
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Verónica Tijero | + 3260 word(s) | 3260 | 2021-06-07 10:46:23 | | | |
| 2 | Amina Yu | Meta information modification | 3260 | 2021-06-15 03:13:25 | | |
Video Upload Options
Review of the main physiological aspects of primary metabolism in apple, such as photosynthesis and metabolite accumulation processes, as well as how the application of agrochemicals affect the first stages of apple development, when most of the fruit final quality is determined.
1. Introduction
With more than 2500 species belonging to 90 different genera, the Rosaceae family is a very extended group, which comprises ornamental to edible crops. Within the latter, fleshy fruits have an economic importance given their nutritional contribution to a healthy diet; hence, there is high consumer demand [1]. Among the fleshy fruits belonging to the Rosaceae family, there are accessory fruits such as strawberry, stone fruits such as cherries, plums, apricots, or peaches, berries such as raspberries, and pomes such as apples and pears [2].
Apples (Malus domestica L. Borkh.) have an important economic impact worldwide. It is known that almost five million hectares of the worldwide area is harvested for apples, with approximately 17 million metric tons stemming from European production, representing 20% of the global production, which was approximately 90 million metric tons in 2019, becoming the fruit with the third largest production, behind citrus and bananas [3]. During their development, apples accumulate several compounds that are deemed valuable by consumers, such as sugars, organic acids, vitamins, fibers, and antioxidants [4]. The concentration of these compounds may vary depending on factors such as climate, genotype (i.e., cultivar), orchard management, harvest and storage conditions, and processing [5][6][7][8].
Due to its high importance, many studies have focused on developing new agricultural and biotechnological tools that may improve apple quality and maintain its freshness after harvest. Moreover, in the last few years, climate change has been a significant concern, causing production loss around the world. The use of different rootstocks, the exogenous application of agrochemicals during tree vegetation or reproduction, agronomic handling, edible coatings, and the employment of genetic resources are some examples of research topics currently studied [9][10].
The objective of this review was to summarize the main physiological aspects of primary metabolism in apple, such as photosynthesis and metabolite accumulation processes, as well as how they are affected by the application of agrochemicals during the first stages of apple development, when most of the final fruit’s quality at harvest is determined.
2. Fruit as a Photosynthetic Organ
Photosynthesis in leaves is one of the most studied processes in plant science, considering that it is a key mechanism allowing the growth and development of different organs, whereby plants convert sunlight energy into biochemical energy [11]; however, photosynthesis is not only specific to foliar organs. Several studies have reported potential carbon assimilation in other tissues, such as seeds, petioles, stems, or fruits [12][13]. Concerning fleshy fruit, photosynthesis may be limited to the first stages of fruit development, when the fruit is still green and immature [14].
Similar to leaves, young apples have a photosynthetic system with active chloroplasts, which are located at the level of the hypodermal and inner perivascular green tissues, exhibiting a flat and elongated structure with stacked thylakoids forming a hypergranum, but which are smaller and fewer than those found in leaves [15][16]. Photosynthetic pigments (chlorophyll a and b and carotenoids β-carotene, lutein, violaxanthin, and neoxanthin) are also found in both the skin (peel) and the cortex (pulp) of apples, but they are less concentrated and irregularly distributed [17]. Although chlorophyll content gradually decreases as the apple develops, these pigments may accept light energy for photosynthesis, as shown in chlorophyll fluorescence images of young apples where the operating efficiency of photosystem II (Fq’/Fm’) indicates functional photosynthetic electron transport [12].
However, young apples not only have a determined number of stomata per fruit that remains constant since flower development, but they are fewer than the stomata that can be found in leaves [16]. The downregulation of chlorophyll biosynthesis genes in tomato was correlated with a reduction in photosynthetic rate, but fruit size and metabolite levels remained unaltered, suggesting that fruit photosynthesis is not essential for growth and development, as the main source of photoassimilates is leaves. However, a delay in seed development was observed, which suggests an important role of CO2 (re)fixation from fruit photosynthesis [18]. Similarly to tomato, this suggests that apple photosynthesis may be mainly used to support seed development and accumulate malate via the malate-CO2 shuttle.
3. Accumulation of Carbohydrates and Other Primary Metabolites
To reach the optimal commercial quality, fruits on the tree must be able to increase their size, especially during the first stages of development, as well as accumulate sugars and other metabolites important to consumers. Throughout its development, apple experiences different stages from a physiological point of view, in which can be categorized as (1) cell division, (2) cell expansion that partially overlaps with cell division and is continuous until the harvest time, (3) maturation, and (4) fruit ripening, representing important stages where the fruit undergoes structural and biochemical changes that result in the conversion of a green and nonpalatable fruit into a high quality and nutritional fruit (Figure 1A) [1][19][20].
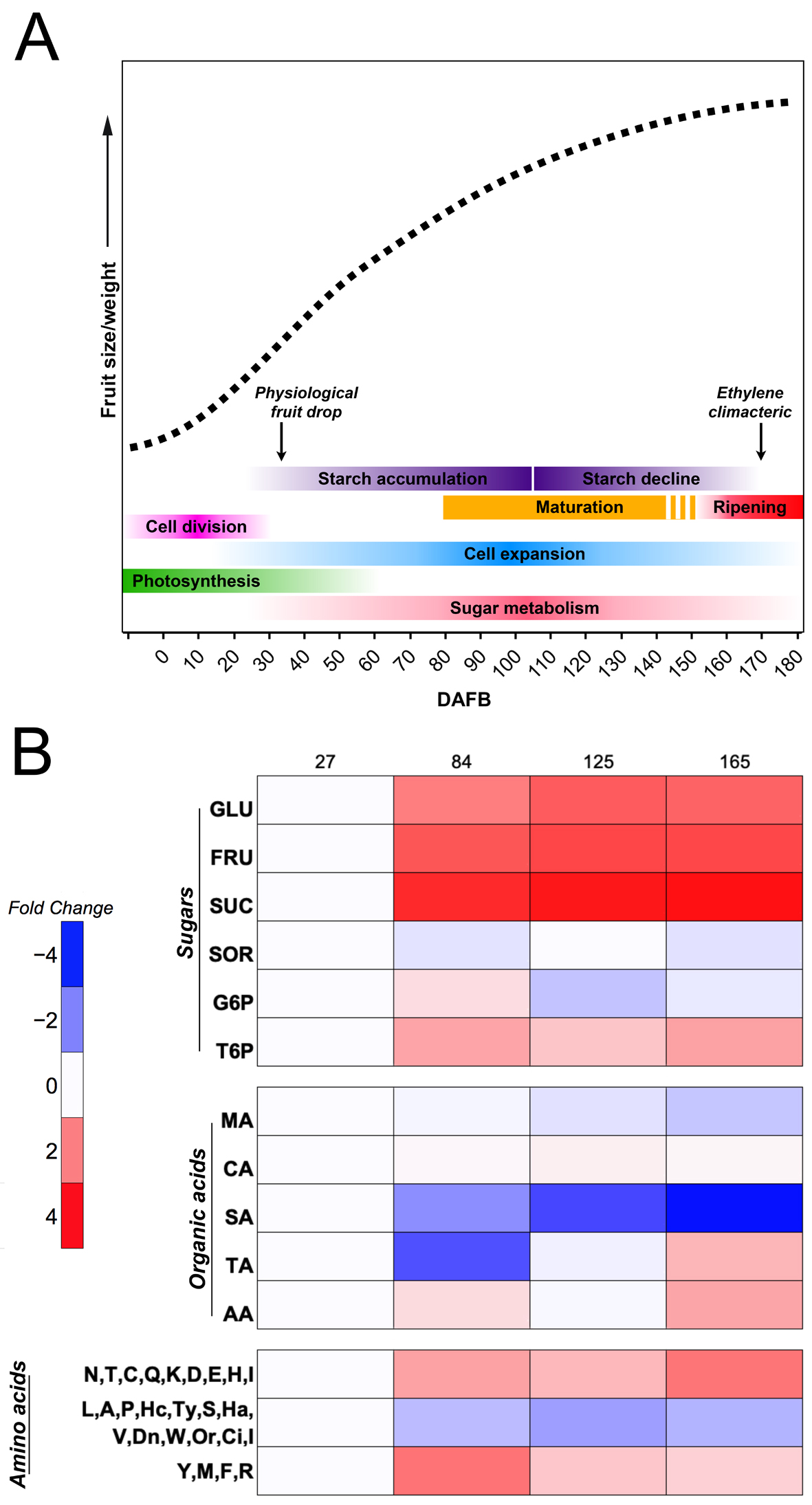 Figure 1. Apple growth curve (dashed line), highlighting main processes and primary metabolites. (A) Simple sigmoid growth pattern based on an expolinear equation for apple. The main physiological processes and events accompanying apple development are indicated. (B) Heatmap showing the time course progression of the levels of some of the most important sugars, organic acids, and amino acids found in apple at four developmental stages (27, 84, 125, and 165 DAFB). Levels were elaborated from references [21][22] and are reported as log2 of the foldchange, keeping the first value equal to 1. GLU, glucose; FRU, fructose; SUC, sucrose; SOR, sorbitol; G6P, glucose-6-phosphate; T6P, trehalose-6-phosphate; MA, malic acid; CA, citric acid; SA, succinic acid; TA, tartaric acid; AA, ascorbic acid.
Figure 1. Apple growth curve (dashed line), highlighting main processes and primary metabolites. (A) Simple sigmoid growth pattern based on an expolinear equation for apple. The main physiological processes and events accompanying apple development are indicated. (B) Heatmap showing the time course progression of the levels of some of the most important sugars, organic acids, and amino acids found in apple at four developmental stages (27, 84, 125, and 165 DAFB). Levels were elaborated from references [21][22] and are reported as log2 of the foldchange, keeping the first value equal to 1. GLU, glucose; FRU, fructose; SUC, sucrose; SOR, sorbitol; G6P, glucose-6-phosphate; T6P, trehalose-6-phosphate; MA, malic acid; CA, citric acid; SA, succinic acid; TA, tartaric acid; AA, ascorbic acid.
Apples show a simple sigmoid curve growth pattern with an exponential initial growth phase, followed by linear growth, based on an expolinear equation that involves fruit diameter [23][24][25] (Figure 1A). During the early developmental stages of apple, sugars are essential to provide the energy needed by the fruitlets in order to endure cell division and subsequent cell expansion; therefore, apple fruitlets become a strong sink organ [26]. However, during the reproductive season, young growing shoots are stronger sinks than fruits, causing an extreme competition for tree resources; as a consequence, the tree is not able to support all growing fruitlets, thus inducing the natural abscission of weaker ones [27][28]. This phenomenon, often called “June drop” or simply “physiological fruit drop”, is not sufficient to achieve a suitable fruit load, thus leading to two main issues: (i) poor fruit quality at harvest, and (ii) alternate bearing (reviewed by Costa et al. [29]).
Outside of mechanical thinning and the use of some caustic agents (i.e., ATS, ammonium thiosulphate), most thinning agents make use of active ingredients with hormone-like activity, such as ethephon (an ethylene releaser), naphthaleneacetic acid (NAA), naphthaleneacetamide (NAD), and the cytokinin-like 6-benzyladenine (BA). Metamitron, a photosynthesis inhibitor, has also been recently released as a thinning agent [29], thus providing growers with new chemical tools that can be applied according to their thinning strategy, i.e., using several thinners at different times in order to achieve an optimal fruit load.
The physiological mechanisms on which the different thinners rely have been deeply investigated, especially with respect to BA and metamitron. While the former stimulates shoot growth and bud outbreak with little direct effect on the fruitlets, metamitron inhibits photosystem II, causing nutritional stress in both cases, thereby enhancing the strong competition for assimilates between the vegetative and reproductive sinks. This new condition is perceived by the weaker fruitlets and, as a result, a complex network is activated at the cortex and seeds of apple fruitlets, in which sugars, mainly sucrose and trehalose, work as the primary signaling molecules. During sugar starvation, there is an accumulation of reactive oxygen species (ROS) molecules, especially hydrogen peroxide (H2O2), which interact with the phytohormones involved in this process, including ethylene and abscisic acid (ABA), thus inducing seed abortion and a subsequent activation of the abscission zone [29][30][31].
Even though the application of agrochemicals is a common practice, there are no health concerns with respect to their usage due to the following reasons: (i) they are mainly applied shortly after full bloom, and the preharvest interval (PHI) is largely respected, as, in most cases, apples are picked after at least 3 months following treatment; (ii) most of the active ingredients are closely similar to endogenous hormones and, consequently, are easily metabolized by both the target trees and the surrounding environment, thus also ruling out any environmental concerns [32][33][34].
In apples, cell division is generally completed 3–4 weeks after pollination [23]. During this short period of time, the carbohydrates produced in the leaf mesophyll and translocated to the immature fruits are sorbitol and sucrose. The loading of both sugars into a complex formed between the companion cell and the sieve element (SE–CC) follows the symplasmic pathway (Figure 2) [35][36]. To meet the energy requirements for cell division, sorbitol, the major carbohydrate transported into young apples and other fruits from the Rosaceae family [37][38][39], is converted into fructose by sorbitol dehydrogenase (SDH) [40]. On the other hand, sucrose conversion into fructose and glucose has two different routes: (1) regulated by the sucrose synthase (SUSY) enzyme, located in the cytoplasm, and (2) regulated by cell-wall invertase (CWINV), situated at the cell-wall space, where hexose transporters (HT) transfer the products from the conversion into the cytosol [41]. G6P transmembrane transporters located at the plastid membrane were found to show a high expression around 40 days after full bloom (DAFB), facilitating G6P import from the cytosol into plastids, where synthesis and accumulation of starch takes place, as several enzymes involved in starch formation, such as starch synthase, starch branching enzyme, and ADP glucose pyrophosphorylase were also co-expressed with G6P transporters [16][21][42]. In addition, cell vacuoles also store sugars that have not been metabolized, such as fructose, glucose, and sucrose, by transporting them into the vacuole space through some tonoplast transporters, e.g., MdSUT4.1, which is significantly associated with sucrose accumulation in apple vacuoles [43][44].
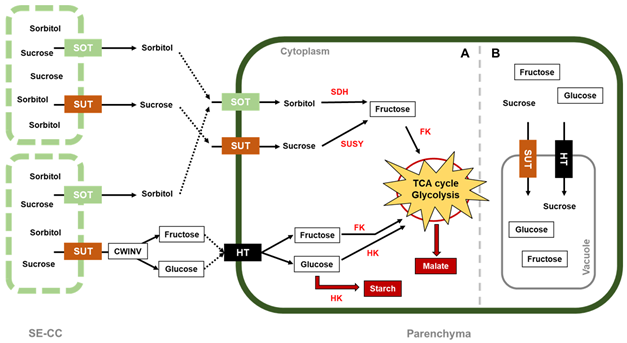 Figure 2. Sugar metabolism in apple. Sorbitol and sucrose are the photoassimilates unloaded to cell fruits via apoplasmic unloading from the sieve element–companion cell complex (SE-CC). With their respective cell-wall transporter, these compounds enter the parenchyma cell where they are converted into hexoses (fructose and glucose), which are phosphorylated by fructokinase and hexokinase, respectively, to enter glycolysis/TCA cycle, with the objective of satisfying the energetic requirements for apple growth. The cytoplasm is divided according to (A) high sugar metabolism that produces energy, starch for storage, and malate used to (re)fix the mitochondrial CO2 via the malate-CO2 shuttle, as well as for accumulation, and (B) slower sugar metabolism that starts to accumulate sucrose, fructose, and glucose inside the vacuoles. In red letters, the enzymes are abbreviated as follows: SDH, sorbitol dehydrogenase; SUSY, sucrose synthase; FK, fructokinase; HK, hexokinase. Sugar transporters are abbreviated as follows: SOT, sorbitol transporter; SUT, sucrose transporter; HT, hexose transporter.
Figure 2. Sugar metabolism in apple. Sorbitol and sucrose are the photoassimilates unloaded to cell fruits via apoplasmic unloading from the sieve element–companion cell complex (SE-CC). With their respective cell-wall transporter, these compounds enter the parenchyma cell where they are converted into hexoses (fructose and glucose), which are phosphorylated by fructokinase and hexokinase, respectively, to enter glycolysis/TCA cycle, with the objective of satisfying the energetic requirements for apple growth. The cytoplasm is divided according to (A) high sugar metabolism that produces energy, starch for storage, and malate used to (re)fix the mitochondrial CO2 via the malate-CO2 shuttle, as well as for accumulation, and (B) slower sugar metabolism that starts to accumulate sucrose, fructose, and glucose inside the vacuoles. In red letters, the enzymes are abbreviated as follows: SDH, sorbitol dehydrogenase; SUSY, sucrose synthase; FK, fructokinase; HK, hexokinase. Sugar transporters are abbreviated as follows: SOT, sorbitol transporter; SUT, sucrose transporter; HT, hexose transporter.
As fruit development progresses over time, apples continue to accumulate a high amount of sugars within vacuoles, thus generating an osmotic pressure that stimulates a high influx of water. Previous studies have shown that isolated vacuoles from immature apple flesh presented a higher turgor pressure than vacuoles from mature apples, as vacuoles from immature fruits contained 706 mM of total sugars (mostly fructose and glucose) in comparison to 67 mM of total sugars accumulated in the apoplast [45], while the content of total sugars in vacuoles from mature fruits was 937 mM compared to 440 mM of total sugars in the apoplast [37]. Therefore, this indicates that, during the early development of apples, there is active cell division that requires a high input of energy from the photoassimilates, sorbitol and sucrose, produced by leaf photosynthesis, which are translocated and rapidly metabolized in immature apple fruits, as a high expression of the enzymes SDH, CWINV, SUSY, FK, and HK was observed on “Greensleeves” apples [46]. Afterward, fruits start to accumulate sugars (Figure 1B) into vacuoles, causing a high turgor pressure that is translated into active cell enlargement and, consequently, an increase in fruit growth that continues over time throughout the development of apples.
In addition to the major sugars present in apple primary metabolism, trehalose 6-phosphate (T6P), which is a precursor product of trehalose biosynthesis and a promoter of plant growth [47], has been found in “Gala” apple [48]. T6P showed low concentration levels during apple development, but it was positively correlated with sorbitol, suggesting the possible regulation of a different starch accumulation pathway. However, further investigation is needed to unravel the role of T6P in sugar metabolism [48][49].
Organic acids are also accumulated into apple cell vacuoles, playing an important role in fruit acidity and its final organoleptic quality [50]. Around 85–90% of total organic acids in apples are represented by malic acid, while acetic acid, oxalic acid, fumaric acid, and citric acid are also found but in minor proportions inside the cell vacuole [45]. There are two pathways used to synthesize malate, the predominant form of malic acid (Figure 3). It has been suggested that cytosolic conversion of OAA into malate is the most likely route for its synthesis, as PEPc is highly expressed during “Greensleeves” apple development [21].
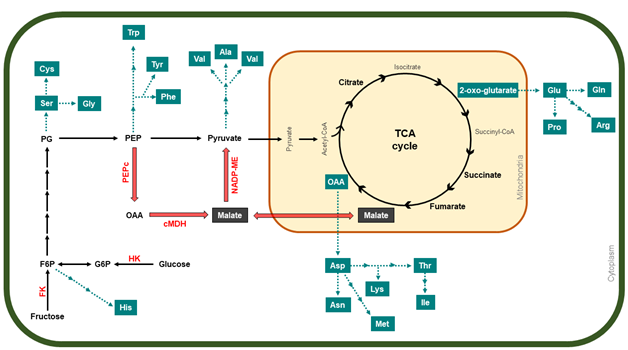 Figure 3. A general description of organic acid and amino-acid metabolism pathway during apple development, which involves glycolysis and the TCA cycle. Red arrows indicate the expected route of malate formation, accumulation, and degradation in apples. Blue boxes and arrows mark the diverse biosynthesis pathways of the different amino acids. In red letters, the enzymes are abbreviated as follows: PEPc, phosphoenolpyruvate carboxylase; cMDH, NAD-dependent malate dehydrogenase; NADP-ME, NADP-dependent malic enzymes; FK, fructokinase; HK, hexokinase
Figure 3. A general description of organic acid and amino-acid metabolism pathway during apple development, which involves glycolysis and the TCA cycle. Red arrows indicate the expected route of malate formation, accumulation, and degradation in apples. Blue boxes and arrows mark the diverse biosynthesis pathways of the different amino acids. In red letters, the enzymes are abbreviated as follows: PEPc, phosphoenolpyruvate carboxylase; cMDH, NAD-dependent malate dehydrogenase; NADP-ME, NADP-dependent malic enzymes; FK, fructokinase; HK, hexokinase
As the fruit continues to metabolize sugars, the malate level is increased and accumulates in the vacuole thanks to the malate transporters localized at the tonoplast, activated by the MYB transcription factors MdMYB1/10 and MdMYB73, which also regulate the activation of the two primary proton pumps, the vacuolar H+-ATPase and the vacuolar pyrophosphatase, both playing an important role in determining fruit acidity [51][52][53]. The highest levels of malate were observed 60 days after bloom in low-acid and high-acid apple genotypes from a cross population of “Toko” and “Fuji”. As the apple continues to grow, malate levels subsequently decrease (Figure 1B) and the activity of NADP-dependent malic enzyme increases, which is in charge of malate degradation [54][55]. By the end of apple development, there is an accumulation of soluble sugars not only due to the decrease in malate content, but also due to the hydrolyzation of the accumulated starch, which provides fructose, glucose, and sucrose [4][56].
Alongside malate, other organic acids from the TCA cycle such as succinic acid, fumaric acid, and citric acid (Figure 3) also present higher concentrations in immature apples; however, as cell expansion begins, their levels decrease throughout apple development [22][57]. In contrast, ascorbic acid is accumulated at a mature stage, as well as tartaric acid (Figure 1B) [22]. Although the concentration of ascorbic acid in apples is low, compared with other fruits such as kiwifruits or blackcurrant [58], it has been reported that apple peel contains more ascorbic acid than the flesh in “Gala” apples, and that it is synthesized from the galactose pathway [59]. Ascorbic acid is a well-known antioxidant and cofactor for several enzymes, and a higher concentration of this organic acid may suggest an antioxidant role during the last stages of apple development, as a response to the increased production of ROS due to high light and high temperature, possibly causing a disorder on apple peel called sunburn [60][61].
Primary metabolism also comprises the accumulation of amino acids which are the constituents of many key proteins associated with fruit development. Amino-acid biosynthesis is linked to carbohydrate metabolism (Figure 3); thus, aromatic amino acids such as phenylalanine, tyrosine and tryptophan are obtained from the shikimate pathway, in which PEP from glycolysis is the main substrate, while F6P is the initial precursor for histidine, formed after a series of reactions starting from phosphoribosylpyrophosphate [62][63]. Moreover, pyruvate is the substrate of branched-chain amino acids valine and leucine, as well as for alanine biosynthesis, while glycine, cysteine, and serine are formed from 3-phosphoglycerate, also from the glycolysis process [64]. Intermediates from the TCA cycle are also involved in the synthesis of amino acids (Figure 3), mainly OAA, which is the precursor of aspartic acid and all its derived amino acids, such as lysine, threonine, methionine, isoleucine, and asparagine, whereas 2-oxo-glutarate is the precursor for glutamic acid, the base for the synthesis of glutamine, arginine, and proline [64][65]. During the early stages of apple development, tyrosine, methionine, phenylalanine, and arginine show a higher accumulation after 84 DAFB, while fruit cells are expanding; however, after reaching their maximum, their levels decrease as the fruit continues to develop. On the other hand, amino acids such as asparagine, threonine, cysteine, glutamine, lysine, aspartic acid, histidine, and glutamic acid exhibit an increasing accumulation trend throughout apple development, reaching their peak at a mature stage. Lastly, the quantification of leucine, alanine, proline, serine, valine, tryptophan, isoleucine, and other amino acids showed minimum levels and a tendency to decrease during the developmental cycle.
4. Future Perspectives and Conclusions
In this review, we aimed to describe the most important pathways and compounds either involved in or derived from primary metabolism, which is important for many developing fruits, as it provides the basis of final fruit size and quality. Considering its importance, research is focused on improving fruit quality and extending shelf-life to fulfil consumers’ high standards, as apples are one of the most produced fruits worldwide. With the release of the apple genome sequence in 2010 [66], many molecular advances have been achieved in recent years, improving apple breeding programs and the comprehension of the most important physiological processes, with relevant positive effects in terms of knowledge transfer to the productive sector, especially with regard to agronomic practices (i.e., thinning and optimization of harvest date). Many studies have demonstrated the effect of these agronomic practices on final fruit quality in terms of primary metabolite content, even though these positive effects are most likely achieved through indirect mechanisms.
The literature is not very exhaustive when it comes to fruit physiology during apple development on the tree, as most studies have sectorially focused on the very beginning of development, i.e., flowering, fruit set, and fruitlet stage, on the later stages, i.e., ripening, or on the postharvest phase, without any semblance of continuity. As an example, we found that the latest review on apple photosynthesis was about 30 years ago [16], despite photosynthesis being a key process not only for fruit development but also for the ignition of primary metabolism. Considering the technological improvements made in the last decades (e.g., in measuring gas exchanges at the orchard, tree, and single organ level), this topic deserves more detailed investigations, as several questions are still unanswered.
It is during the early stages of development, when primary metabolites such as sugars, organic acids, and amino acids play an important role, that most of the final fruit quality is determined, as many significant secondary metabolites are produced by primary metabolites that are the main substrate for many reactions. Within secondary metabolism, plant hormones play a crucial role in apple final quality. In addition to ethylene, the main phytohormone involved in the ripening process for climacteric fruits such as apples, many other hormones are involved such as auxins, cytokinins, or gibberellins, which are common plant growth regulators, or even jasmonates. Therefore, the interplay between different hormones and primary metabolism should be further investigated, representing an important advance in the research of apple physiology, from which future agronomical practices can be inspired.
In conclusion, apple development is a complex process that requires further research focusing on physiological aspects for a full understanding, involving subprocesses ranging from cellular modifications to genetic reprogramming, as well as fruit photosynthesis and climate sensory, sugar, and hormonal signaling.
References
- Farinati, S.; Rasori, A.; Varotto, S.; Bonghi, C. Rosaceae fruit development, ripening and post-harvest: An epigenetic perspective. Front. Plant Sci. 2017, 8, 1247.
- Hummer, K.E.; Janick, J. Rosaceae: Taxonomy, Economic Importance, Genomics. In Genetics and Genomics of Rosaceae. Plant Genetics and Genomics: Crops and Models; Folta, K.M., Gardiner, S.E., Eds.; Springer: New York, NY, USA, 2009; Volume 6.
- FAOSTAT. Available online: (accessed on 23 February 2021).
- Mussachi, S.; Serra, S. Apple fruit quality: Overview on pre-harvest factors. Sci. Hortic. 2018, 234, 409–430.
- van der Sluis, A.A.; Dekker, M.; de Jager, A.; Jongen, W.M. Activity and concentration of polyphenolic antioxidants in apple: Effect of cultivar, harvest year, and storage conditions. J. Agric. Food Chem. 2001, 49, 3606–3613.
- Leja, M.; Mareczek, A.; Ben, J. Antioxidant properties of two apple cultivars during long-term storage. Food Chem. 2003, 80, 303–307.
- Boyer, J.; Liu, R.H. Apple phytochemicals and their health benefits. Nutr. J. 2004, 3, 5.
- Napolitano, A.; Cascone, A.; Graziani, G.; Ferracane, R.; Scalfi, L.; Di Vaio, C.; Ritieni, A.; Fogliano, V. Influence of variety and storage on the polyphenol composition of apple flesh. J. Agric. Food Chem. 2004, 52, 6526–6531.
- Migicovsky, Z.; Gardner, K.M.; Richards, C.; Chao, C.T.; Schwaninger, H.R.; Fazio, G.; Zhong, G.Y.; Myles, S. Genomic consequences of apple improvement. Hortic. Res. 2021, 8, 9.
- Atkinson, R.G.; Sutherland, P.W.; Johnston, S.L.; Gunaseelan, K.; Hallett, I.C.; Mitra, D.; Brummell, D.A.; Schröder, R.; Johnston, J.W.; Schaffer, R.J. Down-regulation of POLYGALACTURONASE1 alters firmness, tensile strength and water loss in apple (Malus x domestica) fruit. BMC Plant Biol. 2012, 12, 129.
- Evans, J.R. Improving photosynthesis. Plant Physiol. 2013, 162, 1780–1793.
- Simkin, A.J.; Faralli, M.; Ramamoorthy, S.; Lawson, T. Photosynthesis in non-foliar tissues: Implications for yield. Plant J. 2020, 101, 1001–1015.
- Henry, R.J.; Furtado, A.; Rangan, P. Pathways of Photosynthesis in Non-Leaf Tissues. Biology 2020, 9, 438.
- Hiratsuka, S.; Suzuki, M.; Nishimura, H.; Nada, K. Fruit photosynthesis in Satsuma mandarin. Plant Sci. 2015, 241, 65–69.
- Phan, C.T. All-granal chloroplasts of apple-fruit. In Advances in Photosynthesis Research. Advances in Agricultural Biotechnology; Sybesma, C., Ed.; Springer: Dordrecht, The Netherlands, 1984; Volume 3.
- Blanke, M.M.; Lenz, F. Fruit photosynthesis. Plant Cell Environ. 1989, 12, 31–46.
- Phan, C.T. Occurrence of active chloroplasts in the internal tissues of apples. Their possible role in fruit maturation. Colloq. Int. CNRS 1975, 238, 49–55.
- Lytovchenko, A.; Eickmeier, I.; Pons, C.; Osorio, S.; Szecowka, M.; Lehmberg, K.; Arrivault, S.; Tohge, T.; Pineda, B.; Anton, M.T.; et al. Tomato fruit photosynthesis is seemingly unimportant in primary metabolism and ripening but plays a considerable role in seed development. Plant Physiol. 2011, 157, 1650–1663.
- Janssen, B.J.; Thodey, K.; Schaeffer, R.J.; Alba, R.; Balakrishnan, L.; Bishop, R.; Bowen, J.H.; Crowhurst, R.N.; Gleavel, A.P.; Ledger, S.; et al. Global gene expression analysis of apple fruit development from the floral bud to ripe fruit. BMC Plant Biol. 2008, 8, 16.
- Eccher, G.; Ferrero, S.; Populin, F.; Colombo, L.; Botton, A. Apple (Malus domestica L. Borkh) as an emerging model for fruit development. Plant Biosyst. 2014, 148, 157–168.
- Li, M.; Li, D.; Feng, F.; Zhang, S.; Ma, F.; Cheng, L. Proteomic analysis reveals dynamic regulation of fruit development and sugar and acid accumulation in apple. J. Exp. Bot. 2016, 67, 5145–5157.
- Xu, J.; Yan, J.; Li, W.; Wang, Q.; Wang, C.; Guo, J.; Geng, D.; Guan, Q.; Ma, F. Integrative Analyses of Widely Targeted Metabolic Profiling and Transcriptome Data Reveals Molecular Insight into Metabolomic variations during apple (Malus domestica) fruit development and ripening. Int. J. Mol. Sci. 2020, 21, 4797.
- Denne, M.P. The Growth of Apple Fruitlets, and the Effect of Early Thinning on Fruit Development. Ann. Bot. Lond. 1960, 24, 397–406.
- Lakso, A.N.; Corelli Grapadelli, L.; Barnard, J.; Goffinet, M.C. An expolinear model of the growth pattern of the apple fruit. J. Hortic. Sci. 1995, 70, 389–394.
- Zadravec, P.; Veberic, R.; Stampar, F.; Schmitzer, V.; Eler, K. Fruit growth patterns of four apple cultivars using nonlinear growth models. Eur. J. Hortic. Sci. 2014, 79, 52–59.
- Gillaspy, G.; Ben-David, H.; Gruissemet, W. Fruits: A developmental perspective. Plant Cell 1993, 5, 1439–1451.
- Byers, R.E. Flower and fruit thinning and vegetative: Fruiting balance. In Apples: Botany, Production and Uses; Ferree, D.C., Warrington, I.J., Eds.; CAB International: Wallingford, UK, 2003; pp. 409–436.
- Costa, G.; Dal Cin, V.; Ramina, A. Physiological, molecular and practical aspects of fruit abscission. Acta Hortic. 2006, 727, 301–310.
- Costa, G.; Botton, A.; Vizzotto, G. Fruit thinning: Advances and trends. In Horticultural Reviews; Warrington, I., Ed.; John Wiley & Sons, Inc.: West Sussex, UK, 2018; Volume 46, pp. 185–226.
- Botton, A.; Eccher, G.; Forcato, C.; Ferrarini, A.; Begheldo, M.; Zermiani, M.; Moscatello, S.; Battistelli, A.; Velasco, R.; Ruperti, B.; et al. Signaling pathways mediating the induction of apple fruitlet abscission. Plant Physiol. 2011, 155, 185–208.
- Eccher, G.; Begheldo, M.; Boschetti, A.; Ruperti, B.; Botton, A. Roles of ethylene production and ethylene receptor expression in regulating apple fruitlet abscission. Plant Physiol. 2015, 169, 125–137.
- European Food Safety Authority. Reasoned opinion on the review of the existing maximum residue levels (MRLs) for 1-naphthylacetamide and 1-naphthylacetic acid according to Article 12 of Regulation (EC) No 396/2005. EFSA J. 2015, 13, 4213.
- Anastassiadou, M.; Bernasconi, G.; Brancato, A.; Carrasco Cabrera, L.; Greco, L.; Jarrah, S.; Kazocina, A.; Leuschner, R.; Magrans, J.O.; Miron, I.; et al. Reasoned Opinion on the review of the existing maximum residue levels for metamitron according to Article 12 of Regulation (EC) No 396/2005. EFSA J. 2020, 18, e05959.
- Anastassiadou, M.; Bernasconi, G.; Brancato, A.; Carrasco Cabrera, L.; Greco, L.; Jarrah, S.; Kazocina, A.; Leuschner, R.; Magrans, J.O.; Miron, I.; et al. Reasoned Opinion on the review of the existing maximum residue levels for 6-benzyladenine according to Article 12 of Regulation (EC) No 396/2005. EFSA J. 2020, 18, e06220.
- Reidel, E.J.; Rennie, E.; Amiard, V.; Cheng, L.; Turgeon, R. Phloem loading strategies in three plant species that transport sugar alcohols. Plant Physiol. 2009, 149, 1601–1608.
- Li, M.; Li, P.; Ma, F.; Dandekar, A.M.; Cheng, L. Sugar metabolism and accumulation in the fruit of transgenic apple trees with decreased sorbitol synthesis. Hortic. Res. 2018, 5.
- Yamaki, S.; Ino, M. Alteration of cellular compartmentation and membrane permeability to sugars in immature and mature apple fruit. J. Am. Soc. Hortic. Sci. 1992, 117, 951–954.
- Lee, J. Sorbitol, Rubus fruit, and misconception. Food Chem. 2015, 166, 616–622.
- Vimolmangkang, S.; Zheng, H.; Peng, Q.; Jiang, Q.; Wang, H.; Fang, T.; Liao, L.; Wang, L.; He, H.; Han, Y. Assessment of sugar components and genes involved in the regulation of sucrose accumulation in peach fruit. J. Agric. Food. Chem. 2016, 64, 6723–6729.
- Park, S.W.; Song, K.J.; Kim, M.Y.; Hwang, J.H.; Shin, Y.U.; Kim, W.C.; Chung, W.I. Molecular cloning and characterization of four cDNAs encoding the isoforms of NAD-dependent sorbitol dehydrogenase from the Fuji apple. Plant Sci. 2002, 162, 513–519.
- Zhang, L.Y.; Peng, Y.B.; Pelleschi-Travier, S.; Fan, Y.; Lu, Y.F.; Lu, Y.M.; Gao, X.P.; Shen, Y.Y.; Delrot, S.; Zhang, D.P. Evidence for apoplasmic phloem unloading in developing apple fruit. Plant Physiol. 2004, 135, 574–586.
- Brookfield, P.; Murphy, P.; Harker, R.; MacRae, E. Starch degradation and starch pattern indices; Interpretation and relationship to maturity. Postharvest Biol. Technol. 1997, 11, 23–30.
- Fan, R.C.; Peng, C.C.; Xu, Y.H.; Wang, X.F.; Li, Y.; Shang, Y.; Du, S.Y.; Zhao, R.; Zhang, X.Y.; Zhang, L.Y.; et al. Apple sucrose transporter SUT1 and sorbitol transporter SOT6 interact with cytochrome b5 to regulate their affinity for substrate sugars. Plant Physiol. 2009, 150, 1880–1901.
- Peng, Q.; Cai, Y.; Lai, E.; Nakamura, M.; Liao, L.; Zheng, B.; Ogutu, C.; Cherono, S.; Han, Y. The sucrose transporter MdSUT4.1 participates in the regulation of fruit sugar accumulation in apple. BMC Plant Biol. 2020, 20, 191.
- Yamaki, S. Isolation of vacuoles from immature apple fruit flesh and compartmentation of sugars, organic acids, phenolic compounds and amino acids. Plant Cell Physiol. 1984, 25, 151–166.
- Li, M.; Feng, F.; Cheng, L. Expression Patterns of Genes Involved in Sugar Metabolism and Accumulation during Apple Fruit Development. PLoS ONE 2012, 7, e33055.
- Lunn, J.E.; Feil, R.; Hendriks, J.H.M.; Gibon, Y.; Morcuende, R.; Osuna, D.; Scheible, W.R.; Carillo, P.; Hajirezaei, M.R.; Stitt, M. Sugar induced increases in trehalose 6-phosphate are correlated with redox activation of ADP glucose pyrophosphorylase and higher rates of starch synthesis in Arabidopsis thaliana. Biochem. J. 2006, 397, 139–148.
- Zhang, W.; Lunn, J.E.; Feil, R.; Wang, Y.; Zhao, J.; Tao, H.; Guo, Y.; Zhao, Z. Trehalose 6-phosphate signal is closely related to sorbitol in apple (Malus domestica Borkh. cv. Gala). Biol. Open 2017, 6, 260–268.
- Tao, H.; Sun, H.; Wang, Y.; Song, X.; Guo, Y. New insights on ‘GALA’ apple fruit development: Sugar and acid accumulation: A transcriptomic approach. J. Plant Growth Regul. 2020, 39, 680–702.
- Harker, F.; Marsh, K.; Young, H.; Murray, S.; Gunson, F.; Walker, S. Sensory interpretation of instrumental measurements 2: Sweet and acid taste of apple fruit. Postharvest Biol. Technol. 2002, 24, 241–250.
- Sweetman, C.; Deluc, L.G.; Cramer, G.R.; Ford, C.M.; Soole, K.L. Regulation of malate metabolism in grape berry and other developing fruits. Phytochemistry 2009, 70, 1329–1344.
- Hu, D.G.; Sun, C.H.; Ma, Q.J.; You, C.X.; Cheng, L.L.; Hao, Y.J. MdMYB1 regulates anthocyanin and malate accumulation by directly facilitating their transport into vacuoles in apples. Plant Physiol. 2016, 170, 1315–1330.
- Hu, D.G.; Li, Y.Y.; Zhang, Q.Y.; Li, M.; Sun, C.H.; Yu, J.Q.; Hao, Y.J. The R2R3-MYB transcription factor MdMYB73 is involved in malate accumulation and vacuolar acidification in apple. Plant J. 2017, 91, 443–454.
- Yao, Y.X.; Li, M.; Liu, Z.; You, C.X.; Wang, D.M.; Zhai, H.; Hao, Y.J. Molecular cloning of three malic acid related genes MdPEPC, MdVHA-A, MdcyME and their expression analysis in apple fruits. Sci. Hortic. Amst. 2009, 122, 404–408.
- Yao, Y.X.; Li, M.; Zhai, H.; You, C.X.; Hao, Y.J. Isolation and characterization of an apple cytosolic malate dehydrogenase gene reveal its function in malate synthesis. J. Plant Physiol. 2011, 168, 474–480.
- Doerflinger, F.C.; Miller, W.B.; Nock, J.F.; Watkins, C.B. Relationships between starch pattern indices and starch concentrations in four apple cultivars. Postharvest Biol. Technol. 2015, 110, 86–95.
- Zhang, Y.; Li, P.; Cheng, L. Developmental changes of carbohydrates, organic acids, amino acids, and phenolic compounds in ‘Honeycrisp’ apple flesh. Food Chem. 2010, 123, 1013–1018.
- Davey, M.W.; Van Montagu, M.; Inze, D.; Sanmartin, M.; Kanellis, A.; Smirnoff, N.; Benzie, I.; Strain, J.; Favell, D.; Fletcher, J. Plant L-ascorbic acid: Chemistry, function, metabolism, bioavailability and effects of processing. J. Sci. Food Agric. 2000, 80, 825–860.
- Li, M.; Ma, F.; Shang, P.; Zhang, M.; Hou, C.; Liang, D. Influence of light on ascorbate formation and metabolism in apple fruits. Planta 2009, 230, 39–51.
- Chen, L.S.; Li, P.; Cheng, L. Effects of high temperature coupled with high light on the balance between photooxidation and photoprotection in the sun-exposed peel of apple. Planta 2008, 228, 745.
- Morales-Quintana, L.; Waite, J.M.; Kalcsits, L.; Torres, C.A.; Ramos, P. Sun injury on apple fruit: Physiological, biochemical and molecular advances, and future challenges. Sci. Hortic. Amst. 2020, 260, 108866.
- Stepansky, A.; Leustek, T. Histidine biosynthesis in plants. Amino Acids 2006, 30, 127–142.
- Maeda, H.; Dudareva, N. The shikimate pathway and aromatic amino acid biosynthesis in plants. Annu. Rev. Plant Biol. 2012, 63, 73–105.
- Forde, B.G.; Lea, P.J. Glutamate in plants: Metabolism, regulation, and signalling. J. Exp. Bot. 2007, 58, 2339–2358.
- Pratelli, R.; Pilot, G. Regulation of amino acid metabolic enzymes and transporters in plants. J. Exp. Bot. 2014, 65, 5535–5556.
- Velasco, R.; Zharkikh, A.; Affourtit, J.; Dhingra, A.; Cestaro, A.; Kalyanaraman, A.; Fontana, P.; Bhatnagar, S.K.; Troggio, M.; Pruss, D.; et al. The genome of the domesticated apple (Malus × domestica Borkh.). Nat. Genet. 2010, 42, 833–839.
- Maeda, H.; Dudareva, N. The shikimate pathway and aromatic amino acid biosynthesis in plants. Annu. Rev. Plant Biol. 2012, 63, 73–105.




