Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Jean-Jacques Rouby | + 1591 word(s) | 1591 | 2021-05-31 12:35:01 | | | |
| 2 | Rita Xu | Meta information modification | 1591 | 2021-06-11 10:45:55 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Rouby, J. Polymyxins. Encyclopedia. Available online: https://encyclopedia.pub/entry/10774 (accessed on 07 February 2026).
Rouby J. Polymyxins. Encyclopedia. Available at: https://encyclopedia.pub/entry/10774. Accessed February 07, 2026.
Rouby, Jean-Jacques. "Polymyxins" Encyclopedia, https://encyclopedia.pub/entry/10774 (accessed February 07, 2026).
Rouby, J. (2021, June 11). Polymyxins. In Encyclopedia. https://encyclopedia.pub/entry/10774
Rouby, Jean-Jacques. "Polymyxins." Encyclopedia. Web. 11 June, 2021.
Copy Citation
Polymyxins are non-ribosomal, cyclic oligopeptide antimicrobials, produced by the Gram-positive, spore-forming rod Bacillus aerosporus that were identified in 1946 from the soil of market gardens in England.
nebulized polymyxin
nebulized colistimethate sodium
colistin
multidrug resistant gram-negative bacteria
1. Introduction
Polymyxins are non-ribosomal, cyclic oligopeptide antimicrobials, produced by the Gram-positive, spore-forming rod Bacillus aerosporus that were identified in 1946 from the soil of market gardens in England [1]. Among the five antibiotics that belong to the polymyxin group, only two can be used in human and veterinary medicine: polymyxin B (PMB) and polymyxin E, also called colistin. Colistin sulfate is available for oral and topical use. PMB sulfate and colistin methanesulfonate sodium (CMS) are available for aerosol use and intravenous administration. PMB sulfate—available in North and South America, Southeast Asia and Japan—combines two components with direct antibacterial activity: PMB1 and PMB2. CMS is an essentially inactive prodrug that is hydrolyzed to multiple components with direct bactericidal activity. Among these colistin components, two are predominant, colistin A and colistin B. Colistin A and B are concentration-dependent antibiotics, active against multidrug-resistant (MDR) Gram-negative bacteria (GNB) such as carbapenemase-producing Enterobacterales, Pseudomonas aeruginosa and Acinetobacter baumannii. CMS is an inexpensive antibiotic massively used worldwide and considered as an essential antimicrobial agent by the World Health Organization [2]. The CMS dose is labelled as “colistin base activity (CBA in mg)” in North and South America, Singapore, Malaysia, New Zealand and Australia and as “international units (IU)” in Europe and India. Milligrams of CBA and IU are expressions of the antibacterial activity measured in vitro and do not reflect a mass unit. The equivalence between absolute mass of CMS and IU or CBA is the following:
~ 80 mg CMS = one million IU CMS = ~ 33.3 mg CBA.
2. Pharmacokinetics
2.1. Pharmacokinetics of Intravenous Colistimethate Sodium
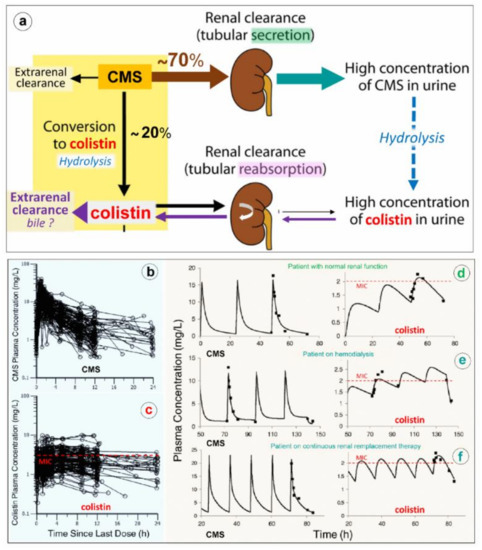
CMS is a complex mixture of up to ~30 methanesulfonated derivatives produced by the reaction of colistin with formaldehyde and sodium bisulfite. The composition of CMS pharmaceutical products may vary from brand-to-brand and even from batch-to-batch. CMS acts as a polyanionic inactive prodrug that is less nephrotoxic than colistin sulfate. As shown in Figure 1b, methanesulfonate moieties are masking the primary amines of the Dab residues, are negatively charged at physiological pH and preclude the interaction of CMS with anionic phospholipids of LPS. As a consequence, CMS lacks any antibacterial activity [3].
As shown in Figure 1a, CMS is rapidly and massively cleared from the blood by glomerular filtration and tubular excretion. Additionally CMS is spontaneously converted to colistin by hydrolysis, a necessary step to achieve antibacterial activity. The renal elimination of CMS is quantitatively greater than its spontaneous hydrolysis into colistin. In patients with normal renal function, approximately 20–25% of CMS is converted to colistin. The various composition in methanesulfonated derivatives of the different pharmaceutical products affects the spontaneous hydrolysis of CMS in biological fluids and is associated with a brand-to-brand and batch-to-batch interindividual variability in the rate of conversion. Adding to the complexity of pharmacokinetics, colistin resulting from the spontaneous hydrolysis of plasma CMS is filtered by the kidney and largely reabsorbed by the renal tubules. Although only a minor fraction is directly excreted in urine, urinary concentrations of colistin can be high, resulting from the spontaneous hydrolysis of CMS within renal tubules and bladder [3]. The mechanism of elimination of polymyxins is far from being elucidated [4]. Biliary excretion is likely the predominant pathway, as the different components of polymyxin B have been detected in bile [5][6][7].

Figure 1. Elimination and pharmacokinetics of colistimethate sodium (CMS) and colistin. (a) Schematic representation of elimination pathways for CMS and colistin. Arrow thickness indicates the relative magnitude of each pathway when kidney function is normal. CMS includes all partially methanesulfonated derivatives of colistin. After intravenous administration of CMS, extensive renal excretion occurs, with some of the excreted CMS being converted to colistin within the urinary tract. Colistin is massively reabsorbed by renal tubules and likely excreted in the biliary tract [8]. (b,c) Plasma concentration time profiles of CMS (Figure 1b) and formed colistin (Figure 1c) with 105 critically ill patients who were treated by intravenous CMS for blood stream infection or pneumonia caused by multidrug-resistant Gram-negative bacteria (89 not on renal replacement, 12 on intermittent hemodialysis and 4 on continuous renal replacement therapy). Doses of replacement therapy (d–f). The dashed line indicates the minimum inhibitory concentrations of susceptible strains [9].
Because renal elimination of CMS is much more rapid than its spontaneous conversion to colistin, it is necessary to administer about 4–5 times the amount of CMS to generate colistin plasma concentrations above the minimum inhibitory concentrations required for a bactericidal activity. As shown in Figure 1d–f, the disposition of CMS is best described by a two compartment linear model, whereas the disposition of colistin is best described by a one compartment model [9][10]. Following a single administration per day, the colistin profile is flatter than the CMS profile, offering the possibility of a longer half-life and a prolonged antibacterial effect if intravenous administrations are repeated 2-3 times a day. As in vivo conversion of CMS to colistin is slow, incomplete and variable, achievement of colistin plasma concentrations ≥ minimum inhibitory concentrations can be facilitated by a loading dose of 9 million IU. However, even with a loading dose and high daily doses (up to three times per day of 3 million IU), the brand-to-brand and batch-to-batch variability in CMS components may decrease the rate of conversion to colistin, although the CMS renal clearance remains rapid and efficient [11]. As a consequence, if renal function is normal, an increase in inter-individual pharmacokinetics variability may result, precluding the achievement of optimal colistin plasma concentrations required to obtain an efficient bactericidal effect. This may have a negative impact on prognosis given the link between delayed initiation of appropriate antibiotic therapy and patient outcome. Thus, the intravenous administration of CMS, a complex prodrug whose conversion to colistin, the active antibiotic, is far slower than its renal clearance and does not create optimal conditions to treat efficiently VAP caused by MDR GNB. As a consequence, CMS selected by physicians ranged between from 2.3 and 12 million IU/day, and plasma concentrations were measured using high-performance liquid chromatography. (d–f) Representative individual population pharmacokinetic model fits of CMS and colistin. Panel d illustrates a critically ill patient not on renal replacement. Panels e and f are representative of a patient on hemodialysis or on continuous renal; if intravenous CMS is used, concentrations should be measured regularly to detect suboptimal dosage. Such a difficult issue can be partially resolved by nebulization of CMS: the prodrug trapped in the distal lung undergoes progressive local conversion to colistin with a low systemic absorption and limited renal elimination.
2.2. Pharmacokinetics of Nebulized Colistimethate Sodium
Pharmacokinetics of nebulized CMS are incompletely understood mainly because of the difficulty to assessing lung interstitial concentrations in human studies. Indeed, the contamination of the bronchoscope by bronchial secretions during the bronchoalveolar procedure skews the interpretation of epithelial lining fluid (ELF) concentrations following antibiotic nebulization [12][13] and leads to a gross overestimation of interstitial space fluid concentrations [14][15]. Animal studies demonstrate that high colistin concentrations measured in post mortem subpleural lung specimens are high following nebulization of CMS 100,000/kg × 2/24 h in ventilated piglets with massive Pseudomonas aeruginosa inoculation pneumonia [16]. Lung homogenate colistin concentrations depend on both the severity of aeration loss and histologic grade (Figure 8a,b) with lower colistin lung concentration associated with a more severe histological grade of pneumonia. Colistin concentrations > five times minimum inhibitory concentrations are exclusively obtained in lung regions with a moderate severity of pneumonia and relative preservation of lung aeration, whereas in pulmonary segments with confluent pneumonia, colistin concentrations are in the range of minimum inhibitory concentrations. These high lung tissue concentrations, which underestimate interstitial space fluid concentrations due to the dilution effect of pulmonary cells and vessels, are associated with a rapid and potent bacterial killing [16].
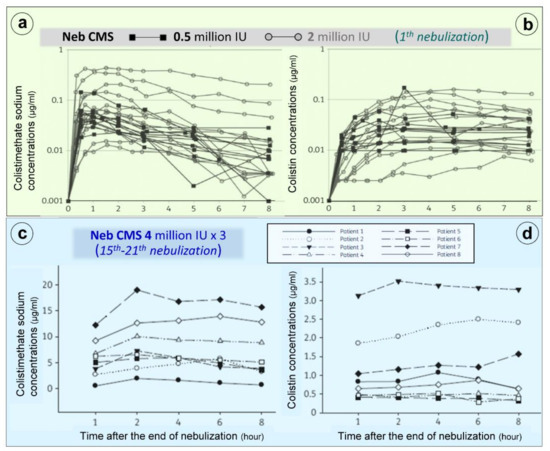
Nebulization of high doses of CMS results in high lung tissue colistin concentrations with correspondingly low plasma colistin concentrations (< 2 µg/mL) (Figure 1c) suggesting limited diffusion into the systemic compartment [17][18][19][16][20][21][22][23][24][25][26][27]. After the initial CMS nebulization of 2 million IU (Figure 8a,b), CMS and colistin plasma concentrations show quite similar pharmacokinetic profiles [24]: an early peak concentration for CMS (30 min), a delayed peak concentration for colistin (3 h) and a slow and progressive decrease in concentrations over the following hours. After 2–3 days of CMS nebulization at a dose of 4 million IU three times a day (Figure 2c,d), similar pharmacokinetic profiles were observed [26]. Repeated nebulized CMS doses result in increased CMS plasma concentrations. In the majority of patients, there was a slight increase in colistin plasma concentration when the nebulized CMS dose was increased from 0.5 to 4 million IU (Figure 2b,d). In a few patients, however, colistin plasma concentrations markedly increase, approaching concentrations observed after intravenous administration and plateauing over time (Figure 2d). These results suggest that CMS and colistin accumulate in the lung compartment. Colistin plasma concentrations remain low (< 2 µg/mL) in the majority of patients due to the slow diffuse of both CMS and colistin into the systemic circulation, rapid renal elimination of CMS and slow hydrolysis of CMS to colistin.

Figure 2. Colistimethate sodium (CMS) and colistin plasma concentrations following nebulization of various doses of CMS. (a,b) Plasma concentrations measured following the initial nebulization of CMS 0.5 and 2 million IU in a series of twelve patients with ventilator-associated pneumonia caused by Gram-negative bacteria [24]. (c,d) Plasma concentrations measured after 2–7 days of CMS nebulization at a dose of 4 million IU three times a day in a series of eight patients with ventilator-associated pneumonia caused by multidrug-resistant Gram-negative bacteria. Plasma concentrations were measured by high-performance liquid chromatography in the 8 h following an individual nebulization [26].
References
- Ainsworth, G.C.; Brown, A.M.; Brownlee, G. “Aerosporin”, an Antibiotic Produced by Bacillus aerosporus Greer. Nature 1947, 159, 263.
- World Health Organization. Critically important antimicrobials for human medicine 3rd Revision 2011. Clin. Infect. Dis. 2012, 55, 712–719. Available online: (accessed on 22 May 2021).
- Nation, R.L.; Forrest, A. Clinical Pharmacokinetics, Pharmacodynamics and Toxicodynamics of Polymyxins: Implications for Therapeutic Use. Adv. Exp. Med. Biol. 2019, 1145, 219–249.
- Avedissian, S.N.; Liu, J.; Rhodes, N.J.; Lee, A.; Pais, G.M.; Hauser, A.R.; Scheetz, M.H. A Review of the Clinical Pharmacokinetics of Polymyxin B. Antibiotics 2019, 8, 31.
- Barnett, M.; Bushby, S.R.M.; Wilkinson, S. Sodium sulphomethyl derivatives of polymyxins. Br. J. Pharm. Chemother. 1964, 23, 552–574.
- Zavascki, A.P.; Goldani, L.Z.; Cao, G.; Superti, S.V.; Lutz, L.; Barth, A.L.; Ramos, F.; Boniatti, M.M.; Nation, R.L.; Li, J. Pharmacokinetics of intravenous polymyxin B in critically ill patients. Clin. Infect. Dis. 2008, 47, 1298–1304.
- Manchandani, P.; Zhou, J.; Ledesma, K.R.; Truong, L.D.; Chow, D.S.; Eriksen, J.L.; Tam, V.H. Characterization of Polymyxin B Biodistribution and Disposition in an Animal Model. Antimicrob. Agents Chemother. 2015, 60, 1029–1034.
- Poirel, L.; Aurélie Jayol, A.; Nordmann, P. Polymyxins: Antibacterial Activity, Susceptibility Testing, and Resistance Mechanisms Encoded by Plasmids or Chromosomes. Clin. Microbiol. Rev. 2017, 30, 557–596.
- Garonzik, S.M.; Li, J.; Thamlikitkul, V.; Paterson, D.L.; Shoham, S.; Jacob, J.; Silveira, F.P.; Forrest, A.; Nation, R.L. Population pharmacokinetics of colistin methanesulfonate and formed colistin in critically ill patients from a multicenter study provide dosing suggestions for various categories of patients. Antimicrob. Agents Chemother. 2011, 55, 3284–3294.
- Viel, A.; Henri, J.; Bouchène, S.; Laroche, J.; Rolland, J.G.; Manceau, J.; Laurentie, M.; Couet, W.; Grégoire, N. A Population WB-PBPK Model of Colistin and its Prodrug CMS in Pigs: Focus on the Renal Distribution and Excretion. Pharm. Res. 2018, 35, 92.
- Bergen, P.J.; Landersdorfer, C.B.; Zhang, J.; Zhao, M.; Lee, H.J.; Nation, R.L.; Li, J. Pharmacokinetics and pharmacodynamics of ‘old’ polymyxins: What is new? Diagn. Microbiol. Infect. Dis. 2012, 74, 213–223.
- Monsel, A.; Torres, A.; Zhu, Y.-G.; Pugin, J.; Rello, J.; Rouby, J.J.; on behalf of the European Investigators Network for Nebulized Antibiotics in Ventilator-associated Pneumonia (ENAVAP). Nebulised antibiotics for ventilator-associated pneumonia: Methodological framework for multicenter randomised controlled trials. Curr. Opin. Infect. Dis. 2021, 34, 156–168.
- Rouby, J.J.; Monsel, A. Nebulized antibiotics: Epithelial lining fluid concentrations overestimate lung tissue concentrations. Anesthesiology 2019, 131, 229–232.
- Rouby, J.J.; Monsel, A.; Leone, M.; Mimoz, O.; Laterre, P.F.; Pugin, J. The IASIS, INHALE and VAPORISE trials. Reasons for a triple failure: Study design, aminoglycosides dosing and technique of nebulisation. Anaesth. Crit. Care Pain Med. 2020, 39, 179–183.
- Rouby, J.J.; Monsel, A.; Ehrmann, S.; Bouglé, A.; Laterre, P.F. The INHALE trial: Multiple reasons for a negative result. Lancet Infect. Dis. 2020, 20, 778–779.
- Lu, Q.; Girardi, C.; Zhang, M.; Bouhemad, B.; Louchahi, K.; Petitjean, O.; Wallet, F.; Becquemin, M.H.; Le Naour, G.; Marquette, C.H.; et al. Nebulized and intravenous colistin in experimental pneumonia caused by Pseudomonas aeruginosa. Intensive Care Med. 2010, 36, 1147–1155.
- Lu, Q.; Luo, R.; Bodin, L.; Yang, J.; Zahr, N.; Aubry, A.; Golmard, J.L.; Rouby, J.J. The Nebulized Antibiotics Study Group. Efficacy of High-dose Nebulized Colistin in Ventilator-associated Pneumonia Caused by Multidrug-resistant Pseudomonas aeruginosa and Acinetobacter baumannii. Anesthesiology 2012, 117, 1335–1347.
- Athanassa, Z.E.; Markantonis, S.L.; Fousteri, M.Z.; Myrianthefs, P.M.; Boutzouka, E.G.; Tsakris, A.; Baltopouloss, G.J. Pharmacokinetics of inhaled colistimethate sodium (CMS) in 417 mechanically ventilated critically ill patients. Intensive Care Med. 2012, 38, 1779–1786.
- Benítez-Cano, A.; de Antonio-Cuscó, M.; Luque, S.; Sorlí, L.; Carazo, J.; Ramos, I.; Grau, S. Systemic pharmacokinetics and safety of high doses of nebulized colistimethate sodium in critically ill patients with hospital-acquired and ventilator-associated pneumonia. J. Antimicrob. Chemother. 2019, 74, 3268–3273.
- Boisson, M.; Jacobs, M.; Grégoire, N.; Gobin, P.; Marchand, S.; Couet, W.; Mimoz, O. Comparison of intrapulmonary and systemic pharmacokinetics of colistin methanesulfonate (CMS) and colistin after aerosol delivery and intravenous administration of CMS in critically ill patients. Antimicrob. Agents Chemother. 2014, 58, 7331–7339.
- Yapa, S.W.; Li, J.; Patel, K.; Wilson, J.W.; Dooley, M.J.; George, J.; Clark, D.; Poole, S.; Williams, E.; Porter, C.J.; et al. Pulmonary and systemic pharmacokinetics of inhaled and intravenous colistin methanesulfonate in cystic fibrosis patients: Targeting advantage of inhalational administration. Antimicrob. Agents Chemother. 2014, 58, 2570–2579.
- Gontijo, A.V.; Grégoire, N.; Lamarche, I.; Gobin, P.; Couet, W.; Marchand, S. Biopharmaceutical characterization of nebulized antimicrobial agents in rats: 2. Colistin. Antimicrob. Agents Chemother. 2014, 58, 3950–3956.
- Marchand, S.; Bouchene, S.; de Monte, M.; Guilleminault, L.; Montharu, J.; Cabrera, M.; Grégoire, N.; Gobin, P.; Diot, P.; Couet, W.; et al. Pharmacokinetics of Colistin Methansulphonate (CMS) and Colistin after CMS Nebulisation in Baboon Monkeys. Pharm. Res. 2015, 32, 3403–3414.
- Boisson, M.; Grégoire, N.; Cormier, M.; Gobin, P.; Marchand, S.; Couet, W.; Mimoz, O. Pharmacokinetics of nebulized colistin methanesulfonate in critically ill patients. J. Antimicrob. Chemother. 2017, 72, 2607–2612.
- Lin, Y.W.; Zhou, Q.T.; Hu, Y.; Onufrak, N.J.; Sun, S.; Wang, J.; Forrest, A.; Chan, H.K.; Li, J. Pulmonary pharmacokinetics of colistin following administration of dry powder aerosols in rats. Antimicrob. Agents Chemother. 2017, 61, e00973–e01017.
- Bihan, K.; Zahr, N.; Becquemin, M.H.; Lu, X.; Bertholon, J.F.; Vezinet, C.; Arbelot, C.; Monsel, A.; Rouby, J.J.; Langeron, O.; et al. Influence of diluent volume of colistimethate sodium on aerosol characteristics and pharmacokinetics in ventilator-associated pneumonia caused by MDR bacteria. J. Antimicrob. Chemother. 2018, 73, 1639–1646.
- Tewes, F.; Brillault, J.; Gregoire, N.; Olivier, J.C.; Lamarche, I.; Adier, C.; Healy, A.M.; Marchand, S. Comparison between Colistin Sulfate Dry Powder and Solution for Pulmonary Delivery. Pharmaceutics 2020, 12, 557.
More
Information
Subjects:
Pharmacology & Pharmacy
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.1K
Revisions:
2 times
(View History)
Update Date:
11 Jun 2021
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




