
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Priscilla Prestes | + 981 word(s) | 981 | 2020-05-26 04:31:41 | | | |
| 2 | Nicole Yin | + 11 word(s) | 992 | 2020-11-06 03:10:59 | | |
Video Upload Options
Cardiovascular disease (CVD) is the leading cause of morbidity and mortality in adults in developed countries. CVD encompass many diseased states including hypertension, coronary artery disease and atherosclerosis. Studies in animal models and human studies have elucidated the contribution of many genetic factors, including non-coding RNAs. Non-coding RNAs are RNAs not translated into protein, involved in gene expression regulation post-transcriptionally and implicated in CVD. These include long non-coding RNAs. Of these, circular RNAs (circRNAs) and micro RNAs. CircRNAs are created by the back-splicing of pre-messenger RNA and have been underexplored as contributors to CVD. These circRNAs may also act as biomarkers of human disease as they can be extracted from whole blood, plasma, saliva and seminal fluid. CircRNAs have recently been implicated in various disease processes, including hypertension and other cardiovascular disease. This review article will explore the promising and emerging roles of circRNAs as potential biomarkers and therapeutic targets in CVD, in particular hypertension.
1. Introduction
CircRNAs are abundant, underexplored ncRNAs. Recent studies revealed that large numbers of circRNAs are endogenous, highly conserved and stable in mammalian cells and prevalent in disease states (Figure 1)[1]. Although mRNA and circRNAs both originate from precursor-mRNAs, they are formed differently, giving them unique characteristics. mRNAs are formed by RNA splicing where introns are removed, and certain exons are included or excluded to create alternative coding mature mRNAs. This process creates linear mRNAs with exposed poly(A) tails. This characteristic leaves them prone to degradation by RNases[2]. Meanwhile, circRNAs are formed by back-splicing, promoting the circularization process where exons and/or introns converge onto each other, potentially protecting them from degradation and conferring a half-life of approximately 48 h, around five times more stable than linear mRNAs (Figure 2)[3].
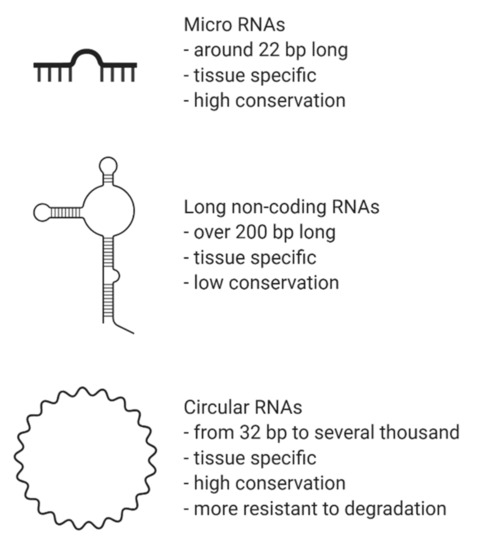
Figure 1. Brief characteristics of non-coding RNAs. Legend: bp, base pairs.
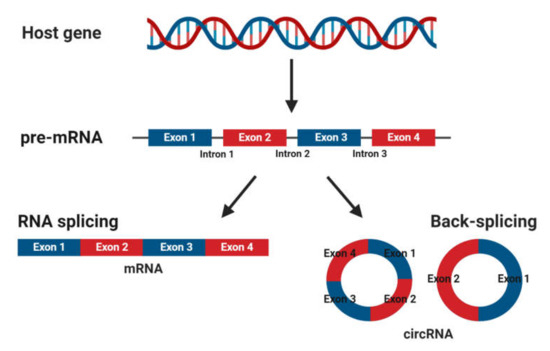
Figure 2. Biogenesis of mature messenger RNA (mRNA) and circular RNA (circRNA) from precursor mRNA (pre-mRNA). Pre-mRNA undergoes RNA splicing to form a mature mRNA or back-splicing to form circRNAs.
2. Founction
The definitive function of circRNAs still remains unclear. It has been proposed that circRNAs regulate the expression of linear mRNA transcripts both directly, via the competition with the splicing machinery[4]; and indirectly, acting as sponges to miRNAs due to the presence of multiple binding sites, allowing them to interact with and sequester cellular miRNAs preventing the performance of their roles on post-transcriptional regulation (Figure 3)[5][6].
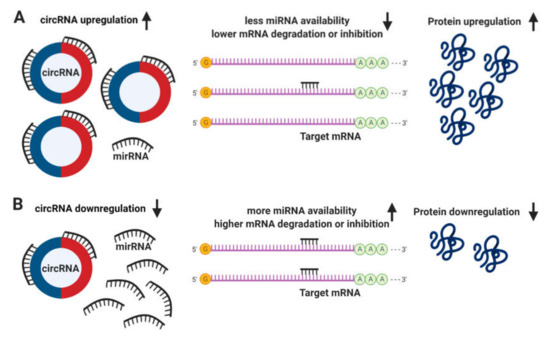
Figure 3. Circular RNA (circRNA) functions. (A) The upregulation of circRNAs leads to a decrease in microRNA (miRNA) availability, messenger RNA (mRNA) degradation or inhibition, resulting in an increase of protein translation. (B) Conversely, the downregulation of circRNAs leads to more miRNA available and higher levels of mRNA degradation or inhibition, leading to a downregulation in protein translation.
Importantly, circRNAs make up 1% of total RNA being expressed widely in various cell types and may have a regulatory function in human disease, with a pivotal role in the initiation and progression of various types of biological processes[5], potentially acting as a biomarker for the discovery and investigation on the progression of disease. However promising, research to identify and characterize circRNAs has mostly been performed using bioinformatics and in silico approaches and a limited number of studies have investigated their function in situ or in vivo to establish their involvement in disease.
The properties described above and promising research in the field of cancer genomics using circRNAs as biomarkers is encouraging and should be explored and translated into cardiovascular genomics research. This is extremely relevant, as conventional methods for controlling risk factors and initiating early treatment in CVD intervention have often led to poor prognosis. Current biomarkers usually detect the disease at later stages of development, increasing the need for the discovery of new biomarkers for prevention or at early onset of disease. This further highlights the benefits of using circRNAs as potential biomarkers in CVD (Table 1).
Emerging evidence of circular RNAs in cardiovascular disorders has demonstrated differential expression in both healthy and diseased hearts[7][8][9][10]. However, the relevance of circular RNAs to the cardiovascular system remains poorly characterised, and an improvement in understanding of circRNA involvement in CVD will form a basis for the development of these RNAs as biomarkers for discovery, prediction and therapeutic agents. Importantly, the combination of genetic sequencing and bioinformatics discovery has enabled the identification of many novel circRNAs.
Table 1. Summary of circRNAs implicated in cardiovascular diseases, including species and sample types studied and differential expression direction.
|
Disease |
Circular RNA |
Species |
Sample |
Expression |
References |
|
Hypertension |
hsa_circ_0005870 |
Human |
Blood |
Down |
[11] |
|
hsa_circ_0037909 |
Human |
Blood |
Up |
[12] |
|
|
hsa_circ_0037911 |
Human |
Blood |
Up |
[13] |
|
|
hsa_circ_0126991 |
Human |
Blood |
Up |
[14] |
|
|
hsa_circ_0014243 |
Human |
Blood |
Up |
[15] |
|
|
Myocardial infarction |
mmu_circ_0001878 |
Mouse |
Cardiomyocytes |
Up |
[16] |
|
circTtc3 |
Rats |
Cardiomyocytes |
Up |
[17] |
|
|
MICRA |
Human |
Blood |
Down |
[18] |
|
|
circNfix |
Mouse |
Cardiomyocytes |
Up |
[19] |
|
|
circFndc3b |
Mouse Human |
Cardiomyocytes |
Down |
[20] |
|
|
Atherosclerosis |
circRNA-0044073 |
Human |
Blood |
Up |
[21] |
|
ocu-ciR-novel-18038 |
Rabbit |
Blood |
Down |
[22] |
|
|
ocu-ciR-novel-18298 |
Rabbit |
Blood |
Up |
||
|
ocu-ciR-novel-15993 |
Rabbit |
Blood |
Up |
||
|
ocu-ciR-novel-17934 |
Rabbit |
Blood |
Down |
||
|
ocu-ciR-novel-17879 |
Rabbit |
Blood |
Up |
||
|
ocu-ciR-novel-18036 |
Rabbit |
Blood |
Up |
||
|
ocu-ciR-novel-14389 |
Rabbit |
Blood |
Up |
||
|
circANRIL |
Human |
Blood |
Up |
[23] |
|
|
circCHFR |
Human |
VSMC |
Up |
[24] |
|
|
circRNA-0003575 |
Mouse |
Endothelial cells |
Up |
[25] |
|
|
Coronary artery disease |
hsa_circ_0001879 |
Human |
Blood |
Up |
[26] |
|
hsa_circ_0004104 |
Human |
Blood |
Up |
||
|
hsa_circ_0124644 |
Human |
Blood |
Up |
[27] |
|
|
hsa_circ_0098964 |
Human |
Blood |
Up |
||
|
hsa_circ_0030769 |
Human |
Blood |
Up |
[28] |
|
|
hsa_circ_0079828 |
Human |
Blood |
Up |
||
|
hsa_circ_15486-161 |
Human |
Blood |
Up |
||
|
hsa_circ_0122274 |
Human |
Blood |
Up |
||
|
hsa_circ_16316-13 |
Human |
Blood |
Up |
||
|
hsa_circ_0140538 |
Human |
Blood |
Up |
||
|
Cardiomyopathy |
mmu_circ_0000254 |
Mouse |
Cardiomyocytes |
Down |
[29] |
|
hsa_circ_0076631 |
Human |
Cardiomyocytes and Serum |
Up |
[30] |
|
|
circRNA_000203 |
Mouse |
Ventricular Cardiomyocytes |
Up |
[31] |
|
|
circSlc8a1 |
Mouse |
Cardiomyocytes |
Up |
[32] |
Legend: ciR, circular; circ, circular; circANRIL, circular antisense non-coding RNA in the INK4 locus; circCHFR, circular checkpoint with fork-head associated and ring finger; circFndc3b, circular fibronectin type III domain containing 3B; circNfix, circular nuclear factor I X; circSlc8a1, circular solute carrier family 8 member a1; circTtc3, circular tetratricopeptide repeat domain 3; hsa, Homo sapiens; MICRA, myocardial infarction-associated circular RNA; mmu, Mus musculus; ocu, Oryctolagus cuniculus; VSMC, vascular smooth muscle cells.
References
- Evangelos Evangelou; The Million Veteran Program; Helen R. Warren; David Mosen-Ansorena; Borbala Mifsud; Raha Pazoki; He Gao; Georgios Ntritsos; Niki Dimou; Claudia P. Cabrera; et al.Ibrahim KaramanFu Liang NgMarina EvangelouKatarzyna WitkowskaEvan TzanisJacklyn N. HellwegeAyush GiriDigna R. Velez EdwardsYan V. SunKelly ChoJ. Michael GazianoPeter W. F. WilsonPhilip S. TsaoCsaba P. KovesdyTonu EskoReedik MägiLili MilaniPeter AlmgrenThibaud BoutinStéphanie DebetteJun DingFranco GiulianiniElizabeth G. HollidayAnne U. JacksonRuifang Li-GaoAndreea IlincaJian’An LuanMassimo ManginoChristopher OldmeadowBram Peter PrinsYong QianMuralidharan SargurupremrajNabi ShahPraveen SurendranSébastien ThériaultNiek VerweijSara M. WillemsJing-Hua ZhaoPhilippe AmouyelJohn ConnellRenée De MutsertAlex S. F. DoneyMartin FarrallCristina MenniAndrew D. MorrisRaymond NoordamGuillaume ParéNeil R. PoulterDenis C. ShieldsAlice V. StantonSimon ThomGonçalo AbecasisNajaf AminDan E. ArkingKristin L. AyersCaterina M. BarbieriChiara BatiniJoshua C. BisTineka BlakeMurielle BochudMichael BoehnkeEric BoerwinkleDorret I. BoomsmaErwin P. BottingerPeter S. BraundMarco BrumatArchie CampbellHarry CampbellAravinda ChakravartiJohn C. ChambersGanesh ChauhanMarina CiulloMassimiliano CoccaFrancis S. CollinsHeather J. CordellGail DaviesMartin H. De BorstEco J.C. De GeusIan J. DearyJoris DeelenFabiola Del Greco M.Cumhur Yusuf DemirkaleMarcus DörrGeorg B. EhretRoberto ElosuaStefan EnrothA. Mesut ErzurumluogluTeresa FerreiraMattias FrånbergOscar H. FrancoIlaria GandinPaolo GaspariniVilmantas GiedraitisChristian GiegerGiorgia GirottoAnuj GoelAlan J. GowVilmundur GudnasonXiuqing GuoUlf GyllenstenAnders HamstenTamara B. HarrisSarah E. HarrisCatharina A. HartmanAki S. HavulinnaAndrew A. HicksEdith HoferAlbert HofmanJouke-Jan HottengaJennifer HuffmanShih-Jen HwangErik IngelssonAlan JamesRick JansenMarjo-Riitta JarvelinRoby JoehanesÅsa JohanssonAndrew D. JohnsonPeter K. JoshiPekka JousilahtiJ. W. JukemaAntti JulaMika KähönenSekar KathiresanBernard D. KeavneyKay-Tee KhawPaul KnektJoanne KnightIvana KolcicJaspal S. KoonerSeppo KoskinenKati KristianssonZoltán KutalikMaris LaanMarty LarsonLenore J. LaunerBenjamin LehneTerho LehtimäkiDavid C. M. LiewaldLi LinLars LindCecilia M. LindgrenYongMei LiuRuth J.F. LoosLorna M. LopezYingchang LuLeo-Pekka LyytikäinenAnubha MahajanChrysovalanto MamasoulaJaume MarrugatJonathan MartenYuri MilaneschiAnna MorganAlanna C. MorrisonPeter J. MunsonMike A. NallsPriyanka NandakumarChristopher P. NelsonTeemu NiiranenIlja M. NolteTeresa NutileAlbertine J. OldehinkelBen A. OostraPaul F. O’ReillyElin OrgSandosh PadmanabhanWalter PalmasAarno PalotieAlison PattieBrenda W. J. H. PenninxMarkus PerolaAnnette PetersOzren PolasekPeter P. PramstallerQuang Tri NguyenOlli T. RaitakariMeixia RenRainer RettigKenneth RicePaul M. RidkerJanina S. RiedHarriëtte RieseSamuli RipattiAntonietta RobinoLynda M. RoseJerome I. RotterIgor RudanDaniela RuggieroYasaman SabaCinzia F. SalaVeikko SalomaaNilesh J. SamaniAntti-Pekka SarinReinhold SchmidtHelena SchmidtNick ShrineDavid SiscovickAlbert V. SmithHarold SniederSiim SõberRossella SoriceJohn M. StarrDavid J. StottDavid P. StrachanRona J. StrawbridgeJohan SundströmMorris A. SwertzKent D. TaylorAlexander TeumerMartin D. TobinMaciej TomaszewskiDaniela TonioloMichela TragliaStella TrompetJaakko TuomilehtoChristophe TzourioAndré G. UitterlindenAhmad VaezPeter J. Van Der MostCornelia M. Van DuijnAnne-Claire VergnaudGermaine C. VerwoertVeronique VitartUwe VölkerPeter VollenweiderDragana VuckovicHugh WatkinsSarah H. WildGonneke WillemsenJames G. WilsonAlan F. WrightJie YaoTatijana ZemunikWeihua ZhangJohn R AttiaAdam S. ButterworthDaniel I. ChasmanDavid ConenFrancesco CuccaJohn DaneshCaroline HaywardJoanna M M HowsonMarkku LaaksoEdward G. LakattaClaudia LangenbergOlle MelanderDennis O. Mook-KanamoriColin N A PalmerLorenz RischRobert A. ScottRodney J. ScottPeter SeverTim D. SpectorPim Van Der HarstNicholas J. WarehamEleftheria ZegginiDaniel LevyPatricia B MunroeChristopher Newton-ChehMorris J. BrownAndres MetspaluAdriana M. HungChristopher J. O’DonnellTodd L. EdwardsBruce M. PsatyIoanna TzoulakiMichael R. BarnesLouise V WainPaul ElliottMark Caulfield Genetic analysis of over 1 million people identifies 535 new loci associated with blood pressure traits. Nature Genetics 2018, 50, 1412-1425, 10.1038/s41588-018-0205-x.
- Douglas L. Black; Mechanisms of Alternative Pre-Messenger RNA Splicing. Annual Review of Biochemistry 2003, 72, 291-336, 10.1146/annurev.biochem.72.121801.161720.
- Shibin Qu; Xisheng Yang; Xiaolei Li; Jianlin Wang; Yuan Gao; Runze Shang; Wei Sun; Kefeng Dou; Haimin Li; Circular RNA: A new star of noncoding RNAs. Cancer Letters 2015, 365, 141-148, 10.1016/j.canlet.2015.06.003.
- Stefan Starke; Isabelle Jost; Oliver Rossbach; Tim Schneider; Silke Schreiner; Lee-Hsueh Hung; Albrecht Bindereif; Exon Circularization Requires Canonical Splice Signals. Cell Reports 2015, 10, 103-111, 10.1016/j.celrep.2014.12.002.
- Salzman, J. Circular RNA Expression: Its Potential Regulation and Function. Trends Genet. 2016, 32, 309–316.
- Chen, N.; Lu, X.; Yang, F.; Xing, N. Circular RNA circHIPK3 promotes cell proliferation and invasion of prostate cancer by sponging miR-193a-3p and regulating MCL1 expression. Cancer Manag. Res. 2019, 11, 1415–1423.
- Li, M.; Ding, W.; Sun, T.; Tariq, M.A.; Xu, T.; Li, P.; Wang, J. Biogenesis of circular RNA s and their roles in cardiovascular development and pathology. FEBS J. 2017, 285, 220–232.
- Fan, X.; Weng, X.; Zhao, Y.; Chen, W.; Gan, T.; Xu, D. Circular RNAs in Cardiovascular Disease: An Overview. BioMed. Res. Int. 2017, 2017, 1–9.
- Tan, W.L.; Lim, B.T.; Anene-Nzelu, C.G.; Ackers-Johnson, M.; Dashi, A.; See, K.; Tiang, Z.; Lee, D.P.; Chua, W.W.; Luu, T.D.; et al. A landscape of circular RNA expression in the human heart. Cardiovasc. Res. 2017, 113, 298–309.
- Stanislas Werfel; Stephan Nothjunge; Thomas Schwarzmayr; Tim-Matthias Strom; Thomas Meitinger; Stefan Engelhardt; Characterization of circular RNAs in human, mouse and rat hearts. Journal of Molecular and Cellular Cardiology 2016, 98, 103-107, 10.1016/j.yjmcc.2016.07.007.
- Wu, N.; Jin, L.; Cai, J. Profiling and bioinformatics analyses reveal differential circular RNA expression in hypertensive patients. Clin. Exp. Hypertens. 2017, 39, 454–459.
- Bao, X.; He, X.; Zheng, S.; Sun, J.; Luo, Y.; Tan, R.; Zhao, J.; Zhong, F.; Zhang, L.-N. Up-regulation of circular RNA hsa_circ_0037909 promotes essential hypertension. J. Clin. Lab. Anal. 2019, 33, e22853.
- Bao, X.; Zheng, S.; Mao, S.; Gu, T.; Liu, S.; Sun, J.; Zhang, L.-N. A potential risk factor of essential hypertension in case-control study: Circular RNA hsa_circ_0037911. Biochem. Biophys. Res. Commun. 2018, 498, 789–794.
- Liu, L.; Gu, T.; Bao, X.; Zheng, S.; Zhao, J.; Zhang, L. Microarray Profiling of Circular RNA Identifies hsa_circ_0126991 as a Potential Risk Factor for Essential Hypertension. Cytogenet. Genome Res. 2019, 157, 203–212.
- Zheng, S.; Gu, T.; Bao, X.; Sun, J.; Zhao, J.; Zhang, T.; Zhang, L.-N. Circular RNA hsa_circ_0014243 may serve as a diagnostic biomarker for essential hypertension. Exp. Ther. Med. 2018, 17, 1728–1736.
- Geng, H.-H.; Li, R.; Su, Y.-M.; Xiao, J.; Pan, M.; Cai, X.-X.; Ji, X.-P. The Circular RNA Cdr1as Promotes Myocardial Infarction by Mediating the Regulation of miR-7a on Its Target Genes Expression. PLoS ONE 2016, 11, e0151753.
- Cai, L.-D.; Qi, B.; Wu, X.; Peng, S.; Zhou, G.; Wei, Y.; Xu, J.; Chen, S.; Liu, S. Circular RNA Ttc3 regulates cardiac function after myocardial infarction by sponging miR-15b. J. Mol. Cell. Cardiol. 2019, 130, 10–22.
- Salgado-Somoza, A.; Zhang, L.; Vausort, M.; Devaux, Y. The circular RNA MICRA for risk stratification after myocardial infarction. IJC Hear. Vasc. 2017, 17, 33–36.
- Huang, S.; Li, X.; Zheng, H.; Si, X.; Li, B.; Wei, G.; Li, C.; Chen, Y.; Chen, Y.; Liao, W.; et al. Loss of Super-Enhancer-Regulated circRNA Nfix Induces Cardiac Regeneration After Myocardial Infarction in Adult Mice. Circulation 2019, 139, 2857–2876.
- Garikipati, V.N.S.; Verma, S.K.; Cheng, Z.; Liang, D.; Truongcao, M.M.; Cimini, M.; Yue, Y.; Huang, G.; Wang, C.; Benedict, C.; et al. Circular RNA CircFndc3b modulates cardiac repair after myocardial infarction via FUS/VEGF-A axis. Nat. Commun. 2019, 10, 4317.
- Shen, L.; Hu, Y.; Lou, J.; Yin, S.; Wang, W.; Wang, Y.; Xia, Y.; Wu, W. CircRNA-0044073 is upregulated in atherosclerosis and increases the proliferation and invasion of cells by targeting miR-107. Mol. Med. Rep. 2019, 19, 3923–3932.
- Zhang, F.; Zhang, R.; Zhang, X.; Wu, Y.; Li, X.; Zhang, S.; Hou, W.; Ding, Y.; Tian, J.; Sun, L.; et al. Comprehensive analysis of circRNA expression pattern and circRNA-miRNA-mRNA network in the pathogenesis of atherosclerosis in rabbits. Aging 2018, 10, 2266–2283.
- Holdt, L.M.; Stahringer, A.; Sass, K.; Pichler, G.; Kulak, N.A.; Wilfert, W.; Kohlmaier, A.; Herbst, A.; Northoff, B.H.; Nicolaou, A.; et al. Circular non-coding RNA ANRIL modulates ribosomal RNA maturation and atherosclerosis in humans. Nat. Commun. 2016, 7, 12429.
- Yang, L.; Yang, F.; Zhao, H.; Wang, M.; Zhang, Y. Circular RNA circCHFR Facilitates the Proliferation and Migration of Vascular Smooth Muscle via miR-370/FOXO1/Cyclin D1 Pathway. Mol. Ther. Nucleic Acids 2019, 16, 434–441.
- Shang, L.; Quan, A.; Sun, H.; Xu, Y.; Sun, G.; Cao, P. MicroRNA-148a-3p promotes survival and migration of endothelial cells isolated from Apoe deficient mice through restricting circular RNA 0003575. Gene 2019, 711, 143948.
- Wang, L.; Shen, C.; Wang, Y.; Zou, T.; Zhu, H.; Lu, X.; Li, L.; Yang, B.; Chen, J.; Chen, S.; et al. Identification of circular RNA Hsa_circ_0001879 and Hsa_circ_0004104 as novel biomarkers for coronary artery disease. Atherosclerosis 2019, 286, 88–96.
- Zhao, Z.; Li, X.; Gao, C.; Jian, D.; Hao, P.; Rao, L.; Li, M. Peripheral blood circular RNA hsa_circ_0124644 can be used as a diagnostic biomarker of coronary artery disease. Sci. Rep. 2017, 7, 39918.
- Lin, F.; Zhao, G.; Chen, Z.; Wang, X.; Lv, F.; Zhang, Y.; Yang, X.; Liang, W.; Cai, R.; Li, J.; et al. circRNA-miRNA association for coronary heart disease. Mol. Med. Rep. 2019, 19, 2527–2536.
- Wang, K.; Long, B.; Liu, F.; Wang, J.-X.; Liu, C.-Y.; Zhao, B.; Zhou, L.-Y.; Sun, T.; Wang, M.; Yu, T.; et al. A circular RNA protects the heart from pathological hypertrophy and heart failure by targeting miR-223. Eur. Hear. J. 2016, 37, 2602–2611.
- Yang, F.; Li, A.; Qin, Y.; Che, H.; Wang, Y.; Lv, J.; Li, Y.; Li, H.; Yue, E.; Ding, X.; et al. A Novel Circular RNA Mediates Pyroptosis of Diabetic Cardiomyopathy by Functioning as a Competing Endogenous RNA. Mol. Ther. Nucleic Acids 2019, 17, 636–643.
- Li, H.; Xu, J.-D.; Fang, X.-H.; Zhu, J.-N.; Yang, J.; Pan, R.; Yuan, S.-J.; Zeng, N.; Yang, Z.-Z.; Yang, H.; et al. Circular RNA circRNA_000203 aggravates cardiac hypertrophy via suppressing miR26b-5p and miR-140-3p binding to Gata4. Cardiovasc. Res. 2019.
- Lim, T.B.; Aliwarga, E.; Luu, T.D.A.; Li, Y.P.; Ng, S.L.; Annadoray, L.; Sian, S.; Ackers-Johnson, M.A.; Foo, R. Targeting the highly abundant circular RNA circSlc8a1 in cardiomyocytes attenuates pressure overload induced hypertrophy. Cardiovasc. Res. 2019, 115, 1998–2007.




