| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Kumar Ganesan | + 5830 word(s) | 5830 | 2020-06-08 08:01:43 |
Video Upload Options
Diet-derived phytochemicals modulate microbiome that is found to offer significant protective effects against colorectal cancer (CRC). The person’s lifestyle and the eating pattern have significant impacts on the CRC in a positive and/or negative way. Phytochemicals are a concoction of various bioactive compounds directing various cell signaling pathways that altered gut microbiota composition. This may support to destroy malignant cells with minor risks of emerging drug resistance. The effectiveness of CRC can be reduced by the use of various dietary phytochemicals or modulating microbiome that reduces or inverses the progression of a tumor, which could be promising and efficient in reducing the burden of CRC. Phytochemicals with modulation of gut microbiome continue to be auspicious investigations in CRC through noticeable anti-tumorigenic effects, which provides new openings for cancer inhibition and treatment.
1. Gut microbiota
The microbiome is the main inhabitants in the human gut, comprises 100 trillion microbes with diverse actions that maintain the integrity of healthy colon [1]. Undigested dietary residues in the colonic lumen are the prime energy sources for the gut microbiota, which digest those dietary residues resulting in the formation of several active metabolites with favorable functions. Imbalance of gut microbiota or dysbiosis can lead to several pathologies, including infectious diseases, gastrointestinal cancers, inflammatory bowel disease, and even obesity and diabetes. Dysbiosis may cause chronic inflammation, recognized as one of the prime causes of CRC. Earlier, our publications have also summarized the functions of gut microbiota, particularly, short-chain fatty acid synthesis with their benefits to the hosts in regulating various diseases such as diabetes, cardiovascular diseases, and cancer [2-4]. Dietary interventions or the consumption of phytochemicals is the beneficial components, proved as effective in treating CRC [5-12]. Taken into the account, we aimed to review in-depth analysis of various diet-derived phytochemicals mediated gut microbiome and its role in CRC prevention and treatment.
Earlier studies suggested the gut microbiota (Bacteroides fragilis, Escherichia coli strain NC101, Desulfovibrio, Helicobacter hepaticus, Clostridium ramosum, Fusobacterium, Campylobacter, Prevotella, etc.) in humans play a significant role and alter the immune function through pro-carcinogenic markers resulting in the etiology of CRC [4]. Altering the immune system in the gut normally enhances tumor microhabitats, and inflammation, ensuing the CRC development [3]. In recent research has also recommended genetically reformed colon bacteria, which are beneficial and are currently employed in experimental cases that outcomes are promising [13]. Furthermore, they can be greatly beneficial to the host as probiotics that inhibit CRC through alterations of microbiota and colon environment.
The consumption of natural products produces essential bioeffects in the body through multifaceted relations with gut microbiota [14,15]. Natural phytochemicals normally have fiber-rich glycosides that exist as complex molecules with the properties of lower bioavailability and lesser solubility [16]. The nature of the phytochemicals could be altered during microbial fermentation in the colon, ensuring high quantities of various byproducts with greater pharmacological activity [17]. Numerous metabolites that derived from gut microbiota may further be subject to various enzymatic cleavage by methylation, glucuronidation, glycination, or sulfation in the hepatocytes, which are then trafficked into the tissues and finally excreted into the gut [16,18]. Gut microbiota converts glucuronides to aglycones by β-glucuronidases, which can be immediately reabsorbed in the colon. Thus, the synthesis of microbial β-glucuronidase and its enterohepatic passage have possible steps to extend the holding period of phytochemicals in the host [16,18]. Rising data suggested the dietary phytometabolites derived from gut microbiota, which are capable of enhancing the bioavailability, antioxidant properties, detoxification of xenobiotics, and prebiotics function [18,19]. Furthermore, these compounds can eliminate gut pathogenic organisms, reduce oxidative DNA damage and pro-inflammatory mediators, and thus regulate normal cell division and apoptosis [20,21]. The effects of phytochemicals on gut microbiota and their anti-inflammatory effects are presented in Table 1.
Table 1. Effects of phytochemicals on gut microbiota and their anti-inflammatory effects.
|
Phytochemicals
|
Compounds |
Model |
Effect on gut microbiota |
Anti-inflammatory effect |
References |
|
Anthocyanins |
Anthocyanins |
C57BL/6 J mice |
Feces of gut microbiota-deficient mice showed an increase in anthocyanins and a decrease in their phenolic acid metabolites, while a corresponding increase was observed in jejunum tissue |
Decreased the inflammatory status of mice |
[22] |
|
Anthocyanins |
Anthocyanins |
C57BL/6 J mice |
Treatment modified the gut microbiota composition |
Effectively reduced the expression levels of IL-6 and TNFα genes, markedly increased SOD and GPx activity |
[23] |
|
Catechins |
Epigallocatechin-3-gallate |
C57BL/6 J mice |
The Firmicutes/Bacteroidetes ratio significantly lowered in HFD + EGCG, but higher in control diet + EGCG |
Potential use for prevention, or therapy, for oxidative stress-induced health risks |
[24] |
|
Catechins |
Epigallocatechin-3-gallate |
C57BL/6 J mice |
Maintained the microbial ecology balance and prevented dysbiosis |
Suppressed the activation of NF-κB and decrease expression of iNOS |
[25] |
|
Catechins |
Epigallocatechin-3-gallate |
Wistar rats |
Affected the growth of certain species of gut microbiota |
Suppressed the activation of NF-κB |
[26] |
|
Catechins |
Quercetin |
C57BL/6 J mice |
Increased Firmicutes/Bacteroidetes ratio and gram-negative bacteria and increased Helicobacter. Regulated gut microbiota balance |
Reverted dysbiosis-mediated TLR-4, NF-κB signaling pathway activation, and related endotoxemia, with subsequent inhibition of inflammasome response and reticulum stress pathway activation |
[27] |
|
Catechins |
Quercetin |
Wistar rats |
Attenuated Firmicutes/Bacteroidetes ratio, inhibiting the growth of bacterial species associated with diet-induced obesity (Erysipelotrichaceae, Bacillus, Eubacterium cylindroides). Quercetin was effective in lessening high-fat sucrose diet-induced gut microbiota dysbiosis |
Suppressed the activation of NF-κB |
[28] |
|
Catechins |
Quercetin |
Fischer 344 rats |
Exerted prebiotic properties by decreased pH, increased butyrate production, and altered gut microbiota |
Suppressed the activation of NF-κB |
[29] |
|
Catechins |
Kaempferol |
3 T3-L1 adipocytes |
Treatment modified the gut microbiota composition |
Reduced LPS pro-inflammatory action, promoted anti-inflammatory and antioxidant effects |
[30] |
|
Flavonones |
Baicalein |
C57BL/6 J mice |
Firmicutes/Bacteroidetes ratio significantly lowered and regulated dysbiosis |
Suppressed the activation of NF-κB and decreased the expression of iNOS and TGF-β |
[31] |
|
Organosulfur compounds |
Garlic essential oil and |
C57BL/6 mice |
Treatment modified the gut microbiota composition |
Significantly decreased the release of pro-inflammatory cytokines in the liver, accompanied by elevated antioxidant capacity via inhibition of cytochrome P450 2E1 expression |
[32] |
|
Phenolic acid
|
Curcumin |
Mice |
A direct effect of bioactive metabolites reaching the adipose tissue rather than from changes in gut microbiota composition |
Nutritional doses of Curcuma longa decreased proinflammatory cytokine expression in subcutaneous adipose tissue |
[33] |
|
Phenolic acid |
Curcumin |
LDLR−/− mice |
Improved intestinal barrier function and prevented the development of metabolic diseases |
Significantly attenuated the Western diet-induced increase in plasma LPS levels |
[34] |
|
Phenolic acid |
Curcumin |
Human IEC lines Caco-2 and HT-29 |
Modulated chronic inflammatory diseases by reducing intestinal barrier dysfunction despite poor bioavailability |
Significantly attenuated LPS-induced secretion of master cytokine IL-1β from IEC and macrophages. Reduced IL-1β-induced activation of p38 MAPK in IEC and subsequent increase in the expression of myosin light-chain kinase |
[35] |
|
Polyphenols |
Polyphenols |
C57BL/6 J ApcMin mice |
Bacterial diversity was higher in the bilberry group than in the other groups |
Attenuation of inflammation in cloudberry-fed mice |
[36] |
|
Stilbenes |
Resveratrol |
Kunming mice |
HF microbiomes were different from those in CT and HF-RES mice. After treatment, Lactobacillus and Bifidobacterium were significantly increased, whereas Enterococcus faecalis was significantly decreased, resulting in a higher abundance of Bacteroidetes and a lower abundance of Firmicutes |
Decreased the inflammatory status of mice |
[37] |
|
Stilbenes |
Resveratrol |
Glp1r−/− mice |
Treatment modified the gut microbiota composition |
Decreased the inflammatory status of mice |
[38] |
|
Stilbenes |
Resveratrol |
Wistar rats |
Trans-resveratrol supplementation alone or in combination with quercetin scarcely modified the gut microbiota profile but acted at the intestinal level, altering mRNA expression of tight-junction proteins and inflammation-associated genes |
Altered mRNA expression of tight-junction proteins and inflammation-associated genes |
[28] |
|
Stilbenes |
Resveratrol
|
Adipocytes |
Treatment modified the gut microbiota composition |
Resveratrol opposed the effect induced by LPS, functioning as an ameliorating factor in disease state |
[39] |
|
Stilbenes |
Resveratrol |
Human |
Steroid metabolism of the affected gut microbiota was studied |
- |
[40] |
|
Stilbenes |
Piceatannol |
C57BL/6 mice |
Altered the composition of the gut microbiota by increasing Firmicutes and Lactobacillus and decreasing Bacteroidetes |
Decreased the inflammatory status of mice |
[41] |
|
Stilbenes |
Piceatannol |
Zucker obese rats |
It did not modify the profusion of the most abundant phyla in gut microbiota, though slight changes were observed in the abundance of several Lactobacillus, Clostridium, and Bacteroides species belonging to Firmicutes and Bacteroidetes |
Showed a tendency to reduce plasma LPS by 30% |
[42] |
Abbreviation: Caco-2—human epithelial colorectal adenocarcinoma cells; CT—control diet; EGCG—Epigallocatechin-3-gallate; GPx—glutathione peroxidase; HF-RES—high-fat diet supplemented with resveratrol; HFD—high-fat diet; IEC—intestinal epithelial cells; IL 6—interleukin 6; iNOS—inducible nitric oxide synthase; LPS—lipopolysaccharides; MAPK—mitogen-activated protein kinase; mRNA—messenger ribonucleic acids; NF-κB—nuclear factor kappa B; SOD—superoxide dismutase; TGF β—transforming growth factor-beta; TLR-4—toll-like receptor 4; TNFα—tumor necrosis factor-alpha; P450 2E1—cytochrome P450 2E1
2. Dietary Polyphenols
Polyphenols are one of the prime classes of chemicals in plants, extensively studied for their health-promoting properties [7-10,12,43-46]. Human diets contain varieties of polyphenols and have significant protective activities against various cancer types. Scavenging of free radicals and reducing oxidative stress are the key mechanisms by which a polyphenol can achieve [38]. Several studies confirmed the actions of polyphenols on CRC inhibition, which often interconnected with the relationship of gut microbiota [47-49]. For instance, an animal study was conducted related to cranberry polyphenols on Akkermansia (mucin-degrading bacterium), which protected the host from obesity, diabetes, and gut inflammation. In this study, the mice were administered with high fat and sugar diet and cranberry extract (CE) (200 mg/kg/day) for eight weeks, and the various gut microbiota were analyzed by the methods of 16S rRNA and 454 pyrosequencing. The outcomes of the study revealed the administration of CE reduced body weight, visceral fat obesity, triglyceride accumulation, and inflammation, and elevated antioxidant properties and insulin sensitivity. Furthermore, the metagenomics study of CE treatment exhibited an increased percentage of Akkermansia [48].
The anti-carcinogenic properties of the gut microbiota are generally attributed based on the two properties, (a) either by improving the host’s immune system or (b) by generating the metabolites, which can interfere with the pathways involving CRC formation. A study demonstrated that the presence of amines, bile acids, and high consumption of meat can reduce some bacterial growth such as Clostridium, which inhibits the development of CRC [49]. By using the alimentary metabolites, gut microbiota produces biologically active short-chain fatty acids. The Rosburia faecis and Eubacterium rectale group of bacteria can normally produce the butyrate, which involves reducing cell apoptosis and diversity [47]. A study showed the polyphenol metabolites modulated microbiota that directly restricted the growth/proliferation of CRC [50]. Another study has also related intestinal metabolites, quercetin, chlorogenic, and caffeic acids to interfering in cyclooxygenase-2 expression resulting in the prevention of DNA damage in the colon [51]. The polyphenols-mediated gut microbiota changes are a potential technique for inhibiting colon cancer, although insufficient trials have been piloted, in which, wine [52], blueberry [53], and cocoa [54] displayed a bifidogenic outcome.
3. Dietary Flavonoids
Flavonoids are mainly present in fruits, vegetables, seeds, and various beverages such as tea, coffee, and red wine. Several medicinal herbs are amongst the richest sources of flavonoids. They are grouped into the following sub-classes-flavonols (quercetin, rutin), flavanols (catechin, epicatechin, and epigallocatechin), flavones (luteolin, apigenin), anthocyanidins (malvidin, cyanidin, and delphinidin), isoflavones (daidzein, genistein, glycetin, and formanantine), and flavanones (naringenin, hesperetin) [55-58]. A hypothesis stated that the presence of beneficial phytochemicals in diets attributes an anticancer property to the respective food. The flavonoids present in the food prevent CRC development by exerting various mechanisms: alleviating DNA damage, reducing the effects of gene mutation, regulation of phase I, and phase II enzymes via modulation in cell signaling pathways, suppressing oncogene expression and regulating inflammatory responses [59-63]. In a recent clinical trial, a flavonoid mixture of 20 mg apigenin along with 20 mg epigallocatechin gallate was given to CRC patients daily for long-term interventions that showed the reduction of CRC relapse [64]. The greater quantities of polymeric flavonoids and the non-absorbed flavonoids passed into the colon region where they underwent breakdown and gut microbiota facilitate converting these flavonoids into simple phenolic acids [65].
The digestion of flavonoids is often mediated by gut microbiota, which is a similar pattern to other phytochemicals. Gut microbiota facilitates converting a large group of flavonoids into simple active metabolites (aromatic catabolites and small phenolic acids) by oxidation and demethylation [66,67]. These active products augment physiological activity and perform various roles in the regulation of the host’s immune system. One best instance for the gut microbiota-mediated metabolite is daidzein-isoflavones, which serves various benefits to the host. Daidzein is found in numerous plants and predominantly occurs in soybeans; daidzein is transformed by bacterial flora into the most active compound equol. In vitro and clinical trials showed that equol is more bioactive than daidzein (food precursor), and the biological effect is significantly improved in patients who produced equol after isoflavone consumption [68]. This result strongly suggested that gut microbiota aid a pivotal function in regulating the biological effects of ingested phytochemicals.
We recognize that the impacts of the gut bacterium on the flavonoids and the effects of flavonoids on the gut microbiota are bidirectional. Flavonoids can change the organization and roles of gut microbiota, and similarly, gut microbiota can enhance the flavonoid breakdown. A case pilot study with 178 elderly people showed the habitual diet, which contributed to bacterial alterations resulted in the improvement of frailty and inflammation [69]. Another fascinating study revealed that 15 women with a two-month dietary intervention connected to alterations of gut microbiota including, Gammaproteobacteria and Erysipelotrichi [70]. A study on the impacts of grape extract (GE) on experimental animals showed the reduction of the Firmicutes-to-Bacteroidetes ratio and an increasing of Akkermansia muciniphila. Supplementation of GE along with gut microbiota significantly reduced inflammatory response and improved insulin sensitivity. These findings offered noteworthy support in favor of colonic bacteria and their substantial role in facilitating the flavonoids on health impacts, which reduced inflammatory response as well as improved the metabolic function. Another interesting clinical study demonstrated that the feeding stable isotope-labeled anthocyanins were ingested by gut microbiota, which yielded high quantities of diverse active metabolites [1,71]. These colonic bioactive phytometabolites exert greater anti-inflammatory functions and maintain vascular integrity when compared to the normal colonic metabolites [72]. This statement complements the belief of the effect of increased activities of phytochemicals on host health, which are the utmost prospective study related to gut microbiota.
4. Phytochemicals modulate Gut microbiome that regulates Wnt/β-catenin signaling pathways
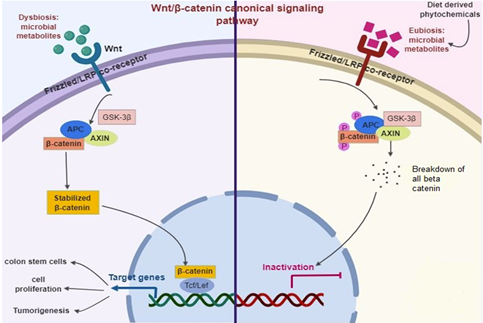
The initiation of colon tumorigenesis is frequently determined by mutations in the Wnt signaling. Wnt is generally a secreted signaling protein. Conversely, the loss of function of adenomatous polyposis coli or gain of function of β-catenin cause the balance of unrestricted β-catenin that provides abnormal Wnt signaling leads to tumorigenesis [73]. Notably, mutation triggering of the Wnt pathway in G-protein-coupled receptor (Lgr5+) cells contributes to intestinal tumors with high competence than other colonic cell tumors [74]. According to the CSC hypothesis, the populace of colon cells can propagate tumor generation, measured as multipotent resulting in the cell of cancer [74,75]. Current data shows that multiple CSC hierarchies occur in the colon, facilitate cell fate in the account for various extrinsic factors including, diet, inflammation, and body anxiety [76]. Additionally, a function of diet in the maintenance of colon CSC has also described [77]. The Wnt signaling generally occurs in an upstream of the β-catenin pathway [78] (Figure 1). Briefly, Wnt ligands largely fix with the complex of Frizzled/LRP co-receptor, which triggers the canonical pathway. Axin, a Wnt signaling inhibitor protein is employed to the cell membrane, resulting in the inactivation of the adenomatous polyposis coli complex succeeding in the equilibrium of β-catenin. When Wnt is triggered, β-catenin is instantly soothed, allowing transfer to the nucleus and fixes with T cell factor and eventually elicits the expression of target genes. Among them, Leucine-rich repeat-containing Lgr5+ genes participated in stem cell proliferation [79]. The adenomatous polyposis coli is normally a tumor-suppressor protein that is mutated in almost 80% of CRC. Thus, the stimulation of Wnt/β-catenin is a primary biomarker of colitis-related CRC [80]. Diet-derived phytochemicals balance the microbiome status (Eubiosis), which inhibits Wnt/β-catenin signaling pathways and successively prevent intestinal infection and inflammation [81,82].
Figure 1. Diet-derived phytochemicals stabilize the microbiome status (Eubiosis) that inhibit Wnt/β-catenin signaling pathways successively prevent intestinal infection and inflammation.
Gut microbiota is chiefly affected by the dietary phytochemicals that can disturb its physiological relations in the host [3]. Through their alimentary canal route, phytochemicals are digested by colonic bacteria and produce several by-products [14]. These phytochemicals are rich in various active principles comprising polyphenols and flavonoids that upsurge the Firmicutes, Bacteroidetes, Actinobacteria, and Proteobacteria, which alters the pH of the colon environment and maintains the balance of the colonic microbiome [4]. Therefore, the effect of colonic bacteria on the dietary phytochemicals targeting dietary intervention which may contribute to host well-being [3,4]. The phytochemicals facilitate colonic bacteria, which may influence as adjuvants to treat cancer, obesity, diabetes, and chronic inflammatory diseases and prove as potentially prophylactics and candidates for the treatment of these diseases [2-4].
5. Concluding Remarks and Prospective
Several matters urgently require to explain before phytochemicals can be successfully transferred from bench to bed. Concerning the good source of bioactive compounds, the succeeding features ought to be measured: (a) they acquire directly from dietary or pharmacological sources (b) can be employed alone or combined with other existing medicine (c) bioavailability and optimization of the individual bioactive compound, which impacts on gut microbiota (d) require adequate clinical trials based on the underlying gastrointestinal inflammatory conditions. (e) Standardize the feasible nutraceutical preparations (methodology and chemical composition) (f) the dosage fixation and route of infusion of the drugs. The existing cumulative data associated with in vitro and animal studies that strongly propose the promising effects of the wide spectrum of phytochemicals related to various gut-associated disease conditions. However, it remains inadequate to achieve in the human trials due to lacking validation of phytochemicals on the gut microbiome. More investigations are highly recommended to focus on the analysis of different diet-derived phytochemicals metabolized by gut microbiota and their impacts on human health. Nevertheless, dietary phytochemicals and their analogs modulate gut microbiota, which can be offered the advancement of better quality drugs and provide the resolution to eradicate various gut-associated diseases. Dietary phytochemicals induced gut microbiota is continued to be an encouraging and dynamic research niche in the upcoming days with evident anti-tumorigenesis effects and propose novel opportunities for CRC prevention and treatment.
References
- Czank, C.; Cassidy, A.; Zhang, Q.; Morrison, D.J.; Preston, T.; Kroon, P.A.; Botting, N.P.; Kay, C.D. Human metabolism and elimination of the anthocyanin, cyanidin-3-glucoside: a 13C-tracer study. Am. J. Clin. Nutr. 2013, 97, 995-1003, doi:10.3945/ajcn.112.049247.
- Jayachandran, M.; Xiao, J.; Xu, B. A critical review on health-promoting benefits of edible mushrooms through gut microbiota. Int. J. Mol. Sci. 2017, 18, 1934, doi:10.3390/ijms18091934.
- Ganesan, K.; Chung, S.K.; Vanamala, J.; Xu, B. Causal relationship between diet-induced gut microbiota changes and diabetes: a novel strategy to transplant Faecalibacterium prausnitzii in preventing diabetes. Int. J. Mol. Sci. 2018, 19, 3720, doi:10.3390/ijms19123720.
- Ganesan, K.; Guo, S.; Fayyaz, S.; Zhang, G.; Xu, B. Targeting programmed Fusobacterium nucleatum Fap2 for colorectal cancer therapy. Cancers 2019, 11, 1592, doi:10.3390/cancers11101592.
- Ganesan; Xu. Anti-Diabetic effects and mechanisms of dietary polysaccharides. Molecules 2019, 24, 2556, doi:10.3390/molecules24142556.
- Ganesan, K.; Xu, B. Anti-obesity effects of medicinal and edible mushrooms. Molecules 2018, 23, 2880, doi:10.3390/molecules23112880.
- Ganesan, K.; Jayachandran, M.; Xu, B. A critical review on hepatoprotective effects of bioactive food components. Crit. Rev. Food Sci. Nutr. 2017, 58, 1165-1229, doi:10.1080/10408398.2016.1244154.
- Ganesan, K.; Xu, B. A critical review on polyphenols and health benefits of black soybeans. Nutrients 2017, 9, doi:10.3390/nu9050455.
- Xu, B.; Ganesan, K.; Mickymaray, S.; Alfaiz, F.A.; Thatchinamoorthi, R.; Aboody, M.S.A. Immunomodulatory and antineoplastic efficacy of common spices and their connection with phenolic antioxidants. Bioactive Comp. Health Dis. 2020, 3, 15, doi:10.31989/bchd.v3i2.687.
- Islam, T.; Ganesan, K.; Xu, B. New insight into mycochemical profiles and antioxidant potential of edible and medicinal mushrooms: A review. Int. J.Med. Mushrooms 2019, 21, 237-251, doi:10.1615/intjmedmushrooms.2019030079.
- Ganesan, K.; Xu, B. Telomerase inhibitors from natural products and their anticancer potential. Int. J. Mol. Sci. 2017, 19, doi:10.3390/ijms19010013.
- Sukalingam, K.; Ganesan, K.; Xu, B. Trianthema portulacastrum L. (giant pigweed): phytochemistry and pharmacological properties. Phytochem. Rev. 2017, 16, 461-478, doi:10.1007/s11101-017-9493-5.
- Abreu, M.T.; Peek, R.M. Gastrointestinal malignancy and the microbiome. Gastroenterology 2014, 146, 1534-1546.e1533, doi:10.1053/j.gastro.2014.01.001.
- Eid, H.M.; Wright, M.L.; Anil Kumar, N.V.; Qawasmeh, A.; Hassan, S.T.S.; Mocan, A.; Nabavi, S.M.; Rastrelli, L.; Atanasov, A.G.; Haddad, P.S. Significance of microbiota in obesity and metabolic diseases and the modulatory potential by medicinal plant and food ingredients. Front. Pharmacol. 2017, 8, doi:10.3389/fphar.2017.00387.
- Espín, J.C.; González-Sarrías, A.; Tomás-Barberán, F.A. The gut microbiota: A key factor in the therapeutic effects of (poly)phenols. Biochem. Pharmacol. 2017, 139, 82-93, doi:10.1016/j.bcp.2017.04.033.
- Bohn, T.; McDougall, G.J.; Alegría, A.; Alminger, M.; Arrigoni, E.; Aura, A.-M.; Brito, C.; Cilla, A.; El, S.N.; Karakaya, S., et al. Mind the gap-deficits in our knowledge of aspects impacting the bioavailability of phytochemicals and their metabolites-a position paper focusing on carotenoids and polyphenols. Mol. Nutr. Food Res. 2015, 59, 1307-1323, doi:10.1002/mnfr.201400745.
- Marín, L.; Miguélez, E.M.; Villar, C.J.; Lombó, F. Bioavailability of dietary polyphenols and gut microbiota metabolism: antimicrobial properties. BioMed Res. Int. 2015, 2015, 1-18, doi:10.1155/2015/905215.
- Louis, P.; Hold, G.L.; Flint, H.J. The gut microbiota, bacterial metabolites and colorectal cancer. Nature Reviews Microbiology 2014, 12, 661-672, doi:10.1038/nrmicro3344.
- Ozdal, T.; Sela, D.A.; Xiao, J.; Boyacioglu, D.; Chen, F.; Capanoglu, E. The Reciprocal Interactions between Polyphenols and Gut Microbiota and Effects on Bioaccessibility. Nutrients 2016, 8, 78, doi:10.3390/nu8020078.
- Del Rio, D.; Rodriguez-Mateos, A.; Spencer, J.P.E.; Tognolini, M.; Borges, G.; Crozier, A. Dietary (Poly)phenolics in human health: structures, bioavailability, and evidence of protective effects against chronic diseases. Antioxid. Redox Signal. 2013, 18, 1818-1892, doi:10.1089/ars.2012.4581.
- Etxeberria, U.; Fernández-Quintela, A.; Milagro, F.I.; Aguirre, L.; Martínez, J.A.; Portillo, M.P. Impact of polyphenols and polyphenol-rich dietary sources on gut microbiota composition. J. Agri.Food Chem. 2013, 61, 9517-9533, doi:10.1021/jf402506c.
- Esposito, D.; Damsud, T.; Wilson, M.; Grace, M.H.; Strauch, R.; Li, X.; Lila, M.A.; Komarnytsky, S. Black currant anthocyanins attenuate weight gain and improve glucose metabolism in diet-induced obese mice with intact, but not disrupted, gut microbiome. J. Agri.Food Chem. 2015, 63, 6172-6180, doi:10.1021/acs.jafc.5b00963.
- Wu, T.; Tang, Q.; Yu, Z.; Gao, Z.; Hu, H.; Chen, W.; Zheng, X.; Yu, T. Inhibitory effects of sweet cherry anthocyanins on the obesity development in C57BL/6 mice. Int. J. Food Sci. Nutr. 2013, 65, 351-359, doi:10.3109/09637486.2013.854749.
- Remely, M.; Ferk, F.; Sterneder, S.; Setayesh, T.; Roth, S.; Kepcija, T.; Noorizadeh, R.; Rebhan, I.; Greunz, M.; Beckmann, J., et al. EGCG Prevents high fat diet-induced changes in gut microbiota, decreases of DNA strand breaks, and changes in expression and DNA methylation of Dnmt1 and MLH1in C57BL/6J male mice. Oxid.Med. Cell. Longevit 2017, 2017, 1-17, doi:10.1155/2017/3079148.
- Cheng, M.; Zhang, X.; Miao, Y.; Cao, J.; Wu, Z.; Weng, P. The modulatory effect of (-)-epigallocatechin 3-O-(3-O-methyl) gallate (EGCG3″Me) on intestinal microbiota of high fat diet-induced obesity mice model. Food Res. Int. 2017, 92, 9-16, doi:10.1016/j.foodres.2016.12.008.
- Unno, T.; Sakuma, M.; Mitsuhashi, S. Effect of Dietary Supplementation of (^|^minus;)-Epigallocatechin gallate on gut microbiota and biomarkers of colonic fermentation in rats. J. Nutr. Sci. Vitaminol. 2014, 60, 213-219, doi:10.3177/jnsv.60.213.
- Porras, D.; Nistal, E.; Martínez-Flórez, S.; Pisonero-Vaquero, S.; Olcoz, J.L.; Jover, R.; González-Gallego, J.; García-Mediavilla, M.V.; Sánchez-Campos, S. Protective effect of quercetin on high-fat diet-induced non-alcoholic fatty liver disease in mice is mediated by modulating intestinal microbiota imbalance and related gut-liver axis activation. Free Rad. Biol. Med. 2017, 102, 188-202, doi:10.1016/j.freeradbiomed.2016.11.037.
- Etxeberria, U.; Arias, N.; Boqué, N.; Macarulla, M.T.; Portillo, M.P.; Martínez, J.A.; Milagro, F.I. Reshaping faecal gut microbiota composition by the intake of trans-resveratrol and quercetin in high-fat sucrose diet-fed rats. J. Nutr. Biochem. 2015, 26, 651-660, doi:10.1016/j.jnutbio.2015.01.002.
- Roldán-Marín, E.; Krath, B.N.; Poulsen, M.; Binderup, M.-L.; Nielsen, T.H.; Hansen, M.; Barri, T.; Langkilde, S.; Pilar Cano, M.; Sánchez-Moreno, C., et al. Effects of an onion by-product on bioactivity and safety markers in healthy rats. Br. J. Nutr. 2009, 102, 1574, doi:10.1017/s0007114509990870.
- Le Sage, F.; Meilhac, O.; Gonthier, M.-P. Anti-inflammatory and antioxidant effects of polyphenols extracted from Antirhea borbonica medicinal plant on adipocytes exposed to Porphyromonas gingivalis and Escherichia coli lipopolysaccharides. Pharmacol. Res. 2017, 119, 303-312, doi:10.1016/j.phrs.2017.02.020.
- Pu, P.; Wang, X.-A.; Salim, M.; Zhu, L.-H.; Wang, L.; Chen, k.-J.; Xiao, J.-F.; Deng, W.; Shi, H.-W.; Jiang, H., et al. Baicalein, a natural product, selectively activating AMPKα2 and ameliorates metabolic disorder in diet-induced mice. Mol. Cell. Endocrinol. 2012, 362, 128-138, doi:10.1016/j.mce.2012.06.002.
- Lai, Y.-S.; Chen, W.-C.; Ho, C.-T.; Lu, K.-H.; Lin, S.-H.; Tseng, H.-C.; Lin, S.-Y.; Sheen, L.-Y. Garlic essential oil protects against obesity-triggered nonalcoholic fatty liver disease through modulation of lipid metabolism and oxidative stress. J. Agri. Food Chem. 2014, 62, 5897-5906, doi:10.1021/jf500803c.
- Neyrinck, A.M.; Alligier, M.; Memvanga, P.B.; Névraumont, E.; Larondelle, Y.; Préat, V.; Cani, P.D.; Delzenne, N.M. Curcuma longa extract associated with White Pepper Lessens high fat diet-induced inflammation in subcutaneous adipose tissue. PLoS ONE 2013, 8, e81252, doi:10.1371/journal.pone.0081252.
- Ghosh, S.S.; Bie, J.; Wang, J.; Ghosh, S. Oral supplementation with non-absorbable antibiotics or curcumin attenuates western diet-induced atherosclerosis and glucose intolerance in LDLR−/− Mice – role of intestinal permeability and macrophage activation. PLoS ONE 2014, 9, e108577, doi:10.1371/journal.pone.0108577.
- Wang, J.; Ghosh, S.S.; Ghosh, S. Curcumin improves intestinal barrier function: modulation of intracellular signaling, and organization of tight junctions. Am. J. Physiol. Cell Physiol. 2017, 312, C438-C445, doi:10.1152/ajpcell.00235.2016.
- Päivärinta, E.; Niku, M.; Maukonen, J.; Storvik, M.; Heiman-Lindh, A.; Saarela, M.; Pajari, A.-M.; Mutanen, M. Changes in intestinal immunity, gut microbiota, and expression of energy metabolism–related genes explain adenoma growth in bilberry and cloudberry-fed Apc Min mice. Nutr. Res. 2016, 36, 1285-1297, doi:10.1016/j.nutres.2016.10.003.
- Qiao, Y.; Sun, J.; Xia, S.; Tang, X.; Shi, Y.; Le, G. Effects of resveratrol on gut microbiota and fat storage in a mouse model with high-fat-induced obesity. Food Funct. 2014, 5, 1241, doi:10.1039/c3fo60630a.
- Dao, T.-M.A.; Waget, A.; Klopp, P.; Serino, M.; Vachoux, C.; Pechere, L.; Drucker, D.J.; Champion, S.; Barthélemy, S.; Barra, Y., et al. Resveratrol increases glucose induced glp-1 secretion in mice: a mechanism which contributes to the glycemic control. PLoS ONE 2011, 6, e20700, doi:10.1371/journal.pone.0020700.
- Nøhr, M.K.; Kroager, T.P.; Sanggaard, K.W.; Knudsen, A.D.; Stensballe, A.; Enghild, J.J.; Ølholm, J.; Richelsen, B.; Pedersen, S.B. SILAC-MS Based characterization of LPS and Resveratrol induced changes in adipocyte proteomics – Resveratrol as Ameliorating factor on LPS induced changes. PLOS ONE 2016, 11, e0159747, doi:10.1371/journal.pone.0159747.
- Korsholm, A.; Kjær, T.; Ornstrup, M.; Pedersen, S. Comprehensive metabolomic analysis in blood, urine, fat, and muscle in men with metabolic syndrome: a randomized, placebo-controlled clinical trial on the effects of Resveratrol after four months’ treatment. Int. J. Mol. Sci. 2017, 18, 554, doi:10.3390/ijms18030554.
- Tung, Y.-C.; Lin, Y.-H.; Chen, H.-J.; Chou, S.-C.; Cheng, A.-C.; Kalyanam, N.; Ho, C.-T.; Pan, M.-H. Piceatannol exerts anti-obesity effects in C57BL/6 mice through modulating adipogenic proteins and gut microbiota. Molecules 2016, 21, 1419, doi:10.3390/molecules21111419.
- Hijona, E.; Aguirre, L.; Pérez-Matute, P.; Villanueva-Millán, M.J.; Mosqueda-Solis, A.; Hasnaoui, M.; Nepveu, F.; Senard, J.M.; Bujanda, L.; Aldámiz-Echevarría, L., et al. Limited beneficial effects of piceatannol supplementation on obesity complications in the obese Zucker rat: gut microbiota, metabolic, endocrine, and cardiac aspects. J. Physiol. Biochem. 2016, 72, 567-582, doi:10.1007/s13105-015-0464-2.
- Ganesan, K.; Xu, B. Polyphenol-rich dry common beans (Phaseolus vulgaris L.) and their health benefits. Int. J. Mol. Sci. 2017, 18, 2331, doi:10.3390/ijms18112331.
- Ganesan, K.; Xu, B. Polyphenol-rich lentils and their health-promoting effects. Int. J. Mol. Sci. 2017, 18, 2390, doi:10.3390/ijms18112390.
- Ganesan, K.; Xu, B. Molecular targets of vitexin and isovitexin in cancer therapy: a critical review. Ann. N. Y. Acad. Sci. 2017, 1401, 102-113, doi:10.1111/nyas.13446.
- Ganesan, K., Xu, B. A critical review on phytochemical profile and health-promoting effects of mung bean (Vigna radiata). Food Sci. Human Wellness 2018, 7, 11-33, doi:https://doi.org/10.1016/j.fshw.2017.11.002.
- Dueñas, M.; Cueva, C.; Muñoz-González, I.; Jiménez-Girón, A.; Sánchez-Patán, F.; Santos-Buelga, C.; Moreno-Arribas, M.; Bartolomé, B. Studies on modulation of gut microbiota by wine polyphenols: from isolated cultures to omic approaches. Antioxidants 2015, 4, 1-21, doi:10.3390/antiox4010001.
- Anhê, F.F.; Roy, D.; Pilon, G.; Dudonné, S.; Matamoros, S.; Varin, T.V.; Garofalo, C.; Moine, Q.; Desjardins, Y.; Levy, E., et al. A polyphenol-rich cranberry extract protects from diet-induced obesity, insulin resistance and intestinal inflammation in association with increasedAkkermansiaspp. population in the gut microbiota of mice. Gut 2014, 64, 872-883, doi:10.1136/gutjnl-2014-307142.
- Pahle, J.; Menzel, L.; Niesler, N.; Kobelt, D.; Aumann, J.; Rivera, M.; Walther, W. Rapid eradication of colon carcinoma by Clostridium perfringens Enterotoxin suicidal gene therapy. BMC Cancer 2017, 17, doi:10.1186/s12885-017-3123-x.
- Duda-Chodak, A.; Tarko, T.; Satora, P.; Sroka, P. Interaction of dietary compounds, especially polyphenols, with the intestinal microbiota: a review. Eur. J. Nutr. 2015, 54, 325-341, doi:10.1007/s00394-015-0852-y.
- Miene, C.; Weise, A.; Glei, M. Impact of polyphenol metabolites produced by colonic microbiota on expression of COX-2 and GSTT2 in human colon cells (LT97). Nutr. Cancer 2011, 63, 653-662, doi:10.1080/01635581.2011.552157.
- Barron, C.C.; Moore, J.; Tsakiridis, T.; Pickering, G.; Tsiani, E. Inhibition of human lung cancer cell proliferation and survival by wine. Cancer Cell Int. 2014, 14, 6, doi:10.1186/1475-2867-14-6.
- Yi, W.; Fischer, J.; Krewer, G.; Akoh, C.C. Phenolic compounds from blueberries can inhibit colon cancer cell proliferation and induce apoptosis. J. Agri. Food Chem. 2005, 53, 7320-7329, doi:10.1021/jf051333o.
- Martín, M.; Goya, L.; Ramos, S. Preventive effects of cocoa and cocoa antioxidants in colon cancer. Diseases 2016, 4, 6, doi:10.3390/diseases4010006.
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: an overview. J.Nutr. Sci. 2016, 5, doi:10.1017/jns.2016.41.
- Ganesan, K.; Xu, B. Ethnobotanical studies on folkloric medicinal plants in Nainamalai, Namakkal District, Tamil Nadu, India. Trend. Phytochem. Res. 2017, 1, 153-168.
- Ganesan, K.; Sukalingam, K.; Xu, B. Solanum trilobatum L. ameliorate thioacetamide-induced oxidative stress and hepatic damage in albino rats. Antioxidants (Basel) 2017, 6, doi:10.3390/antiox6030068.
- Sukalingam, K.; Ganesan, K.; Xu, B. Protective effect of aqueous extract from the leaves of Justicia tranquebariesis against thioacetamide-induced oxidative stress and hepatic fibrosis in rats. Antioxidants 2018, 7, 78, doi:10.3390/antiox7070078.
- Wang, W.; VanAlstyne, P.C.; Irons, K.A.; Chen, S.; Stewart, J.W.; Birt, D.F. Individual and interactive effects of apigenin analogs on G2/M cell-cycle arrest in human colon carcinoma cell lines. Nutr. Cancer 2004, 48, 106-114, doi:10.1207/s15327914nc4801_14.
- Aron, P.M.; Kennedy, J.A. Flavan-3-ols: Nature, occurrence and biological activity. Mol. Nutr. Food Res. 2008, 52, 79-104, doi:10.1002/mnfr.200700137.
- Zhang, T.; Jayachandran, M.; Ganesan, K.; Xu, B. Black truffle aqueous extract attenuates oxidative stress and inflammation in STZ-induced hyperglycemic rats via Nrf2 and NF-κB pathways. Front. Pharmacol. 2018, 9, doi:10.3389/fphar.2018.01257.
- Jayachandran, M.; Zhang, T.; Ganesan, K.; Xu, B.; Chung, S.S.M. Isoquercetin ameliorates hyperglycemia and regulates key enzymes of glucose metabolism via insulin signaling pathway in streptozotocin-induced diabetic rats. Eur.J. Pharmacol. 2018, 829, 112-120, doi:10.1016/j.ejphar.2018.04.015.
- Jayachandran, M.; Wu, Z.; Ganesan, K.; Khalid, S.; Chung, S.M.; Xu, B. Isoquercetin upregulates antioxidant genes, suppresses inflammatory cytokines and regulates AMPK pathway in streptozotocin-induced diabetic rats. Chem. Biol. Interact. 2019, 303, 62-69, doi:10.1016/j.cbi.2019.02.017.
- Hoensch, H.; Groh, B.; Edler, L.; Kirch, W. Prospective cohort comparison of flavonoid treatment in patients with resected colorectal cancer to prevent recurrence. World J. Gastroenterol. 2008, 14, 2187, doi:10.3748/wjg.14.2187.
- Murota, K.; Nakamura, Y.; Uehara, M. Flavonoid metabolism: the interaction of metabolites and gut microbiota. Biosci. Biotechnol. Biochem. 2018, 82, 600-610, doi:10.1080/09168451.2018.1444467.
- Clavel, T.; Henderson, G.; Alpert, C.A.; Philippe, C.; Rigottier-Gois, L.; Dore, J.; Blaut, M. Intestinal bacterial communities that produce active estrogen-like compounds enterodiol and enterolactone in humans. Appl. Environ. Microbiol. 2005, 71, 6077-6085, doi:10.1128/aem.71.10.6077-6085.2005.
- Singh, R.K.; Chang, H.-W.; Yan, D.; Lee, K.M.; Ucmak, D.; Wong, K.; Abrouk, M.; Farahnik, B.; Nakamura, M.; Zhu, T.H., et al. Influence of diet on the gut microbiome and implications for human health. J. Translat. Med. 2017, 15, doi:10.1186/s12967-017-1175-y.
- Setchell, K.D.R.; Brown, N.M.; Lydeking-Olsen, E. The Clinical importance of the metabolite equol—a clue to the effectiveness of soy and its isoflavones. J. Nutr. 2002, 132, 3577-3584, doi:10.1093/jn/132.12.3577.
- Claesson, M.J.; Jeffery, I.B.; Conde, S.; Power, S.E.; O’Connor, E.M.; Cusack, S.; Harris, H.M.B.; Coakley, M.; Lakshminarayanan, B.; O’Sullivan, O., et al. Gut microbiota composition correlates with diet and health in the elderly. Nature 2012, 488, 178-184, doi:10.1038/nature11319.
- Spencer, M.D.; Hamp, T.J.; Reid, R.W.; Fischer, L.M.; Zeisel, S.H.; Fodor, A.A. Association between composition of the human gastrointestinal microbiome and development of fatty liver with choline deficiency. Gastroenterology 2011, 140, 976-986, doi:10.1053/j.gastro.2010.11.049.
- de Ferrars, R.M.; Czank, C.; Zhang, Q.; Botting, N.P.; Kroon, P.A.; Cassidy, A.; Kay, C.D. The pharmacokinetics of anthocyanins and their metabolites in humans. Br. J. Pharmacol. 2014, 171, 3268-3282, doi:10.1111/bph.12676.
- Gil-Cardoso, K.; Ginés, I.; Pinent, M.; Ardévol, A.; Blay, M.; Terra, X. Effects of flavonoids on intestinal inflammation, barrier integrity and changes in gut microbiota during diet-induced obesity. Nutr. Res. Rev. 2016, 29, 234-248, doi:10.1017/s0954422416000159.
- Polakis, P. Wnt Signaling in Cancer. Cold Spring Harbor Perspect. Biol. 2012, 4, a008052-a008052, doi:10.1101/cshperspect.a008052.
- Seidel, D.V.; Azcárate-Peril, M.A.; Chapkin, R.S.; Turner, N.D. Shaping functional gut microbiota using dietary bioactives to reduce colon cancer risk. Sem. Cancer Biol. 2017, 46, 191-204, doi:10.1016/j.semcancer.2017.06.009.
- Rycaj, K.; Tang, D.G. Cell-of-Origin of cancer versus cancer stem cells: assays and interpretations. Cancer Res. 2015, 75, 4003-4011, doi:10.1158/0008-5472.can-15-0798.
- Tetteh, P.W.; Farin, H.F.; Clevers, H. Plasticity within stem cell hierarchies in mammalian epithelia. Trends Cell Biol. 2015, 25, 100-108, doi:10.1016/j.tcb.2014.09.003.
- Beyaz, S.; Mana, M.D.; Roper, J.; Kedrin, D.; Saadatpour, A.; Hong, S.-J.; Bauer-Rowe, K.E.; Xifaras, M.E.; Akkad, A.; Arias, E., et al. High-fat diet enhances stemness and tumorigenicity of intestinal progenitors. Nature 2016, 531, 53-58, doi:10.1038/nature17173.
- Haegebarth, A.; Clevers, H. Wnt Signaling, Lgr5, and stem cells in the intestine and skin. Am. J. Pathol. 2009, 174, 715-721, doi:10.2353/ajpath.2009.080758.
- Barker, N.; Clevers, H. Leucine-Rich Repeat-Containing G-Protein-Coupled Receptors as Markers of Adult Stem Cells. Gastroenterology 2010, 138, 1681-1696, doi:10.1053/j.gastro.2010.03.002.
- Claessen, M.M.; Vleggaar, F.P.; Schipper, M.; Oldenburg, B.; Offerhaus, J.; Siersema, P.D. 915 Wnt-Pathway activation in early IBD-associated colorectal carcinogenesis: A biomarker for colonic surveillance. Gastroenterology 2008, 134, A-132, doi:10.1016/s0016-5085(08)60615-0.
- Schwabe, R.F.; Jobin, C. The microbiome and cancer. Nat. Rev. Cancer 2013, 13, 800-812, doi:10.1038/nrc3610.
- Sauceda, A.E.Q.; Pacheco-Ordaz, R.; Ayala-Zavala, J.F.; Mendoza, A.H.; González-Córdova, A.F.; Vallejo-Galland, B.; González-Aguilar, G.A. Impact of fruit dietary fibers and polyphenols on modulation of the human gut microbiota. In Fruit and Vegetable Phytochemicals, John Wiley & Sons, Ltd: 2017; 10.1002/9781119158042.ch19pp 405-422.