| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Nemany A.N. Hanafy | + 2618 word(s) | 2618 | 2021-06-09 07:56:34 | | | |
| 2 | Peter Tang | Meta information modification | 2618 | 2021-06-17 04:39:48 | | |
Video Upload Options
Hydrogels are widely used materials which have many medical applications. Their ability to absorb aqueous solutions and biological fluids gives them innovative characterizations resulting in increased compatibility with biological activity. In this sense, they are used extensively for encapsulation of several targets such as biomolecules, viruses, bacteria, and mammalian cells.
1. Introduction
2. Structure of Mucus Layers
|
Mucus Membrane |
Mucus Surface Area |
Thickness of Mucus Layer |
Layers |
Turnover Time |
|---|---|---|---|---|
|
Buccal |
30 cm2 |
0.1–0.7 mm |
Epithelium, basement membrane, and connective tissues |
5–6 days |
|
Nasal |
160 cm2 |
5–20 μm |
Both keratinized and nonkeratinized epithelial cells |
10–15 min |
|
Ocular |
3–10 μm |
Pithelium, Bowman’s layer, stroma, Descemet’s membrane, and endothelium |
15–20 h |
|
|
Vaginal |
6–10 cm2 |
10–15 layers |
The epithelial layer consists of the lamina propia and stratified squamous epithelium |
7 days |
|
Rectal |
300 cm2 |
50 µm |
Single layer of cylindrical cells and goblet cells secreting mucus |
90 h |
3. Mucus Barrier Resistance to Drugs and Delivery Systems
3.1. The Dynamic Barrier
3.2. The Steric Barrier
3.3. The Interactive Barrier
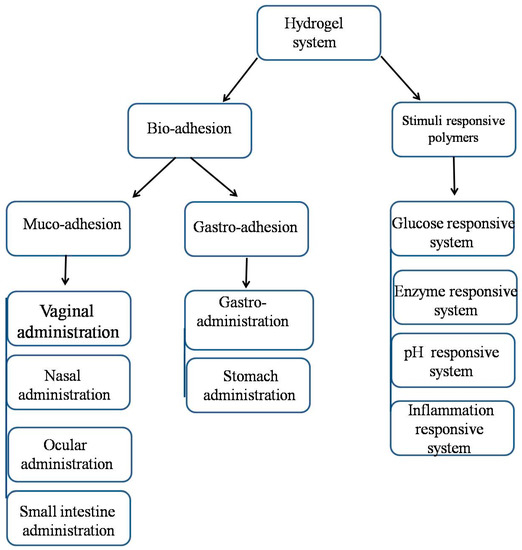
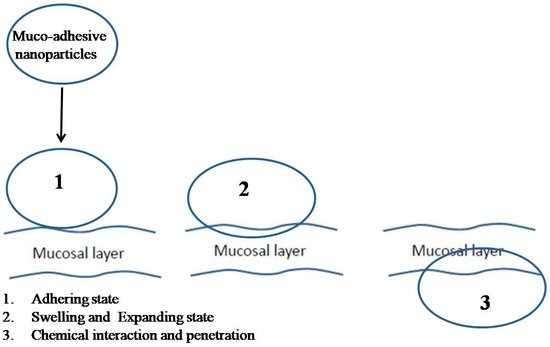
4. Mucoadhesive System
5. The Theories of Mucoadhesion
|
Theory |
Chemical and Physical Reaction |
Biological Reactive System |
Result |
|---|---|---|---|
|
Electronic theory |
Electron transfer reaction |
Mucus and the mucoadhesive system |
Electrical double layer of charges at the mucus and mucoadhesiveinterface |
|
Adsorption theory |
Hydrogen bonding reaction |
Mucus and the mucoadhesive system |
Adhesive interaction betweenthe substrate surfaces |
|
Diffusion theory |
Adhesive force |
Mucus and the mucoadhesive system |
Interpenetration of both polymer and mucin chains to a sufficient depth |
|
Wetting theory |
Spreading property |
Mucus and the mucoadhesive system |
Attachment regarding to contact angle |
|
Fracture theory |
Detachment of polymer moieties |
Mucus and polymer moieties |
Relates the force for polymer detachment |
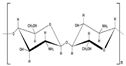
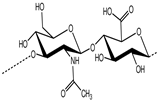
6. Mucoadhesion and Its Relation with Certain Polymers
|
Polymer |
Chemical Structure |
Charge |
Hydrogel Groups |
Structure |
|---|---|---|---|---|
|
Chitosan |
Poly[β-(1,4)-2-amino-2-deoxy-D-glucopyranose] |
Cationic |
NH2 and OH |
|
|
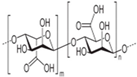
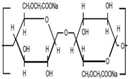
Hyaluronic acid |
D-glucuronic acid and N-acetyl–D-glucosamine linked together through alternating β(1,3) and β(1,4) glycosidic bonds |
Anionic |
COOH and OH |
|
|
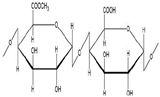
Alginic acid |
β-(1,4)-D-mannuronic acid (M) and α-(1,4)-L-guluronic acid (G) units |
Anionic |
COOH and OH |
|
|
Polygalacturonic acid |
Heterogeneous structure bonded via α (1,4) glycosidic linkage. |
Anionic |
COOH and OH |
|
|
Carboxymethyl cellulose |
Cellulose derivatives |
Anionic |
COOH and OH |
|
|
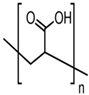
Poly(acrylic acid) |
Polyacrylates |
Anionic |
COOH and OH |
|
References
- Ottenbrite, R.M.; Park, K.; Okano, T.; Peppas, N.A. Biomedical Applications of Hydrogels Handbook, 2010th ed.; Springer: New York, NY, USA, 2010; p. 432.
- Hoffman, A.S. Hydrogels for biomedical applications. Adv. Drug Deliv. Rev. 2002, 43, 3–12.
- Vashist, A.; Gupta, Y.K.; Ahmad, S. Recent advances in hydrogel based drug delivery systems for the human body. J. Mater. Chem. 2014, 2, 147–166.
- Biondi, M.; Borzacchiello, A.; Mayol, L.; Luigi Ambrosio, L. Nanoparticle-Integrated Hydrogels as Multifunctional Composite Materials for Biomedical Applications. Gels 2015, 1, 162–178.
- Patel, B.H.; Patel, L.H.; Shah, H.Z.; Modasiya, K.M. Review on Hydrogel Nanoparticles in Drug Delivery. Am. J. Pharm. Technol. Res. 2011, 1, 19–38.
- Panyam, J.; Labhasetwar, V. Biodegradable nanoparticles for drug and gene delivery to cells and tissue. Adv. Drug Deliv. Rev. 2003, 55, 329–347.
- Matanovi’c, M.R.; Kristl, J.; Grabnar, P.A. Thermoresponsive polymers: Insights into decisive hydrogel characteristics, mechanisms of gelation, and promising biomedical applications. Int. J. Pharm. 2014, 472, 262–275.
- Plieva, F.M.; Galaev, I.Y.; Noppe, W.; Mattiasson, B. Cryogel applications in microbiology. Trends Microbiol. 2008, 16, 543–551.
- Sheridan, M.; Shea, L.; Peters, M.; Mooney, D. Bio-absorbable polymer scaffolds for tissue engineering capable of sustained growth factor delivery. J. Control Release 2000, 64, 91–102.
- Zhou, S.; Bismarck, A.; Steinke, J.H. Ion-responsive alginate based macroporous injectable hydrogel scaffolds prepared by emulsion templating. J. Mater. Chem. B 2013, 1, 4736–4745.
- Hassan, C.M.; Peppas, N.A. Structure and morphology of freeze/thawed PVA hydrogels. Macromolecules 2000, 33, 2472–2479.
- Huebsch, N.; Lippens, E.; Lee, K.; Mehta, M.; Koshy, S.T.; Darnell, M.C.; Desai, R.M.; Madl, C.M.; Xu, M.; Zhao, X.; et al. Matrix elasticity of void-forming hydrogels controls transplanted-stem-cell-mediated bone formation. Nat. Mater. 2015, 14, 1269–1277.
- Lin, C.-C.; Metters, A.T. Hydrogels in controlled release formulations: Network design and mathematical modeling. Adv. Drug Deliv. Rev. 2006, 58, 1379–1408.
- Guilherme, M.R.; Reis, A.V.; Takahashi, S.H.; Rubira, A.F.; Feitosa, J.P.A.; Muniz, E.C. Synthesis of a novel superabsorbent hydrogel by copolymerization of acrylamide and cashew gum modified with glycidyl methacrylate. Carbohydr. Polym. 2005, 61, 464–471.
- Patachia, S.; Valente, A.J.M.; Baciu, C. Effect of non-associated electrolyte solutions on the behaviour of poly(vinyl alcohol)-based hydrogels. Eur. Polym. J. 2007, 43, 460–467.
- Chai, Q.; Jiao, Y.; Yu, X. Hydrogels for Biomedical Applications: Their Characteristics and the Mechanisms behind Them. Gels 2017, 3, 6.
- Khanna, K.V. Targeted Delivery of Nanomedicines. ISRN Pharmacol. 2012, 2012, 571394.
- Pandey, P.; Saini, M.; Neeta. Mucoadhesive drug delivery system: An overview. Pharm. Biol. Eval. 2017, 4, 183–187.
- Kharia, A.A.; Singhai, A.A. Stomach specific mucoadhesive nanoparticles as a controlled release drug delivery system. Mintage J. Pharm. Med Sci. 2014, 1, 12–24.
- Senyiğit, Z.A.; Karavana, S.Y.; Eraç, B.; Gürsel, O.; Limoncu, M.H.; Baloğlu, E. Evaluation of chitosan based vaginal bioadhesive gel formulations for antifungal drugs. Acta Pharm. 2014, 64, 139–156.
- Kraisit, P.; Limmatvapirat, S.; Luangtana-Anan, M.; Sriamornsak, P. Buccal administration of mucoadhesive blend films saturated with propranolol loaded nanoparticles. Asian J. Pharm. Sci. 2017, 13, 34–43.
- Zhang, X.; Zhang, H.; Wu, Z.; Wang, Z.; Niu, H.; Li, C. Nasal absorption enhancement of insulin using PEG-grafted chitosan nanoparticles. Eur. J. Pharm. Biopharm. 2008, 68, 526–534.
- Marriot, C.; Gregory, N. Bioadhesive Drug Delivery Systems; CRC Press: Boca Raton, FL, USA, 1990.
- Boegh, M.; Nielsen, H.M. Mucus as a barrier to drug delivery—Understanding and mimicking the barrier properties. BasicClin. Pharmacol. Toxicol. 2015, 116, 179–186.
- Johansson, M.E.; Sjovall, H.; Hansson, G.C. The gastrointestinal mucus system in health and disease. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 352–361.
- Bennett, E.P.; Mandel, U.; Clausen, H.; Gerken, T.A.; Fritz, T.A.; Tabak, L.A. Control of mucin-type O-glycosylation: A classification of the polypeptide GalNAc-transferase gene family. Glycobiology 2012, 22, 736–756.
- Cone, R.A. Barrier properties of mucus. Adv. Drug Deliv. Rev. 2009, 61, 75–85.
- Svensson, O.; Arnebrant, T. Mucin layers and multilayers—Physicochemical properties and applications. Curr. Opin. Colloid Interface Sci. 2010, 15, 395–405.
- Shaikh, R.; RajSingh, T.R.; Garland, M.J.; Woolfson, A.D.; Donnelly, R.F. Mucoadhesive drug delivery systems. J. Pharm. Bioallied. Sci. 2011, 3, 89–100.
- Fröhlich, E.; Roblegg, E. Mucus as barrier for drug delivery by nanoparticles. J. Nanosci. Nanotechnol. 2014, 14, 126–136.
- Ways, T.M.M.; Lau, W.M.; Khutoryanskiy, V.V. Chitosan and its derivatives for application in mucoadhesive drug delivery systems. Polymers 2018, 10, 267.
- Boddupalli, B.M.; Mohammed, Z.N.; Nath, R.A.; Banji, D. Mucoadhesive drug delivery system: An overview. J. Adv. Pharm. Technol. Res. 2010, 1, 381–387.
- European Pharmacopoeia, 8th ed.; Council of Europe: Strasbourg, France, 2014; Volume 1.
- Andrews, G.P.; Laverty, T.P.; Jones, D.S. Mucoadhesive polymeric platforms for controlled drug delivery. Eur. J. Pharm. Biopharm. 2009, 71, 505–518.
- Bansil, R.; Turner, B. Mucin structure, aggregation, physiological functions and biomedical applications. Curr. Opin. Colloid Interface Sci. 2006, 11, 164–170.
- Hägerström, H.; Edsman, K.; Strømme, M. Low-frequency dielectric spectroscopy as a tool for studying the compatibility between pharmaceutical gels and mucus tissue. J. Pharm. Sci. 2003, 92, 1869–1881.
- Chaturvedi, M.; Kumar, M.; Pathak, K. A review on mucoadhesive polymer used in nasal drug delivery system. J. Adv. Pharm. Technol. Res. 2011, 2, 215.
- Lee, B.P.; Messersmith, P.B.; Israelachvili, J.N.; Waite, J.H. Mussel-inspired adhesives and coatings. Ann. Rev. Mater. Res. 2011, 41, 99.
- Xu, J.; Strandman, S.; Zhu, J.X.; Barralet, J.; Cerruti, M. Genipin-crosslinked catechol-chitosan mucoadhesive hydrogels for buccal drug delivery. Biomaterials 2015, 37, 395–404.
- Martínez-Carmona, M.; Lozano, D.; Colilla, M.; Vallet-Regí, M. Lectin-conjugated pH-responsive mesoporous silica nanoparticles for targeted bone cancer treatment. Acta Biomater. 2018, 65, 393–404.
- Cho, I.S.; Oh, H.M.; Cho, M.O.; Jang, B.S.; Cho, J.K.; Park, K.H.; Kang, S.W.; Huh, K.M. Synthesis and characterization ofthiolatedhexanoyl glycol chitosan as a mucoadhesivethermogellingpolymer. Biomater. Res. 2018, 22, 30.
- Sosnik, A.; DasNeves, J.; Sarmento, B. Mucoadhesive polymers in the design of nano-drug delivery systems for administration by non-parenteral routes: A review. Prog. Polym. Sci. 2014, 39, 2030–2075.
- Bernkop-Schnürch, A.; Greimel, A. Thiomers: The next generation of mucoadhesivepolymers. Am. J. Drug Deliv. 2005, 3, 141–154.
- Sreenivas, S.A.; Pai, K.V. Thiolated chitosan: Noval polymer and mucoadhesive drug delivery—A review. Trop. J. Pharm. Res. 2008, 7, 1077–1088.
- Sharma, S.; Kulkarni, J.; Pawar, A.P. Permeation enhancers in the transmucosal delivery of macromolecules. Pharmazie 2006, 61, 495–504.
- Schipper, N.G.M.; Varum, K.M.; Stenberg, P.; Ockind, G.; Lennernäis, H.; Artursson, P. Chitosan as absorption enhancers for poorly absorbable drugs 3: Influence of mucus on absorption enhancement. Eur. J. Pharm. Sci. 1999, 8, 335–343.
- Menchicchi, B.; Fuenzalida, J.P.; BobbiliK, B.A.; Hensel, A.; Swamy, M.J.; Goycoolea, F.M. Structure of chitosan determines its interactions with mucin. Biomacromolecules 2014, 15, 3550–3558.
- Salamat-Miller, N.; Chittchang, M.; Johnston, T.P. The use of mucoadhesive polymers in buccal drug delivery. Adv. Drug Deliv. Rev. 2005, 57, 1666–1691.
- Hanafy, N.A.N.; Quarta, A.; Ferraro, M.M.; Dini, L.; Nobile, C.; De Giorgi, M.L.; Carallo, S.; Citti, C.; Gaballo, A.; Cannazza, G.; et al. Polymeric Nano-MicellesasNovel Cargo-Carriers for LY2157299 LiverCancerCells Delivery. Int. J. Mol. Sci. 2018, 6, 19.
- Ralet, M.C.; Dronnet, V.; Buchholt, H.C.; Thibault, J.F. Enzymatically and chemically de-esterified lime pectins: Characterisation, polyelectrolyte behaviour and calcium binding properties. Carbohydr. Res. 2001, 336, 117–125.
- Chivite, A.T. Ionic Complexes of Biodegradable Polyelectrolytes. Ph.D. Thesis, Universitat Politècnica de Catalunya, Barcelona, Spain, 2014.
- Liu, L.; Fishman, M.L.; Kost, J.; Hicks, K.B. Pectin-based systems for colon-specific drug delivery via oral route. Biomaterials 2003, 24, 3333–3343.
- Sriamornsak, P.; Wattanakorn, N.; Takeuchi, H. Study on the mucoadhesion mechanism of pectin by atomic force microscopy and mucin-particle method. Carbohydr. Polym. 2010, 79, 54–59.
- Huang, Y.; Peppas, N.A. Nanoscale analysis of mucus–carrier interactions for improved drug absorption. In Nanotechnology in Therapeutics; Thomas, J.B., Ed.; HorizonBioscience: Wymondham, UK, 2007; pp. 109–129.
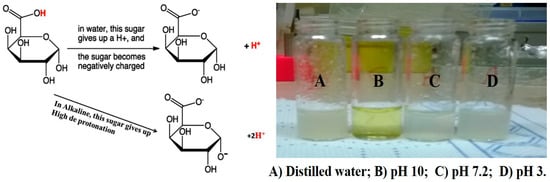
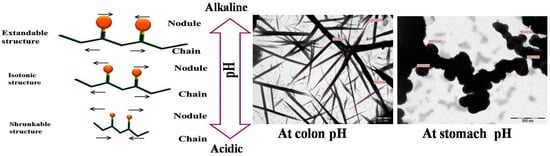
- Fang, X.; Somasundaran, P. Swelling of Poly(acrylic acid) in Concentrated Sodium Carbonate Solution. J. Chem. Eng. Data 2010, 55, 3555–3559.
- Hanafy, N.A.N.; El-Kemary, M.; Leporatti, S. Reduction diameter of CaCO3 crystals by using poly acrylic acid might improve cellular uptake of encapsulated curcumin in breast cancer. J. Nanomed. Res. 2018, 7, 235–239.
- Hanafy, N.A.N.; El-Kemary, M.; Leporatti, S. Micelles structure development as a strategy to improve drug delivery system. Cancers 2018, 10, 238.
- Hanafy, N.A.; DeGiorgi, M.L.D.; Nobile, C.; Rinaldi, R.; Leporatti, S. Control of Colloidal CaCO3 suspension by using biodegradable polymers during fabrication. Beni-Suef Univ. J. Basic Appl. Sci. 2015, 4, 60–70.
- Peppas, N.A.; Wood, K.M.; Blanchette, J.O. Hydrogels for oral delivery of therapeutic proteins. Expert Opin. Biol. Ther. 2004, 4, 881–887.
- Bastakotin, B.P.; Liao, S.H.; Inoue, M.; Yusa, S.I.; Imura, M.; Nakashima, K.; Wu, K.C.; Yamauchi, Y. pH-responsive polymeric micelles with Core-shell corona architectures as intracellular anti-cancer drug carriers. Sci. Technol. Adv. Mater. 2013, 14, 044402.
- Iatridi, Z.; Tsitsilianis, C. Water-Soluble Stimuli Responsive Star-Shape Segmented Macromolecules. Polymers 2011, 3, 1911–1933.
- Bhattarai, N.; Gunn, J.; Zhang, M. Chitosan-based hydrogels for controlled, localized drug delivery. Adv. Drug Deliv. Rev. 2010, 62, 83–99.
- Hanafy, N.A.N. Development and production of multifunctional bio nanoengineered drug delivery systems loaded by TGFβ1 inhibitors for delivering into hepatocellular carcinoma cells. Ph.D. Thesis, Salento University, Lecce, Italy, 2017.
- Dünnhaupt, S.; Barthelmes, J.; Kollner, S.; Sakloetsakun, D.; Shahnaz, G.; Düregger, A.; Bernkop-Schnürch, A. Thiolatednanocarriers for oral delivery of hydrophilic macromolecular drugs. Carbohydr. Polym. 2015, 117, 577–584.
- Parodi, B.; Russo, E.; Gatti, P.; Cafaggi, S.; Bignardi, G. Development and in vitro evaluation of buccoadhesive tablets using a new model substrate for bioadhesionmeasures: The eggshellmembrane. Drug Dev. Ind. Pharm. 1999, 25, 289–295.
- Hanafy, N.A.; Ferraro, M.M.; Gaballo, A.; Dini, L.; Tasco, V.; Nobile, C.; DeGiorgi, M.L.; Carallo, S.; Rinaldi, R.; Leporatti, S. Fabrication and Characterization of ALK1fc-Loaded Fluoro-Magnetic Nanoparticles Rods for Inhibiting TGF β1 in HCC. RSC Adv. 2016, 6, 48834–48842.
- Liao, Y.H.; Jones, S.A.; Forbes, B.; Martin, G.P.; Brown, M.B. Hyaluronan: Pharmaceutical characterization and drug delivery. Drug Deliv. 2005, 12, 327–342.
- Jørgensen, L.; Klösgen, B.; Simonsen, A.C.; Borch, J.; Hagesaether, E. New insights into the mucoadhesion of pectins by AFM roughness parameters in combination with SPR. Int. J. Pharm. 2011, 411, 162–168.
- Malik, A.; Gupta, M.; Gupta, V.; Gogoi, H.; Bhatnagar, R. Novel application of trimethyl chitosan as an adjuvant in vaccine delivery. Int. J. Nanomed. 2018, 13, 7959–7970.
- Li, D.; Fu, D.; Kang, H.; Rong, G.; Jin, Z.; Wang, X.; Zhao, K. Advances and Potential Applications of Chitosan Nanoparticles as a Delivery Carrier for the Mucosal Immunity of Vaccine. Curr. Drug Deliv. 2017, 14, 27–35.
- Anil, A.; Sudheer, P. Design and Evaluation of Mucoadhesive Buccal Patch of Ramipril. Int. J. Pharm. Sci. Rev. Res. 2018, 50, 121–129.