
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Seunghyung Lee | + 3507 word(s) | 3507 | 2021-06-10 10:32:44 | | | |
| 2 | Lily Guo | Meta information modification | 3507 | 2021-06-11 04:41:19 | | |
Video Upload Options
The corpus luteum is a temporary endocrine gland in the ovary. In the ovarian cycle, repeated patterns of specific cellular proliferation, differentiation, and transformation occur that accompany the formation and regression of the corpus luteum. Molecular mechanism events in the ovarian microenvironment, such as angiogenesis and apoptosis, are complex. Recently, we focused on the role of RAS protein in the ovarian corpus luteum. RAS protein plays a vital role in the modulation of cell survival, proliferation, and differentiation by molecular pathway signaling. Additionally, reproductive hormones regulate RAS activity in the cellular physiological function of ovarian follicles during pre-ovulatory maturation and ovulation. Thus, we have reviewed the role of RAS protein related to the biological events of the corpus luteum in the ovary.
1. Introduction
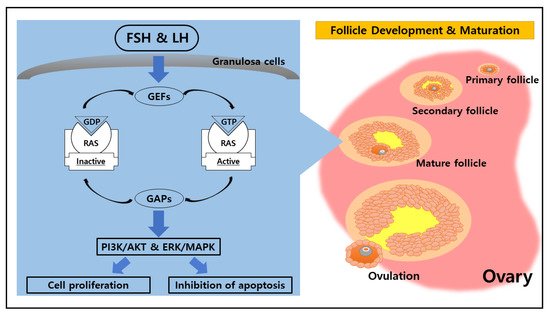
2. Potential Function of RAS in the Ovary
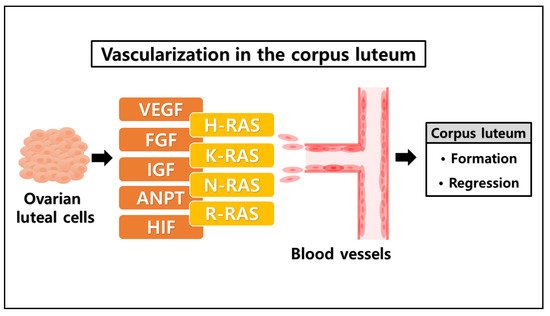
3. The Likelihood of a Novel Role of RAS in the Ovarian Corpus Luteum
| Conditions | Factors | Functions |
|---|---|---|
| Formation of corpus luteum | Vascular endothelial growth factor (VEGF) | Vasculogenesis [57] |
| Insulin-like growth factor (IGF) | Angiogenesis [58] | |
| Fibroblast growth factor (FGF) | Angiogenic factor [59] | |
| Angiopoietins (ANPT) | Cellular growth factor [60] | |
| Hypoxia-inducible factor (HIF) | Transcription [61] | |
| Regression of corpus luteum | Prostaglandin F2α (PGF2α) | Luteolysis [62] Apoptosis [63] |
| RAS family | H-RAS | Cellular signal transduction [64] |
| K-RAS | ERK pathway [65] | |
| N-RAS | PI3K-AKT pathway [66] | |
| R-RAS | RAS-MAPK pathway [67] |
References
- Telfer, E.E.; Anderson, R.A. The existence and potential of germline stem cells in the adult mammalian ovary. Climacteric 2019, 22, 22–26.
- Berisha, B.; Schams, D.; Rodler, D.; Pfaffl, M.W. Angiogenesis in the ovary–the most important regulatory event for follicle and corpus luteum development and function in cow—an overview. Anat. Histol. Embryol. 2016, 45, 124–130.
- Richards, J.S. The ovarian cycle. Vitam. Horm. 2018, 107, 1–25.
- Csépányi-Kömi, R.; Lévay, M.; Ligeti, E. Small G proteins and their regulators in cellular signalling. Mol. Cell. Endocrinol. 2012, 353, 10–20.
- Nakhaei-Rad, S.; Haghighi, F.; Nouri, P.; Rezaei Adariani, S.; Lissy, J.; Kazemein Jasemi, N.S.; Ahmadian, M.R. Structural fingerprints, interactions, and signaling networks of RAS family proteins beyond RAS isoforms. Crit. Rev. Biochem. Mol. Biol. 2018, 53, 130–156.
- Serna-Blasco, R.; Sanz-Álvarez, M.; Aguilera, Ó.; García-Foncillas, J. Targeting the RAS-dependent chemoresistance: The Warburg connection. Semin. Cancer Biol. 2019, 54, 80–90.
- Pylayeva-Gupta, Y.; Grabocka, E.; Bar-Sagi, D. RAS oncogenes: Weaving a tumorigenic web. Nat. Rev. Cancer 2011, 11, 761.
- Quilliam, L.A. Encyclopedia of Biological Chemistry, 2nd ed.; Elsevier Science: Kidlington, UK, 2013; pp. 12–16.
- Simanshu, D.K.; Nissley, D.V.; McCormick, F. RAS proteins and their regulators in human disease. Cell 2017, 170, 17–33.
- Johnson, D.S.; Chen, Y.H. Ras family of small GTPases in immunity and inflammation. Curr. Opin. Pharmacol. 2012, 12, 458–463.
- Cherfils, J.; Zeghouf, M. Regulation of small GTPases by GEFs, GAPs, and GDIs. Physiol. Rev. 2013, 93, 269–309.
- Erickson, K.E.; Rukhlenko, O.S.; Posner, R.G.; Hlavacek, W.S.; Kholodenko, B.N. New insights into RAS biology reinvigorate interest in mathematical modeling of RAS signaling. Semin. Cancer Biol. 2019, 54, 162–173.
- Mishra, A.K.; Lambright, D.G. Invited review: Small GTPases and their GAPs. Biopolymers 2016, 105, 431–448.
- Dror, R.O.; Mildorf, T.J.; Hilger, D.; Manglik, A.; Borhani, D.W.; Arlow, D.H.; Hubbell, W.L. Structural basis for nucleotide exchange in heterotrimeric G proteins. Science 2015, 348, 1361–1365.
- Llavero, F.; Arrazola Sastre, A.; Luque Montoro, M.; Martín, M.A.; Arenas, J.; Lucia, A.; Zugaza, J.L. Small GTPases of the Ras superfamily and glycogen phosphorylase regulation in T cells. Small GTPases 2019, 12, 106–113.
- Hancock, J.F. Ras proteins: Different signals from different locations. Nat. Rev. Mol. Cell Biol. 2003, 4, 373.
- Chavan, T.S.; Muratcioglu, S.; Marszalek, R.; Jang, H.; Keskin, O.; Gursoy, A.; Nussinov, R.; Gaponenko, V. Plasma membrane regulates Ras signaling networks. Cell. Logist. 2016, 5, e1136374.
- Squires, E.J. Hormone and Receptor Structure and Function, 2nd ed.; CABI Publishing: Wallingford, UK, 2010; pp. 1–46.
- Norris, D.O.; Lopez, K.H. The Endocrinology of the Mammalian Ovary; Academic Press: London, UK, 2011; pp. 59–72.
- Krsmanovic, L.Z.; Hu, L.; Leung, P.K.; Feng, H.; Catt, K.J. The hypothalamic GnRH pulse generator: Multiple regulatory mechanisms. Trends Endocrinol. Metab. 2009, 20, 402–408.
- Thompson, I.R.; Kaiser, U.B. GnRH pulse frequency-dependent differential regulation of LH and FSH gene expression. Mol. Cell. Endocrinol. 2014, 385, 28–35.
- Richards, J.S.; Pangas, S.A. The ovary: Basic biology and clinical implications. J. Clin. Invest. 2010, 120, 963–972.
- Jiang, X.; Dias, J.A.; He, X. Structural biology of glycoprotein hormones and their receptors: Insights to signaling. Mol. Cell. Endocrinol. 2014, 382, 424–451.
- Howles, C.M. Role of LH and FSH in ovarian function. Mol. Cell. Endocrinol. 2000, 161, 25–30.
- Gromoll, J.; Simoni, M. Genetic complexity of FSH receptor function. Trends Endocrinol. Metab. 2005, 16, 368–373.
- Ulloa-Aguirre, A.; Reiter, E.; Crepieux, P. FSH receptor signaling: Complexity of interactions and signal diversity. Endocrinology 2018, 159, 3020–3035.
- Rao, C.V.; Lei, Z.M. Consequences of targeted inactivation of LH receptors. Mol. Cell. Endocrinol. 2002, 187, 57–67.
- Lalioti, M.D. Impact of follicle stimulating hormone receptor variants in fertility. Curr. Opin. Obstet. Gynecol. 2011, 23, 158–167.
- Bramble, M.S.; Goldstein, E.H.; Lipson, A.; Ngun, T.; Eskin, A.; Gosschalk, J.E.; Arboleda, V.A. A novel follicle-stimulating hormone receptor mutation causing primary ovarian failure: A fertility application of whole exome sequencing. Hum. Reprod. 2016, 31, 905–914.
- Casarini, L.; Crepieux, P. Molecular mechanisms of action of FSH. Front. Endocrinol. 2019, 10, 305.
- Wei, S.; Shen, X.; Lai, L.; Liang, H.; Deng, Y.; Gong, Z.; Che, T. FSH receptor binding inhibitor impacts K-Ras and c-Myc of ovarian cancer and signal pathway. Oncotarget 2018, 9, 22498.
- Yang, J.; Gong, Z.; Shen, X.; Bai, S.; Bai, X.; Wei, S. FSH receptor binding inhibitor depresses carcinogenesis of ovarian cancer via decreasing levels of K-Ras, c-Myc and FSHR. Anim. Biotechnol. 2021, 32, 84–91.
- Hunzicker-Dunn, M.E.; Lopez-Biladeau, B.; Law, N.C.; Fiedler, S.E.; Carr, D.W.; Maizels, E.T. PKA and GAB2 play central roles in the FSH signaling pathway to PI3K and AKT in ovarian granulosa cells. Proc. Natl. Acad. Sci. USA 2012, 109, E2979–E2988.
- Duggavathi, R.; Murphy, B.D. Ovulation signals. Science 2009, 324, 890–891.
- Su, Y.Q.; Sugiura, K.; Li, Q.; Wigglesworth, K.; Matzuk, M.M.; Eppig, J.J. Mouse oocytes enable LH-induced maturation of the cumulus-oocyte complex via promoting EGF receptor-dependent signaling. Mol. Endocrinol. 2010, 24, 1230–1239.
- Woods, D.C.; Johnson, A.L. Protein kinase C activity mediates LH-induced ErbB/Erk signaling in differentiated hen granulosa cells. Reproduction 2007, 133, 733–741.
- Fan, H.Y.; Liu, Z.; Shimada, M.; Sterneck, E.; Johnson, P.F.; Hedrick, S.M.; Richards, J.S. MAPK3/1 (ERK1/2) in ovarian granulosa cells are essential for female fertility. Science 2009, 324, 938–941.
- Fan, H.Y.; Liu, Z.; Mullany, L.K.; Richards, J.S. Consequences of RAS and MAPK activation in the ovary: The good, the bad and the ugly. Mol. Cell. Endocrinol. 2012, 356, 74–79.
- Richards, J.S.; Ascoli, M. Endocrine, paracrine, and autocrine signaling pathways that regulate ovulation. Trends Endocrinol. Metab. 2018, 29, 313–325.
- Fan, H.Y.; Richards, J.S. Minireview: Physiological and pathological actions of RAS in the ovary. Mol. Endocrinol. 2010, 24, 286–298.
- Davis, J.S.; LaVoie, H.A. The Ovary, 3rd ed.; Academic Press: London, UK, 2019; pp. 237–253.
- Devoto, L.; Henríquez, S.; Kohen, P.; Strauss, J.F. The significance of estradiol metabolites in human corpus luteum physiology. Steroids 2017, 123, 50–54.
- Niswender, G.D.; Juengel, J.L.; Silva, P.J.; Rollyson, M.K.; McIntush, E.W. Mechanisms controlling the function and life span of the corpus luteum. Physiol. Rev. 2000, 80, 1–29.
- Maalouf, S.W.; Smith, C.L.; Pate, J.L. Changes in microRNA expression during maturation of the bovine corpus luteum: Regulation of luteal cell proliferation and function by microRNA-34a. Biol. Reprod. 2016, 94, 71.
- Robinson, R.S.; Woad, K.J.; Hunter, M.G.; Sinclair, K.D.; Laird, M.; Joseph, C.; Mann, G.E. Corpus luteum development and angiogenesis. Bioscientifica Proc. 2019, 8, 327–343.
- Wiltbank, M.C.; Meidan, R.; Ochoa, J.; Baez, G.M.; Giordano, J.O.; Ferreira, J.C.P.; Sartori, R. Maintenance or regression of the corpus luteum during multiple decisive periods of bovine pregnancy. Anim. Reprod. 2018, 13, 217–233.
- Woad, K.J.; Robinson, R.S. Luteal angiogenesis and its control. Theriogenology 2016, 86, 221–228.
- Galvão, A.M.; Ferreira-Dias, G.; Skarzynski, D.J. Cytokines and angiogenesis in the corpus luteum. Mediat. Inflamm. 2013, 2013, 420186.
- Robinson, R.S. The critical importance of ovarian angiogenesis. Reprod. Fertil. Dev. 2013, 25, 3–5.
- Kaessmeyer, S.; Huenigen, H.; Al Masri, S.; Dieckhoefer, P.; Richardson, K.; Plendl, J. Corpus luteal angiogenesis in a high milk production dairy breed differs from that of cattle with lower milk production levels. Vet. Med. 2016, 61, 497–503.
- Robinson, R.S.; Woad, K.J.; Hammond, A.J.; Laird, M.; Hunter, M.G.; Mann, G.E. Angiogenesis and vascular function in the ovary. Reproduction 2009, 138, 869–881.
- Meidan, R.; Klipper, E.; Zalman, Y.; Yalu, R. The role of hypoxia-induced genes in ovarian angiogenesis. Reprod. Fertil. Dev. 2013, 25, 343–350.
- Folkman, J.; Klagsbrun, M. Angiogenic factors. Science 1987, 235, 442–447.
- Tamanini, C.; De Ambrogi, M. Angiogenesis in developing follicle and corpus luteum. Reprod. Domest. Anim. 2004, 39, 206–216.
- Ferrara, N.; Gerber, H.P.; LeCouter, J. The biology of VEGF and its receptors. Nat. Med. 2003, 9, 669.
- Mishra, S.R.; Parmar, M.S.; Chouhan, V.S.; Rajesh, G.; Yadav, V.P.; Bharti, M.K.; Dangi, S.S. Expression and localization of fibroblast growth factor (FGF) family in corpus luteum during different stages of estrous cycle and synergistic role of FGF2 and vascular endothelial growth factor (VEGF) on steroidogenesis, angiogenesis and survivability of cultured buffalo luteal cells. Agri. Gene 2016, 1, 53–68.
- Simons, M.; Gordon, E.; Claesson-Welsh, L. Mechanisms and regulation of endothelial VEGF receptor signalling. Nat. Rev. Mol. Cell Biol. 2016, 17, 611–625.
- Schams, D.; Berisha, B. Regulation of corpus luteum function in cattle–an overview. Reprod. Domest. Anim. 2004, 39, 241–251.
- Lu, E.; Li, C.; Wang, J.; Zhang, C. Inflammation and angiogenesis in the corpus luteum. J. Obstet. Gynaecol. Res. 2019, 45, 1967–1974.
- Duncan, W.C.; Myers, M.; Dickinson, R.E.; Van Den Driesche, S.; Fraser, H.M. Paracrine regulation of luteal development and luteolysis in the primate. Anim. Reprod. 2018, 6, 34–46.
- Berisha, B.; Schams, D.; Rodler, D.; Sinowatz, F.; Pfaffl, M.W. Expression pattern of HIF 1alpha and vasohibins during follicle maturation and corpus luteum function in the bovine ovary. Reprod. Domest. Anim. 2017, 52, 130–139.
- Meidan, R.; Girsh, E.; Mamluk, R.; Levy, N.; Farberov, S. The Life Cycle of the Corpus Luteum; Springer Cham: Basingstoke, UK, 2017; pp. 159–182.
- Hojo, T.; Piotrowska-Tomala, K.K.; Jonczyk, A.W.; Lukasik, K.; Jankowska, K.; Okuda, K.; Skarzynski, D.J. Receptor interacting protein kinases-dependent necroptosis as a new, potent mechanism for elimination of the endothelial cells during luteolysis in cow. Theriogenology 2019, 128, 193–200.
- Li, W.; Liang, R.R.; Zhou, C.; Wu, M.Y.; Lian, L.; Yuan, G.F.; Chen, K. The association between expressions of Ras and CD68 in the angiogenesis of breast cancers. Cancer Cell Int. 2015, 15, 17.
- Wei, F.; Liu, Y.; Bellail, A.C.; Olson, J.J.; Sun, S.Y.; Lu, G.; Hao, C. K-Ras mutation-mediated IGF-1-induced feedback ERK activation contributes to the rapalog resistance in pancreatic ductal adenocarcinomas. Cancer Lett. 2012, 322, 58–69.
- Katoh, M.; Nakagama, H. FGF receptors: Cancer biology and therapeutics. Med. Res. Rev. 2014, 34, 280–300.
- Hardy, K.M.; Yatskievych, T.A.; Konieczka, J.H.; Bobbs, A.S.; Antin, P.B. FGF signalling through RAS/MAPK and PI3K pathways regulates cell movement and gene expression in the chicken primitive streak without affecting E-cadherin expression. BMC Dev. Biol. 2011, 11, 20.
- Watson, E.D.; Fraser, H.M. Angiogenesis and vascular endothelial growth factor expression in the equine corpus luteum. Reproduction 2003, 125, 259–270.
- Maia, V.N.; Batista, A.M.; Neto, S.C.; Silva, D.M.; Adrião, M.; Wischral, A. Expression of angiogenic factors and luteinizing hormone receptors in the corpus luteum of mares induced to ovulate with deslorelin acetate. Theriogenology 2016, 85, 461–465.
- Schams, D.; Berisha, B. Angiogenic factors in the bovine corpus luteum. J. Reprod. Dev. 2002, 48, 233–242.
- Boonyaprakob, U.; Gadsby, J.E.; Hedgpeth, V.; Routh, P.A.; Almond, G.W. Expression and localization of hypoxia inducible factor-1alpha mRNA in the porcine ovary. Can. J. Vet. Res. 2005, 69, 215–222.
- Wu, L.; Zhang, Z.; Pan, X.; Wang, Z. Expression and contribution of the HIF-1α/VEGF signaling pathway to luteal development and function in pregnant rats. Mol. Med. Rep. 2015, 12, 7153–7159.
- Tanaka, J.; Acosta, T.J.; Berisha, B.; Tetsuka, M.; Matsui, M.; Kobayashi, S.; Miyamoto, A. Relative changes in mRNA expression of angiopoietins and receptors tie in bovine corpus luteum during estrous cycle and prostaglandin F2α-induced luteolysis: A possible mechanism for the initiation of luteal regression. J. Reprod. Dev. 2004, 50, 619–626.
- Stocco, C.; Telleria, C.; Gibori, G. The molecular control of corpus luteum formation, function, and regression. Endocr. Rev. 2007, 28, 117–149.
- Skarzynski, D.J.; Ferreira-Dias, G.; Okuda, K. Regulation of luteal function and corpus luteum regression in cows: Hormonal control, immune mechanisms and intercellular communication. Reprod. Domest. Anim. 2008, 43, 57–65.
- Miyamoto, A.; Shirasuna, K. Luteolysis in the cow: A novel concept of vasoactive molecules. Anim. Reprod. 2009, 6, 47–59.
- Neuvians, T.P.; Berisha, B.; Schams, D. Vascular endothelial growth factor (VEGF) and fibroblast growth factor (FGF) expression during induced luteolysis in the bovine corpus luteum. Mol. Reprod. Dev. 2004, 67, 389–395.
- Pan, X.Y.; Zhang, Z.H.; Wu, L.X.; Wang, Z.C. Effect of HIF-1a/VEGF signaling pathway on plasma progesterone and ovarian prostaglandin F. Genet. Mol. Res. 2015, 14, 8796–8809.
- Vonnahme, K.A.; Redmer, D.A.; Borowczyk, E.; Bilski, J.J.; Luther, J.S.; Johnson, M.L.; Grazul-Bilska, A.T. Vascular composition, apoptosis, and expression of angiogenic factors in the corpus luteum during prostaglandin F2α-induced regression in sheep. Reproduction 2006, 131, 1115–1126.
- Neuvians, T.P.; Pfaffl, M.W.; Berisha, B.; Schams, D. The mRNA expression of the members of the IGF-system in bovine corpus luteum during induced luteolysis. Domest. Anim. Endocrinol. 2003, 25, 359–372.
- Berisha, B.; Meyer, H.H.; Schams, D. Effect of prostaglandin F2 alpha on local luteotropic and angiogenic factors during induced functional luteolysis in the bovine corpus luteum. Biol. Reprod. 2010, 82, 940–947.
- Moura, M.M.; Cavaco, B.M.; Leite, V. RAS proto-oncogene in medullary thyroid carcinoma. Endocr. Relat. Cancer 2015, 22, R235–R252.
- Kranenburg, O.; Gebbink, M.F.; Voest, E.E. Stimulation of angiogenesis by Ras proteins. Biochim. Biophys. Acta-Rev. Cancer 2004, 1654, 23–37.
- Karar, J.; Maity, A. PI3K/AKT/mTOR pathway in angiogenesis. Front. Mol. Neurosci. 2011, 4, 51.
- Yoshikawa, Y.; Takano, O.; Kato, I.; Takahashi, Y.; Shima, F.; Kataoka, T. Ras inhibitors display an anti-metastatic effect by downregulation of lysyl oxidase through inhibition of the Ras-PI3K-Akt-HIF-1α pathway. Cancer let. 2017, 410, 82–91.
- Larcher, F.; Franco, M.; Bolontrade, M.; Rodriguez-Puebla, M.; Casanova, L.; Navarro, M.; Conti, C.J. Modulation of the angiogenesis response through Ha-ras control, placenta growth factor, and angiopoietin expression in mouse skin carcinogenesis. Mol. Carcinogen. 2003, 37, 83–90.
- Sawada, J.; Urakami, T.; Li, F.; Urakami, A.; Zhu, W.; Fukuda, M.; Komatsu, M. Small GTPase R-Ras regulates integrity and functionality of tumor blood vessels. Cancer Cell 2012, 22, 235–249.
- Ii, M.; Li, H.; Adachi, Y.; Yamamoto, H.; Ohashi, H.; Taniguchi, H.; Shinomura, Y. The efficacy of IGF-I receptor monoclonal antibody against human gastrointestinal carcinomas is independent of k-ras mutation status. Clin. Cancer Res. 2011, 17, 5048–5059.
- Lee, S.H.; Lee, S. Change of Ras and its guanosine triphosphatases during development and regression in bovine corpus luteum. Theriogenology 2020, 144, 16–26.




