.I.; Pfeiffer, E.; Metzler, M. Glucuronidation of curcuminoids by human microsomal and recombinant UDP-glucuronosyltransferases. Mol. Nutr. Food Res. 2007, 51, 932–938.
- Portnoi, G.; Chng, L.-A.; Karimi-Tabesh, L.; Koren, G.; Tan, M.P.; Einarson, A. Prospective comparative study of the safety and effectiveness of ginger for the treatment of nausea and vomiting in pregnancy. Am. J. Obstet. Gynecol. 2003, 189, 1374–1377.
- Heitmann, K.; Nordeng, H.; Holst, L. Safety of ginger use in pregnancy: Results from a large population-based cohort study. Eur. J. Clin. Pharmacol. 2013, 69, 269–277.
- Govindarajan, V.S. Ginger-chemistry, technology, and quality evaluation: Part 2. Crit. Rev. Food Sci. Nutr. 1982, 17, 189–258.
- Fernandes, R.V.B.; Botrel, D.A.; Silva, E.K.; Pereira, C.G.; do Carmo, E.L.; Dessimoni, A.L.A.; Borges, S.V. Microencapsulated ginger oil properties: Influence of operating parameters. Drying Technol. 2017, 35, 1098–1107.
- Toure, A.; Xiaoming, Z.; Jia, C.-S.; Zhijian, D. Microencapsulation and Oxidative Stability of Ginger Essential Oil in Maltodextrin/Whey Protein Isolate (MD/WPI). Int. J. Dairy Sci. 2007, 2, 387–392.
- Jayanudin; Rochmadi; Fahrurrozi, M.; Wirawan, S.K. Microencapsulation Technology of Ginger Oleoresin with Chitosan as Wall Material: A review. J. Appl. Pharm. Sci. 2016, 12, 209–223.
- Jayanudin; Rochmadi. Encapsulation of red ginger oleoresin (Zingiber officinale var rubrum) with chitosan as wall material. Int. J. Pharm. Pharm. Sci. 2017, 9, 29–34.
- Jayanudin; Rochmadi; Wiratni; Yulvianti, M.; Barleany, D.R.; Ernayati, W. Encapsulation Red Ginger Oleoresin (Zingiber officinale var. Rubrum) with Chitosan-alginate as Wall Material Using Spray Drying. Res. J. Appl. Sci. Eng. Technol. 2015, 10, 1370–1378.
- Tanaka, K.; Arita, M.; Sakurai, H.; Ono, N.; Tezuka, Y. Analysis of Chemical Properties of Edible and Medicinal Ginger by Metabolomics Approach. BioMed Res. Int. 2015, 2015, 671058.
- Purathrive. Could Ginger Be Used Medicinally? purathrive.com, 2019. Available online: (accessed on 12 March 2019).
- Synchro. Gold (Lemon ginger). besynchro.com, 2019. Available online: (accessed on 12 March 2019).
- Ganji, S.; Sayyed-Alangi, S.Z. Encapsulation of ginger ethanolic extract in nanoliposome and evaluation of its antioxidant activity on sunflower oil. Chem. Pap. 2017, 71, 1781–1789.
- Baskar, V.; Selvakumar, K.; Madhan, R.; Srinivasan, G.; Muralidharan, M. Study on improving bioavailability ratio of anti-inflammatory compound from ginger through nano transdermal delivery. Asian J. Pharm. Clin. Res. 2012, 5, 241–246.
- Ju, S.; Mu, J.; Dokland, T.; Zhuang, X.; Wang, Q.; Jiang, H.; Xiang, X.; Deng, Z.B.; Wang, B.; Zhang, L.; et al. Grape exosomelike nanoparticles induce intestinal stem cells and protect mice from dss-induced colitis. Mol. Ther. 2013, 21, 1345–1357.
- Wang, B.; Zhuang, X.; Deng, Z.B.; Jiang, H.; Mu, J.; Wang, Q.; Xiang, X.; Guo, H.; Zhang, L.; Dryden, G.; et al. Targeted drug delivery to intestinal macrophages by bioactive nanovesicles released from grapefruit. Mol. Ther. 2014, 22, 522–534.
- Mu, J.; Zhuang, X.; Wang, Q.; Jiang, H.; Deng, Z.B.; Wang, B.; Zhang, L.; Kakar, S.; Jun, Y.; Miller, D.; et al. Interspecies communication between plant and mouse gut host cells through edible plant derived exosome-like nanoparticles. Mol. Nutr. Food Res. 2014, 58, 1561–1573.
- Zhang, M.; Wang, X.; Han, M.K.; Collins, J.F.; Merlin, D. Oral administration of ginger-derived nanolipids loaded with siRNA as a novel approach for efficient siRNA drug delivery to treat ulcerative colitis. Nanomedicine 2017, 12, 1927–1943.
- Zhuang, X.; Deng, Z.B.; Mu, J.; Zhang, L.; Yan, J.; Miller, D.; Feng, W.; McClain, C.J.; Zhang, H.G. Ginger-derived nanoparticles protect against alcohol-induced liver damage. J. Extracell. Vesicles 2015, 4, 28713.
- Zhang, M.; Xiao, B.; Wang, H.; Han, M.K.; Zhang, Z.; Viennois, E.; Xu, C.; Merlin, D. Edible Ginger-derived Nano-lipids Loaded with Doxorubicin as a Novel Drug-delivery Approach for Colon Cancer Therapy. Mol. Ther. 2016, 24, 1783–1796.




