
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Paweł Kowalczyk | + 4722 word(s) | 4722 | 2021-06-01 05:14:00 | | | |
| 2 | Bruce Ren | -21 word(s) | 4701 | 2021-06-15 10:47:16 | | |
Video Upload Options
A pandemic of acute respiratory infections, due to a new type of coronavirus, can cause Severe Acute Respiratory Syndrome 2 (SARS-CoV-2) and has created the need for a better understanding of the clinical, epidemiological, and pathological features of COVID-19, especially in high-risk groups, such as pregnant women. Viral infections in pregnant women may have a much more severe course, and result in an increase in the rate of complications, including spontaneous abortion, stillbirth, and premature birth—which may cause long-term consequences in the offspring. In this review, we focus on the mother-fetal-placenta interface and its role in the potential transmission of SARS-CoV-2, including expression of viral receptors and proteases, placental pathology, and the presence of the virus in neonatal tissues and fluids.
1. Introduction SARS-CoV-2 Infection
Receptor Recognition Is the First Step of Viral Infection That Determines a Cell/Tissue Tropism
| Clinical Course of Infection | Asymptomatic/Mild COVID-19 (Appropriate Immune Response) |
Severe COVID-19 (Defective Immune Response) |
|---|---|---|
| Immune response |
|
|
| Immunopathology | None/Mild |
virus-specific T-cells central memory phenotype;
|
| Clinical outcomes | Infection resolution |
|
2. COVID-19 during Pregnancy—The Role of the Placenta
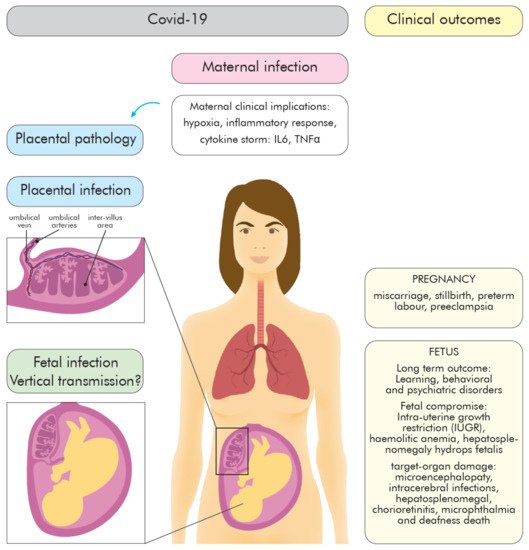
2.1. Maternal Immune Changes during Pregnancy
2.2. Inflammatory Response to SARS-CoV-2 during Pregnancy—Infection Outcomes
3. COVID 19—Placenta
3.1. The Maternal–Fetal Physical and Immunological Barrier
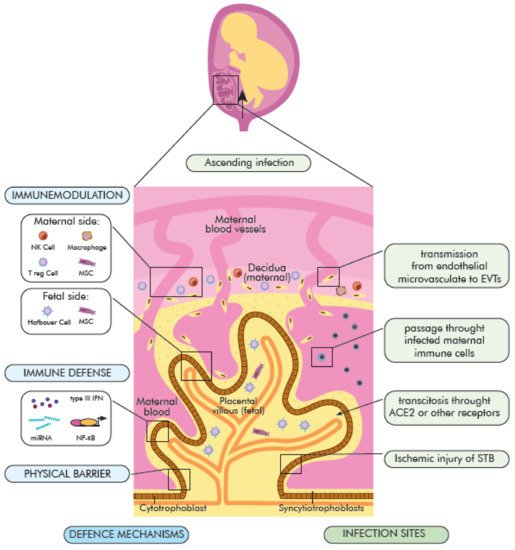
3.2. The Role of the Placenta in COVID-19
3.3. The Possible Mechanisms of SARS-CoV-2 Vertical Transmission
- (1)
-
direct infection of STB syncytiotrophoblasts and their rupture, virion transcytosis via immune receptors ACE2 and Fc (FcR),
- (2)
-
passage through endothelial microcirculation into the intravascular extravascular trophoblasts (EVT) or other placental cells, as well as
- (3)
-
passing through infected maternal immune cells and
- (4)
-
ascending vaginal infection (placental barrier) (Figure 2).
3.4. Placenta Pathology
3.5. The Vertical Transmission Rate
References
- Wang, Y.; Grunewald, M.; Perlman, S. Coronaviruses: An updated overview of their replication and pathogenesis. Methods Mol. Biol. 2020, 2203, 1–29.
- Lu, R.; Zhao, X.; Li, J.; Niu, P.; Yang, B.; Wu, H.; Wang, W.; Song, H.; Huang, B.; Zhu, N.; et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: Implications for virus origins and receptor binding. Lancet 2020, 395, 565–574.
- Tang, J.W.; Tambyah, P.A.; Hui, D.S. Emergence of a novel coronavirus causing respiratory illness from Wuhan, China. J. Infect. 2020, 80, 350–371.
- Fisher, D.; Heymann, D. Q&A: The novel coronavirus outbreak causing COVID-19. BMC Med. 2020, 18, 1–3.
- Guan, W.J.; Ni, Z.Y.; Hu, Y.; Liang, W.H.; Ou, C.Q.; He, J.X.; Liu, L.; Shan, H.; Lei, C.L.; Hui, D.S.C.; et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N. Engl. J. Med. 2020, 382, 1708–1720.
- Zhou, P.; Yang, X.-L.; Wang, X.-G.; Hu, B.; Zhang, L.; Zhang, W.; Si, H.-R.; Zhu, Y.; Li, B.; Huang, C.-L.; et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020, 579, 270–273.
- Hoffmann, M.; Keine-Weber, H.; Schroeder, S.; Kruger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.H.; Nitsche, A.; et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 2020, 181, 271–280.
- Ou, X.; Liu, Y.; Lei, X.; Li, P.; Mi, D.; Ren, L.; Guo, L.; Guo, R.; Chen, T.; Hu, J.; et al. Characterization of spike glycoprotein of SARS-CoV-2 on virus entry and its immune cross-reactivity with SARS-CoV. Nat. Commun. 2020, 11, 1620.
- Shang, J.; Wan, Y.; Luo, C.; Ye, G.; Geng, Q.; Auerbach, A.; Li, F. Cell entry mechanisms of SARS-CoV-2. Proc. Natl. Acad. Sci. USA 2020, 117, 11727–11734.
- Sungnak, W.; Huang, N.; Becavin, C.; Berg, M.; Queen, R.; Litvinukova, M.; Talavera-Lopez, C.; Maatz, H.; Reichart, D.; Sampaziotis, F.; et al. SARS-CoV-2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes. Nat. Med. 2020, 26, 681–687.
- Lukassen, S.; Chua, R.L.; Trefzer, T.; Kahn, N.C.; Schneider, M.A.; Muley, T.; Winter, H.; Meister, M.; Veith, C.; Boots, A.W.; et al. SARS -CoV-2 receptor ACE 2 and TMPRSS 2 are primarily expressed in bronchial transient secretory cells. EMBO J. 2020, 39, e105114.
- Mathew, D.; Giles, J.R.; Baxter, A.E.; Oldridge, D.A.; Greenplate, A.R.; Wu, J.E.; Alanio, C.; Kuri-Cervantes, L.; Pampena, M.B.; D’Andrea, K.; et al. Deep immune profiling of COVID-19 patients reveals distinct immunotypes with therapeutic implications. Science 2020, 369, eabc8511.
- Minakshi, R.; Padhan, K.; Rani, M.; Khan, N.; Ahmad, F.; Jameel, S. The SARS coronavirus 3a protein causes endoplasmic reticulum stress and induces ligand-independent downregulation of the type 1 interferon receptor. PLoS ONE 2009, 4, e8342.
- Blanco-Melo, D.; Nilsson-Payant, B.E.; Liu, W.C.; Uhl, S.; Hoagland, D.; Moller, R.; Jordan, T.X.; Oishi, K.; Panis, M.; Sachs, D.; et al. Imbalanced host response to SARS-CoV-2 drives development of COVID-19. Cell 2020, 181, 1036–1045.
- Tay, M.Z.; Poh, C.M.; Rénia, L.; Macary, P.A.; Ng, L.F.P. The trinity of COVID-19: Immunity, inflammation and intervention. Nat. Rev. Immunol. 2020, 20, 363–374.
- Vareille, M.; Kieninger, E.; Edwards, M.R.; Regamey, N. The airway epithelium: Soldier in the fight against respiratory viruses. Clin. Microbiol. Rev. 2011, 24, 210–229.
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506.
- Ruan, Q.; Yang, K.; Wang, W.; Jiang, L.; Song, J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020, 46, 846–848.
- Thevarajan, I.; Nguyen, T.H.O.; Koutsakos, M.; Druce, J.; Caly, L.; Van De Sandt, C.E.; Jia, X.; Nicholson, S.; Catton, M.; Cowie, B.; et al. Breadth of concomitant immune responses prior to patient recovery: A case report of non-severe COVID-19. Nat. Med. 2020, 26, 453–455.
- Qin, C.; Zhou, L.; Hu, Z.; Zhang, S.; Yang, S.; Tao, Y.; Xie, C.; Ma, K.; Shang, K.; Wang, W.; et al. Dysregulation of immune response in patients with Coronavirus 2019 (COVID-19) in Wuhan, China. Clin. Infect. Dis. 2020, 71, 762–768.
- Wilczyński, J.R. Th1/Th2 cytokines balance–Yin and yang of reproductive immunology. Eur. J. Obstet. Gynecol. Reprod. Biol. 2005, 122, 136–143.
- Aghaeepour, N.; Ganio, E.A.; McIlwain, D.; Tsai, A.S.; Tingle, M.; Van Gassen, S.; Gaudilliere, D.K.; Baca, Q.; McNeil, L.; Okada, R.; et al. An immune clock of human pregnancy. Sci. Immunol. 2017, 2, eaan2946.
- Chen, M.; Zeng, J.; Liu, X.; Sun, G.; Gao, Y.; Liao, J.; Yu, J.; Luo, X.; Qi, H. Changes in physiology and immune system during pregnancy and coronavirus infection: A review. Eur. J. Obstet. Gynecol. Reprod. Biol. 2020, 255, 124–128.
- Rasmussen, S.A.; Smulian, J.C.; Lednicky, J.A.; Wen, T.S.; Jamieson, D.J. Coronavirus disease 2019 (COVID-19) and pregnancy: What obstetricians need to know. Am. J. Obstet. Gynecol. 2020, 222, 415–426.
- De Souza Silva, G.A.; da Silva, S.P.; da Costa, M.A.S.; da Silva, A.R.; de Vasconcelos Alves, R.R.; Tenorio, F.d.C.A.M.; da Silva Melo, A.R.; de Freitas, A.C.; de Melo, C.M.L. SARS-CoV, MERS-CoV and SARS-CoV-2 infections in pregnancy and fetal development. J. Gynecol. Obstet. Hum. Reprod. 2020, 49, 101846.
- Rasmussen, S.A.; Jamieson, D.J.; Uyeki, T.M. Effects of influenza on pregnant women and infants. Am. J. Obstet. Gynecol. 2012, 207, S3–S8.
- Zaigham, M.; Andersson, O. Maternal and perinatal outcomes with COVID-19: A systematic review of 108 pregnancies. Acta Obstet. Gynecol. Scand. 2020, 99, 823–829.
- Patanè, L.; Morotti, D.; Giunta, M.R.; Sigismondi, C.; Piccoli, M.G.; Frigerio, L.; Mangili, G.; Arosio, M.; Cornolti, G. Vertical transmission of coronavirus disease 2019: Severe acute respiratory syndrome coronavirus 2 RNA on the fetal side of the placenta in pregnancies with coronavirus disease 2019–positive mothers and neonates at birth. Am. J. Obstet. Gynecol. MFM 2020, 2, 100145.
- Schwartz, D.A.; Morotti, D. Placental pathology of COVID-19 with and without fetal and neonatal infection: Trophoblast necrosis and chronic histiocytic intervillositis as risk factors for transplacental transmission of SARS-CoV-19. Viruses 2020, 12, 1308.
- Facchetti, F.; Bugatti, M.; Drera, E.; Tripodo, C.; Sartori, E.; Cancila, V.; Papaccio, M.; Castellani, R.; Casola, S.; Boniotti, M.B.; et al. SARS-CoV2 vertical transmission with adverse effects on the newborn revealed through integrated im-munohistochemical, electron microscopy and molecular analyses of Placenta. EBioMedicine 2020, 59, 102951.
- Hosier, H.; Farhadian, S.F.; Morotti, R.A.; Deshmukh, U.; Lu-Culligan, A.; Campbell, K.H.; Yasumoto, Y.; Vogels, C.B.; Casanovas-Massana, A.; Vijayakumar, P.; et al. SARS-CoV-2 infection of the placenta. J. Clin. Investig. 2020, 130, 4947–4953.
- Gilbert, J.S.; Ryan, M.J.; LaMarca, B.B.; Sedeek, M.; Murphy, S.R.; Granger, J.P. Pathophysiology of hypertension during preeclampsia: Linking placental ischemia with endothelial dysfunction. Am. J. Physiol. Heart Circ. Physiol. 2008, 294, H541–H550.
- Koga, K.; Cardenas, I.; Aldo, P.; Abrahams, V.M.; Peng, B.; Fill, S.; Romero, R.; Mor, G. ORIGINAL ARTICLE: Activation of TLR3 in the trophoblast is associated with preterm delivery. Am. J. Reprod. Immunol. 2009, 61, 196–212.
- Wong, S.F.; Chow, K.M.; Leung, T.N.; Ng, W.F.; Ng, T.K.; Shek, C.C.; Ng, P.C.; Lam, P.W.; Ho, L.C.; To, W.W.; et al. Pregnancy and perinatal outcomes of women with severe acute respiratory syndrome. Am. J. Obstet. Gynecol. 2004, 191, 292–297.
- Wong, Y.P.; Khong, T.Y.; Tan, G.C. The effects of COVID-19 on placenta and pregnancy: What do we know so far? Diagnostics 2021, 11, 94.
- Villar, J.; Ariff, S.; Gunier, R.B.; Thiruvengadam, R.; Rauch, S.; Kholin, A.; Roggero, P.; Prefumo, F.; do Vale, M.S.; Cardona-Perez, J.A.; et al. Maternal and neonatal morbidity and mortality among pregnant women with and without COVID-19 infection: The INTERCOVID Multinational Cohort Study. JAMA Pediatr. 2021.
- Littauer, E.Q.; Esser, E.S.; Antao, O.Q.; Vassilieva, E.V.; Compans, R.W.; Skountzou, I. H1N1 influenza virus infection results in adverse pregnancy outcomes by disrupting tissue-specific hormonal regulation. PLOS Pathog. 2017, 13, e1006757.
- Naidu, S.A.G.; Clemens, R.A.; Pressman, P.; Zaigham, M.; Kadkhoda, K.; Davies, K.J.A.; Naidu, A.S. COVID-19 during Pregnancy and Postpartum: I) Pathobiology of Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2) at Maternal-Fetal Interface. J. Diet Suppl. 2020, 1–28.
- Lurie, S.; Rahamim, E.; Piper, I.; Golan, A.; Sadan, O. Total and differential leukocyte counts percentiles in normal pregnancy. Eur. J. Obstet. Gynecol. Reprod. Biol. 2008, 136, 16–19.
- Mandala, W.L.; Gondwe, E.N.; Molyneux, M.E.; MacLennan, J.M.; MacLennan, C.A. Leukocyte counts and lymphocyte subsets in relation to pregnancy and HIV infection in Malawian women. Am. J. Reprod. Immunol. 2017, 78.
- Kraus, T.A.; Engel, S.M.; Sperling, R.S.; Kellerman, L.; Lo, Y.; Wallenstein, S.; Escribese, M.M.; Garrido, J.L.; Singh, T.; Loubeau, M.; et al. Characterizing the pregnancy immune phenotype: Results of the viral immunity and pregnancy (VIP) study. J. Clin. Immunol. 2012, 32, 300–311.
- Yang, F.; Feng, C.; Zhang, X.; Lu, J.; Zhao, Y. The diverse biological functions of neutrophils, beyond the defense against infections. Inflammation 2016, 40, 311–323.
- Piccinni, M.-P.; Romagnani, S. Regulation of fetal allograft survival by hormone-controlled Th1- and Th2-type cytokines. Immunol. Res. 1996, 15, 141–150.
- Chen, H.; Guo, J.; Wang, C.; Luo, F.; Yu, X.; Zhang, W.; Li, J.; Zhao, D.; Xu, D.; Gong, Q.; et al. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: A retrospective review of medical records. Lancet 2020, 395, 809–815.
- Ye, Q.; Wang, B.; Mao, J. The pathogenesis and treatment of the ‘Cytokine Storm’ in COVID-19. J. Infect. 2020, 80, 607–613.
- Elshafeey, F.; Magdi, R.; Hindi, N.; Elshebiny, M.; Farrag, N.; Mahdy, S.; Sabbour, M.; Gebril, S.; Nasser, M.; Kamel, M.; et al. A systematic scoping review of COVID-19 during pregnancy and childbirth. Int. J. Gynecol. Obstet. 2020, 150, 47–52.
- Li, N.; Han, L.; Peng, M.; Lv, Y.; Ouyang, Y.; Liu, K.; Yue, L.; Li, Q.; Sun, G.; Chen, L.; et al. Maternal and neonatal outcomes of pregnant women with Coronavirus disease 2019 (COVID-19) pneumonia: A case-control study. Clin. Infect. Dis. 2020, 71, 2035–2041.
- Amirchaghmaghi, E.; Taghavi, S.A.; Shapouri, F.; Saeidi, S.; Rezaei, A.; Aflatoonian, R. The role of toll like receptors in pregnancy. Int. J. Fertil. Steril. 2013, 7, 147–154.
- Alberca, R.W.; Pereira, N.Z.; Oliveira, L.; Gozzi-Silva, S.C.; Sato, M.N. Pregnancy, viral infection, and COVID-19. Front. Immunol. 2020, 11, 1672.
- Shende, P.; Gaikwad, P.; Gandhewar, M.; Ukey, P.; Bhide, A.; Patel, V.; Bhagat, S.; Bhor, V.; Mahale, S.; Gajbhiye, R.; et al. Persistence of SARS-CoV-2 in the first trimester placenta leading to transplacental transmission and fetal demise from an asymptomatic mother. Hum. Reprod. 2021, 36, 899–906.
- La Cour Freiesleben, N.; Egerup, P.; Hviid, K.V.R.; Severinsen, E.R.; Kolte, A.M.; Westergaard, D.; Olsen, L.F.; Praetorius, L.; Zedeler, A.; Christiansen, A.M.H.; et al. SARS-CoV-2 in first trimester pregnancy: A cohort study. Hum. Reprod. 2021, 36, 40–47.
- De Sousa, A.F.L.; de Carvalho, H.E.F.; de Oliveira, L.B.; Schneider, G.; Camargo, E.L.S.; Watanabe, E.; de Andrade, D.; Fernandes, A.F.C.; Mendes, I.A.C.; Fronteira, I. Effects of COVID-19 Infection during Pregnancy and Neonatal Prognosis: What Is the Evidence? Int. J. Environ. Res. Public Health 2020, 17, 4176.
- Brandt, J.S.; Hill, J.; Reddy, A.; Schuster, M.; Patrick, H.S.; Rosen, T.; Sauer, M.V.; Boyle, C.; Ananth, C.V. Epidemiology of coronavirus disease 2019 in pregnancy: Risk factors and associations with adverse maternal and neonatal outcomes. Am. J. Obstet. Gynecol. 2021, 224, 389.e1–389.e9.
- Turan, O.; Hakim, A.; Dashraath, P.; Jeslyn, W.J.L.; Wright, A.; Abdul-Kadir, R. Clinical characteristics, prognostic factors, and maternal and neonatal outcomes of SARS-CoV-2 infection among hospitalized pregnant women: A systematic review. Int. J. Gynaecol. Obstet. 2020, 151, 7–16.
- Juan, J.; Gil, M.M.; Rong, Z.; Zhang, Y.; Yang, H.; Poon, L.C. Effect of coronavirus disease 2019 (COVID-19) on maternal, perinatal and neonatal outcome: Systematic review. Ultrasound Obstet. Gynecol. 2020, 56, 15–27.
- Makvandi, S.; Mahdavian, M.; Kazemi-Nia, G.; Vahedian-Azimi, A.; Guest, P.C.; Karimi, L.; Sahebkar, A. The 2019 novel Coronavirus disease in pregnancy: A systematic review. Adv. Exp. Med. Biol. 2021, 1321, 299–307.
- Della Gatta, A.N.; Rizzo, R.; Pilu, G.; Simonazzi, G. Coronavirus disease 2019 during pregnancy: A systematic review of reported cases. Am. J. Obstet. Gynecol. 2020, 223, 36–41.
- Smith, V.; Seo, D.; Warty, R.; Payne, O.; Salih, M.; Chin, K.L.; Ofori-Asenso, R.; Krishnan, S.; Costa, F.D.S.; Vollenhoven, B.; et al. Maternal and neonatal outcomes associated with COVID-19 infection: A systematic review. PLoS ONE 2020, 15, e0234187.
- Matar, R.; Alrahmani, L.; Monzer, N.; Debiane, L.G.; Berbari, E.; Fares, J.; Fitzpatrick, F.; Murad, M.H. Clinical presentation and outcomes of pregnant women with coronavirus disease 2019: A systematic review and meta-analysis. Clin. Infect. Dis. 2021, 72, 521–533.
- Huntley, B.; Huntley, E.; Di Mascio, D.; Chen, T.; Berghella, V.; Chauhan, S. Rates of maternal and perinatal mortality and vertical transmission in pregnancies complicated by severe acute respiratory syndrome Coronavirus 2 (SARS-Co-V-2) infection: A systematic review. Obstet. Anesth. Dig. 2021, 41, 58.
- Kayem, G.; Lecarpentier, E.; Deruelle, P.; Bretelle, F.; Azria, E.; Blanc, J.; Bohec, C.; Bornes, M.; Ceccaldi, P.-F.; Chalet, Y.; et al. A snapshot of the Covid-19 pandemic among pregnant women in France. J. Gynecol. Obstet. Hum. Reprod. 2020, 49, 101826.
- Knight, M.; Bunch, K.; Vousden, N.; Morris, E.; Simpson, N.; Gale, C.; O’Brien, P.; Quigley, M.; Brocklehurst, P.; Kurinczuk, J.J.; et al. Characteristics and outcomes of pregnant women admitted to hospital with confirmed SARS-CoV-2 infection in UK: National population based cohort study. BMJ 2020, 369, m2107.
- Takemoto, M.L.S.; Menezes, M.D.O.; Andreucci, C.B.; Nakamura-Pereira, M.; Amorim, M.M.; Katz, L.; Knobel, R. The tragedy of COVID-19 in Brazil: 124 maternal deaths and counting. Int. J. Gynecol. Obstet. 2020, 151, 154–156.
- Wu, A.; Peng, Y.; Huang, B.; Ding, X.; Wang, X.; Niu, P.; Meng, J.; Zhu, Z.; Zhang, Z.; Wang, J.; et al. Genome Composition and Divergence of the Novel Coronavirus (2019-nCoV) Originating in China. Cell Host Microbe 2020, 27, 325–328.
- Werenberg Dreier, J.; Andersen, A.M.N.; Hvolby, A.; Garne, E.; Andersen, P.K.; Berg-Beckhoff, G. Fever and infections in pregnancy and risk of attention deficit/hyperactivity disorder in the off-spring. J. Child Psychol. Psychiatry 2015, 57, 540–548.
- Panisi, C.; Guerini, F.R.; Abruzzo, P.M.; Balzola, F.; Biava, P.M.; Bolotta, A.; Brunero, M.; Burgio, E.; Chiara, A.; Clerici, M.; et al. Autism spectrum disorder from the womb to adulthood: Suggestions for a paradigm shift. J. Pers. Med. 2021, 11, 70.
- Azizieh, F.Y.; Raghupathy, R.G. Tumor necrosis factor-a and pregnancy complications: A prospective study. Med. Princ. Pr. 2014, 24, 165–170.
- Knöfler, M.; Haider, S.; Saleh, L.; Pollheimer, J.; Gamage, T.K.J.B.; James, J. Human placenta and trophoblast development: Key molecular mechanisms and model systems. Cell. Mol. Life Sci. 2019, 76, 3479–3496.
- Herrick, E.J.; Bordoni, B. Embryology, Placenta; StatPearls: Treasure Island, FL, USA, 2021.
- Thaler, C.J.; Labarrere, C.A.; Hunt, J.S.; McIntyre, J.A.; Faulk, W. Immunological studies of lactoferrin in human placentae. J. Reprod. Immunol. 1993, 23, 21–39.
- Thorley, J.A.; McKeating, J.A.; Rappoport, J.Z. Mechanisms of viral entry: Sneaking in the front door. Protoplasma 2010, 244, 15–24.
- Burton, G.J.; Fowden, A.L. The placenta: A multifaceted, transient organ. Philos. Trans. R. Soc. B: Biol. Sci. 2015, 370, 20140066.
- Robbins, J.R.; Skrzypczynska, K.M.; Zeldovich, V.B.; Kapidzic, M.; Bakardjiev, A.I. Placental syncytiotrophoblast constitutes a major barrier to vertical transmission of listeria monocytogenes. PLoS Pathog. 2010, 6, e1000732.
- Ockleford, C.; Wakely, J.; Badley, R.A. Morphogenesis of human placental chorionic villi: Cytoskeletal, syncytioskeletal and extracellular matrix proteins. Proc. R. Soc. London. Ser. B Boil. Sci. 1981, 212, 305–316.
- Robbins, J.R.; Bakardjiev, A.I. Pathogens and the placental fortress. Curr. Opin. Microbiol. 2012, 15, 36–43.
- Mohanty, S.; Anderson, C.; Robinson, J. The expression of caveolin-1 and the distribution of caveolae in the murine placenta and yolk sac: Parallels to the human placenta. Placenta 2010, 31, 144–150.
- Aplin, J.D.; Jones, C.J.; Harris, L.K. Adhesion molecules in human trophoblast–A review. I. Villous trophoblast. Placenta 2009, 30, 293–298.
- Trundley, A.; Moffett, A. Human uterine leukocytes and pregnancy. Tissue Antigens 2003, 63, 1–12.
- Moffett-King, A. Natural killer cells and pregnancy. Nat. Rev. Immunol. 2002, 2, 656–663.
- Nancy, P.; Erlebacher, A. T cell behavior at the maternal-fetal interface. Int. J. Dev. Biol. 2014, 58, 189–198.
- Gardner, L. Dendritic cells in the human decidua. Biol. Reprod. 2003, 69, 1438–1446.
- Pique-Regi, R.; Romero, R.; Tarca, A.L.; Sendler, E.D.; Xu, Y.; Garcia-Flores, V.; Leng, Y.; Luca, F.; Hassan, S.S.; Gomez-Lopez, N. Single cell transcriptional signatures of the human placenta in term and preterm parturition. eLife 2019, 8, e52004.
- Mor, G.; Cardenas, I. The immune system in pregnancy: A unique complexity. Am. J. Reprod. Immunol. 2010, 63, 425–433.
- Ander, S.E.; Diamond, M.S.; Coyne, C.B. Immune responses at the maternal-fetal interface. Sci. Immunol. 2019, 4, eaat6114.
- Manaster, I.; Mandelboim, O. The unique properties of uterine NK cells. Am. J. Reprod. Immunol. 2010, 63, 434–444.
- Ventura Ferreira, M.S.; Bienert, M.; Muller, K.; Rath, B.; Goecke, T.; Oplander, C.; Braunschweig, T.; Mela, P.; Brummendorf, T.H.; Beier, F.; et al. Comprehensive characterization of chorionic villi-derived mesenchymal stromal cells from human placenta. Stem Cell Res. Ther. 2018, 9, 28.
- León-Juárez, M.; Martínez–Castillo, M.; González-García, L.D.; Helguera-Repetto, A.C.; Zaga-Clavellina, V.; García-Cordero, J.; Pliego, A.F.; Herrera-Salazar, A.; Vázquez-Martínez, E.R.; Reyes-Muñoz, E. Cellular and molecular mechanisms of viral infection in the human placenta. Pathog. Dis. 2017, 75.
- Naidu, S.A.G.; Clemens, R.A.; Pressman, P.; Zaigham, M.; Davies, K.J.A.; Naidu, A.S. COVID-19 during Pregnancy and Postpartum: II) Antiviral Spectrum of Maternal Lactoferrin in Fetal and Neonatal Defense. J. Diet. Suppl. 2020, 1–37.
- Koga, K.; Izumi, G.; Mor, G.; Fujii, T.; Osuga, Y. Toll-like receptors at the maternal-fetal interface in normal pregnancy and pregnancy complications. Am. J. Reprod. Immunol. 2014, 72, 192–205.
- Levy, O. Innate immunity of the newborn: Basic mechanisms and clinical correlates. Nat. Rev. Immunol. 2007, 7, 379–390.
- Banchereau, J.; Steinman, R.M. Dendritic cells and the control of immunity. Nature 1998, 392, 245–252.
- Koga, K.; Aldo, P.B.; Mor, G. Toll-like receptors and pregnancy: Trophoblast as modulators of the immune response. J. Obstet. Gynaecol. Res. 2009, 35, 191–202.
- Abrahams, V.M.; Visintin, I.; Aldo, P.B.; Guller, S.; Romero, R.; Mor, G. A Role for TLRs in the regulation of immune cell migration by first trimester trophoblast cells. J. Immunol. 2005, 175, 8096–8104.
- Alexopoulou, L.; Holt, A.C.; Medzhitov, R.; Flavell, R.A. Recognition of double-stranded RNA and activation of NF-κB by Toll-like receptor 3. Nature 2001, 413, 732–738.
- Bayer, A.; Lennemann, N.J.; Ouyang, Y.; Bramley, J.C.; Morosky, S.; Marques, E.T.D.A.; Cherry, S.; Sadovsky, Y.; Coyne, C.B. Type III interferons produced by human placental trophoblasts confer protection against Zika virus infection. Cell Host Microbe 2016, 19, 705–712.
- Tian, Y.; Kuo, C.F.; Akbari, O.; Ou, J.H.J. Maternal-derived hepatitis B virus e antigen alters macrophage function in offspring to drive viral persis-tence after vertical transmission. Immunity 2016, 44, 1204–1214.
- Yockey, L.J.; Iwasaki, A. Interferons and proinflammatory cytokines in pregnancy and fetal development. Immunity 2018, 49, 397–412.
- Roopenian, D.C.; Akilesh, S. FcRn: The neonatal Fc receptor comes of age. Nat. Rev. Immunol. 2007, 7, 715–725.
- Kreis, N.N.; Ritter, A.; Louwen, F.; Yuan, J. A message from the human placenta: Structural and immunomodulatory defense against SARS-CoV-19. Cells 2020, 9, 1777.
- Abdel-Ghany, S.E.; Pilon, M. MicroRNA-mediated systemic down-regulation of copper protein expression in response to low copper availability in Arabidopsis. J. Biol. Chem. 2008, 283, 15932–15945.
- DeDiego, M.L.; Nieto-Torres, J.L.; Regla-Nava, J.A.; Jimenez-Guardeno, J.M.; Fernandez-Delgado, R.; Fett, C.; Castano-Rodriguez, C.; Perlman, S.; Enjuanes, L. Inhibition of NF-κB-mediated inflammation in severe acute respiratory syndrome corona-virus-infected mice increases survival. J. Virol. 2014, 88, 913–924.
- Dosch, S.F.; Mahajan, S.D.; Collins, A.R. SARS Coronavirus spike protein-induced innate immune response occurs via activation of the NF-κB pathway in human monocyte macrophages in vitro. Virus Res. 2009, 142, 19–27.
- Mor, G.; Cardenas, I.; Abrahams, V.; Guller, S. Inflammation and pregnancy: The role of the immune system at the implantation site. Ann. N. Y. Acad. Sci. 2011, 1221, 80–87.
- Schwartz, D.A. An analysis of 38 pregnant women with COVID-19, their newborn infants, and maternal-fetal transmission of SARS-CoV-2: Maternal Coronavirus infections and pregnancy outcomes. Arch. Pathol. Lab. Med. 2020, 144, 799–805.
- Liu, H.; Wang, L.-L.; Zhao, S.-J.; Kwak-Kim, J.; Mor, G.; Liao, A.-H. Why are pregnant women susceptible to COVID-19? An immunological viewpoint. J. Reprod. Immunol. 2020, 139, 103122.
- Wastnedge, E.A.N.; Reynolds, R.M.; Van Boeckel, S.R.; Stock, S.J.; Denison, F.C.; Maybin, J.A.; Critchley, H.O.D. Pregnancy and COVID-19. Physiol. Rev. 2021, 101, 303–318.
- Karimi-Zarchi, M.; Neamatzadeh, H.; Dastgheib, S.A.; Abbasi, H.; Mirjalili, S.R.; Behforouz, A.; Ferdosian, F.; Bahrami, R. Vertical transmission of Coronavirus Disease 19 (COVID-19) from infected pregnant mothers to neonates: A review. Fetal Pediatr. Pathol. 2020, 39, 246–250.
- Wenling, Y.; Junchao, Q.; Xiao, Z.; Ouyang, S. Pregnancy and COVID-19: Management and challenges. Rev. Inst. Med. Trop. São Paulo 2020, 62, e62.
- Delorme-Axford, E.; Sadovsky, Y.; Coyne, C.B. The placenta as a barrier to viral infections. Annu. Rev. Virol. 2014, 1, 133–146.
- Jing, Y.; Run-Qian, L.; Hao-Ran, W.; Hao-Ran, C.; Ya-Bin, L.; Yang, G.; Fei, C. Potential influence of COVID-19/ACE2 on the female reproductive system. Mol. Hum. Reprod. 2020, 26, 367–373.
- Valdés, G.; Neves, L.; Anton, L.; Corthorn, J.; Chacón, C.; Germain, A.; Merrill, D.; Ferrario, C.; Sarao, R.; Penninger, J.; et al. Distribution of Angiotensin-(1–7) and ACE2 in human placentas of normal and pathological pregnancies. Placenta 2006, 27, 200–207.
- Li, M.; Chen, L.; Zhang, J.; Xiong, C.; Li, X. The SARS-CoV-2 receptor ACE2 expression of maternal-fetal interface and fetal organs by single-cell transcriptome study. PLoS ONE 2020, 15.
- Pique-Regi, R.; Romero, R.; Tarca, A.L.; Luca, F.; Xu, Y.; Alazizi, A.; Leng, Y.; Hsu, C.D.; Gomez-Lopez, N. Does the human placenta express the canonical cell entry mediators for SARS-CoV-2? Elife 2020, 9, e58716.
- Gordon, D.E.; Jang, G.M.; Bouhaddou, M.; Xu, J.; Obernier, K.; White, K.M.; O’Meara, M.J.; Rezelj, V.V.; Guo, J.Z.; Swaney, D.L.; et al. A SARS-CoV-2-human protein-protein interaction map reveals drug targets and potential drug-repurposing. bioRxiv 2020, 583, 459–568.
- Portela, L.M.; Santos, S.A.; Constantino, F.B.; Camargo, A.C.; Colombelli, K.T.; Fioretto, M.N.; Barquilha, C.N.; Périco, L.L.; Hiruma-Lima, C.A.; Scarano, W.R.; et al. Increased oxidative stress and cancer biomarkers in the ventral prostate of older rats submitted to maternal malnutrition. Mol. Cell. Endocrinol. 2021, 523, 111148.
- Li, Y.; Zhang, Z.; Yang, L.; Lian, X.; Xie, Y.; Li, S.; Xin, S.; Cao, P.; Lu, J. The MERS-CoV receptor DPP4 as a candidate binding target of the SARS-CoV-2 spike. iScience 2020, 23.
- Chen, C.-P.; Chen, L.F.; Yang, S.R.; Chen, C.Y.; Ko, C.C.; Chang, G.D.; Chen, H. Functional characterization of the human placental fusogenic membrane protein syncytin 2. Biol. Reprod. 2008, 79, 815–823.
- Zhou, Z.; Wang, R.; Yang, X.; Lu, X.Y.; Zhang, Q.; Wang, Y.L.; Wang, H.; Zhu, C.; Lin, H.Y.; Wang, H. The cAMP-responsive element binding protein (CREB) transcription factor regulates furin expression during human trophoblast syncytialization. Placenta 2014, 35, 907–918.
- Xiong, Y.; Liu, Y.; Cao, L.; Wang, D.; Guo, M.; Jiang, A.; Guo, D.; Hu, W.; Yang, J.; Tang, Z.; et al. Transcriptomic characteristics of bronchoalveolar lavage fluid and peripheral blood mononuclear cells in COVID-19 patients. Emerg. Microbes Infect. 2020, 9, 761–770.
- Chen, S.; Huang, B.; Luo, D.J.; Yang, F.; Li, X.; Zhao, Y.; Nie, X.; Huang, B.X. Pregnancy with new coronavirus infection: Clinical characteristics and placental pathological analysis of three cases. Zhonghua Bing Li Xue Za Zhi 2020, 49, 418–423.
- Ysrafil, A.I. Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2): An overview of viral structure and host response. Diabetes Metab. Syndr. 2020, 14, 407–412.
- Mulvey, J.J.; Magro, C.M.; Ma, L.X.; Nuovo, G.J.; Baergen, R.N. Analysis of complement deposition and viral RNA in placentas of COVID-19 patients. Ann. Diagn. Pathol. 2020, 46, 151530.
- Algarroba, G.N.; Rekawek, P.; Vahanian, S.A.; Khullar, P.; Palaia, T.; Peltier, M.R.; Chavez, M.R.; Vintzileos, A.M. Visualization of severe acute respiratory syndrome coronavirus 2 invading the human placenta using electron microscopy. Am. J. Obstet. Gynecol. 2020, 223, 275–278.
- Penfield, C.A.; Brubaker, S.G.; Limaye, M.A.; Lighter, J.; Ratner, A.J.; Thomas, K.M.; Meyer, J.A.; Roman, A. Detection of severe acute respiratory syndrome coronavirus 2 in placental and fetal membrane samples. Am. J. Obstet. Gynecol. MFM 2020, 2, 100133.
- Best Rocha, A.; Stroberg, E.; Barton, L.M.; Duval, E.J.; Mukhophadhyay, S.; Yarid, N.; Caza, T.; Wilson, J.D.; Kenan, D.J.; Kuperman, M.; et al. Detection of SARS-CoV-2 in formalin-fixed paraffin-embedded tissue sections using commercially available reagents. Lab. Investig. 2020, 100, 1485–1489.
- Nunes, V.; Cross, J.; Speich, J.E.; Morgan, D.R.; Strauss, J.F., 3rd; Ramus, R.M. Fetal membrane imaging and the prediction of preterm birth: A systematic review, current issues, and future directions. BMC Pregnancy Childbirth 2016, 16, 387.
- Baergen, R.N.; Heller, D.S. Placental pathology in Covid-19 positive mothers: Preliminary findings. Pediatr. Dev. Pathol. 2020, 23, 177–180.
- Shanes, E.D.; Mithal, L.B.; Otero, S.; Azad, A.H.; Miller, E.S.; Goldstein, A.J. Placental pathology in COVID-19. Am. J. Clin. Pathol. 2020, 154, 23–32.
- Connors, J.M.; Levy, J.H. COVID-19 and its implications for thrombosis and anticoagulation. Blood 2020, 135, 2033–2040.
- Di Mascio, D.; Khalil, A.; Saccone, G.; Rizzo, G.; Buca, D.; Liberati, M.; Vecchiet, J.; Nappi, L.; Scambia, G.; Berghella, V.; et al. Outcome of coronavirus spectrum infections (SARS, MERS, COVID-19) during pregnancy: A systematic review and meta-analysis. Am. J. Obstet. Gynecol. MFM 2020, 2, 100107.
- Smithgall, M.C.; Liu-Jarin, X.; Hamele-Bena, D.; Cimic, A.; Mourad, M.; Debelenko, L.; Chen, X. Third-trimester placentas of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)-positive women: Histomorphology, including viral immunohistochemistry and in-situ hybridization. Histopathology 2020, 77, 994–999.
- Littauer, E.Q.; Skountzou, I. Hormonal regulation of physiology, innate immunity and antibody response to H1N1 influenza virus infection during pregnancy. Front. Immunol. 2018, 9, 2455.
- Vivanti, A.J.; Vauloup-Fellous, C.; Prevot, S.; Zupan, V.; Suffee, C.; Cao, J.D.; Benachi, A.; de Luca, D. Transplacental transmission of SARS-CoV-2 infection. Nat. Commun. 2020, 11, 3572.
- Baud, D.; Greub, G.; Favre, G.; Gengler, C.; Jaton, K.; Dubruc, E.; Pomar, L. Second-trimester miscarriage in a pregnant woman with SARS-CoV-2 infection. JAMA 2020, 323, 2198–2200.
- Dong, L.; Tian, J.; He, S.; Zhu, C.; Wang, J.; Liu, C.; Yang, J. Possible vertical transmission of SARS-CoV-2 from an infected mother to her newborn. JAMA 2020, 323, 1846–1848.
- Jin, Y.; Wang, M.; Zuo, Z.; Fan, C.; Ye, F.; Cai, Z.; Wang, Y.; Cui, H.; Pan, K.; Xu, A. Diagnostic value and dynamic variance of serum antibody in coronavirus disease 2019. Int. J. Infect. Dis. 2020, 94, 49–52.
- Xiong, X.; Wei, H.; Zhang, Z.; Chang, J.; Ma, X.; Gao, X.; Chen, Q.; Pang, Q. Vaginal delivery report of a healthy neonate born to a convalescent mother with COVID-19. J. Med. Virol. 2020, 92, 1657–1659.
- Peng, Z.; Wang, J.; Mo, Y.; Duan, W.; Xiang, G.; Yi, M.; Bao, L.; Shi, Y. Unlikely SARS-CoV-2 vertical transmission from mother to child: A case report. J. Infect. Public Health 2020, 13, 818–820.
- Li, Y.; Zhao, R.; Zheng, S.; Chen, X.; Wang, J.; Sheng, X.; Zhou, J.; Cai, H.; Fang, Q.; Yu, F.; et al. Lack of Vertical Transmission of Severe Acute Respiratory Syndrome Coronavirus 2, China. Emerg. Infect. Dis. 2020, 26, 1335–1336.
- Gidlöf, S.; Savchenko, J.; Brune, T.; Josefsson, H. COVID-19 in pregnancy with comorbidities: More liberal testing strategy is needed. Acta Obstet. Gynecol. Scand. 2020, 99, 948–949.
- Zamaniyan, M.; Ebadi, A.; Mir, S.A.; Rahmani, Z.; Haghshenas, M.; Azizi, S. Preterm delivery, maternal death, and vertical transmission in a pregnant woman with COVID-19 infection. Prenat. Diagn. 2020, 40, 1759–1761.
- Zeng, H.; Xu, C.; Fan, J.; Tang, Y.; Deng, Q.; Zhang, W.; Long, X. Antibodies in infants born to mothers With COVID-19 pneumonia. JAMA 2020, 323, 1848–1849.
- Alzamora, M.C.; Paredes, T.; Caceres, D.; Webb, C.M.; Valdez, L.M.; La Rosa, M. Severe COVID-19 during pregnancy and possible vertical transmission. Am. J. Perinatol. 2020, 37, 861–865.
- Costa, S.; Buonsenso, D.; Sanguinetti, M.; Cattani, P.; Posteraro, B.; Marchetti, S.; Carducci, B.; Lanzone, A.; Tamburrini, E.; Vento, G.; et al. Neonatal late onset infection with severe acute respiratory syndrome Coronavirus. Am. J. Perinatol. 2020, 37, 869–872.
- Mahyuddin, A.P.; Kanneganti, A.; Wong, J.J.L.; Dimri, P.S.; Su, L.L.; Biswas, A.; Illanes, S.E.; Mattar, C.N.Z.; Huang, R.Y.J.; Choolani, M. Mechanisms and evidence of vertical transmission of infections in pregnancy including SARS-CoV-2s. Prenat. Diagn. 2020, 40, 1655–1670.
- Jafari, M.; Pormohammed, A.; Neshin, S.A.S.; Ghorbani, S.; Bose, D.; Alimohammadi, S.; Basirjafari, S.; Mohammedi, M.; Rasmussen-Ivey, C.; Razizadeh, M.H.; et al. Clinical characteristics and outcomes of pregnant women with COVID-19 and comparison with control patients: A systematic review and meta-analysis. Rev. Med. Virol. 2021, e2208.
- Kotlyar, A.M.; Grechukhina, O.; Chen, A.; Popkhadze, S.; Grimshaw, A.; Tal, O.; Taylor, H.S.; Tal, R. Vertical transmission of Coronavirus disease 2019: A systematic review and meta-analysis. Am. J. Obstet. Gynecol. 2021, 224, 35–53.
- Chi, J.; Gong, W.; Gao, Q. Clinical characteristics and outcomes of pregnant women with COVID-19 and the risk of vertical transmission: A systematic review. Arch. Gynecol. Obstet. 2021, 303, 337–345.
- Lamouroux, A.; Attie-Bitach, T.; Martinovic, J.; Leruez-Ville, M.; Ville, Y. Evidence for and against vertical transmission for severe acute respiratory syndrome coronavirus. Am. J. Obstet. Gynecol. 2020, 223, 91.e1–91.e4.
- Sukhikh, G.; Petrova, U.; Prikhodko, A.; Straodubtseva, N.; Chingin, K.; Chen, H.; Bugrova, A.; Kononikhin, A.; Bourmenskaya, O.; Brzhozovskiy, A.; et al. Vertical transmission of SARS-CoV-2 in second trimester associated with severe neonatal pathology. Viruses 2021, 13, 447.
- WHO. Definition and Categorization of the Timing of Mother-to-Child Transmission of SARS-Cov-2; WHO: Geneva, Switzerland, 2021.
- Valdespino-Vazquez, M.Y.; Helguera-Repetto, C.A.; Leon-Juarez, M.; Villavicencio-Carrisoza, O.; Flores-Pliego, A.; Moreno-Verduzco, E.R.; Diaz-Perez, D.L.; Villegas-Mota, I.; Carrasco-Ramirez, E.; Lopez-Martinez, I.E.; et al. Fetal and placental infection with SARS-CoV-2 in early pregnancy. J. Med. Virol. 2021, 93, 4480–5587.
- Gulersen, M.; Prasannan, L.; Tam, H.T.; Metz, C.N.; Rochelson, B.; Meirowitz, N.; Shan, W.; Edelman, M.; Millington, K.A. Histopathologic evaluation of placentas after diagnosis of maternal severe acute respiratory syndrome coronavirus 2 infection. Am. J. Obstet. Gynecol. MFM 2020, 2, 100211.
- Menter, T.; Mertz, K.D.; Jiang, S.; Chen, H.; Monod, C.; Tzankov, A.; Waldvogel, S.; Schulzke, S.M.; Hosli, I.; Bruder, E. Placental pathology findings during and after SARS-CoV-2 infection: Features of villitis and malperfusion. Pathobiology 2021, 88, 69–77.





