Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Muhammad Ahmad | + 1690 word(s) | 1690 | 2021-06-07 11:53:46 | | | |
| 2 | Rita Xu | Meta information modification | 1690 | 2021-06-10 10:47:30 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Ahmad, M. Therapeutic Attributes of Endocannabinoid System. Encyclopedia. Available online: https://encyclopedia.pub/entry/10706 (accessed on 04 March 2026).
Ahmad M. Therapeutic Attributes of Endocannabinoid System. Encyclopedia. Available at: https://encyclopedia.pub/entry/10706. Accessed March 04, 2026.
Ahmad, Muhammad. "Therapeutic Attributes of Endocannabinoid System" Encyclopedia, https://encyclopedia.pub/entry/10706 (accessed March 04, 2026).
Ahmad, M. (2021, June 10). Therapeutic Attributes of Endocannabinoid System. In Encyclopedia. https://encyclopedia.pub/entry/10706
Ahmad, Muhammad. "Therapeutic Attributes of Endocannabinoid System." Encyclopedia. Web. 10 June, 2021.
Copy Citation
In humans, various sites like cannabinoid receptors (CBR) having a binding affinity with cannabinoids are distributed on the surface of different cell types, where endocannabinoids (ECs) and derivatives of fatty acid can bind. The binding of these substance(s) triggers the activation of specific receptors required for various physiological functions, including pain sensation, memory, and appetite.
endocannabinoid system
CB1 and CB2 receptors
cannabis
cancer
1. Introduction—Problem and Opportunities
The endocannabinoid system (ECS) is composed of endocannabinoids (ECs), associated receptors of cannabinoid, and metabolizing enzymes. ECs are endogenous lipid-based retrograde neurotransmitters in a biological system. They are bound to cannabinoid receptors (CBR), and cannabinoid receptor proteins are expressed via the vertebrate central nervous system (CNS) and peripheral nervous system. Cannabinoids are known as a group of terpene phenolic compounds and found in the Cannabis sativa (marijuana plant). Commonly, three types of cannabinoids are identified: (i) phytocannabinoids observed distinctively in the Cannabis sativa plant, (ii) endogenous cannabinoids found in mammals (i.e., humans and animals), and (iii) laboratory-based cannabinoids (i.e., synthetic) [1][2][3][4]. Living organisms respond to complex stimuli, and an evolutionarily conserved form of ECS exists from plants to mammals. The cannabinoids (over 80) are produced from Cannabis sativa. Their broad-spectrum characterization classifies them as an assembly of substances with a substantial structural correlation with Delta-9-tetrahydrocannabinol (Δ9-THC) and binds to the CBR. Marijuana is a primary active component of Cannabis sativa, which has been found highly effective to treat wide-ranging syndrome in patients with cancers, AIDS, CNS disorders (i.e., multiple sclerosis). Moreover, glaucoma is also included in the list of treatments by those who believed in the medicinal aspects of marijuana [5][6][7][8][9]. The chemistry of these substances shows various classes of particular chemicals, such as the close structural similarity of classical cannabinoids to the Δ9-THC, non-classical categorized cannabinoids, the aryl sulphonamides, the ECs related eicosanoids, the aminoalkylindoles, and the quinoles [10][11]. There are additional compounds not categorized into these standard classes due to specific physicochemical characteristics, even though those exhibit the binding affinity to CBR (CB1 and CB2). Multi-dimensional characterization of marijuana on their potential medical effects can be selected during the evaluation parameters of marijuana and cannabinoids concurrence of specific human diseases, with fewer side effects. In the previous decades, the endocannabinoid pathway and the physiological impacts of cannabinoids have been studied extensively. Cannabinoids exhibit immunomodulatory effects, and their application, along with prospective roles as an autoimmune or inflammatory therapy, has widely been explored [12].
2. The Endocannabinoid System
An ECS comprises endogenous ligands, associated CBR (particularly CB1 and CB2), and metabolic enzymes. Endocannabinoid receptors were named CBR after the recognition of endogenous ligands. The ECs are obtained from the membrane that is composed of phospholipids. Therefore, they are known as bioactive lipid mediators. After the identification of the first lipid mediator, arachidonoyl-ethanolamide (AEA) of the ECS, (also known as anandamide) [13][14][15], different biomolecules associated with this family were discovered. The most vital molecules are 2-arachidonoyl-glycerol (2AG) and its isomer 1AG among monoacyl-glycerols; palmitoyl-ethanolamide (PEA); oleyl-ethanolamide (OEA), and the N-acyl-ethanolamides [16][17][18]. The cannabinoid receptor type-1 and type-2, both 2AG and AEA are engaged in different biological functions; however, the AEA metabolism and attachment of the peroxisome proliferator-activated receptor-α is influenced by PEA and OEA [8][19][20][21][22][23]. These all biomolecules are explained in detail in the endocannabinoid related compounds. Partial or full agonists of CB1 receptors in terms of anandamide depend on tissue and biological response type. However, CB2 receptors can attach but have an intermittent effect and can perform like an antagonist [19][20][24].
2.1. Cannabinoids Receptor Agonists
Cannabinoids receptors can be classified into four groups based on different chemical structures named as (i) classical (ii) non-classical (iii) aminoalkylindole (AAIs), and (iv) eicosanoid compounds. These groups are mostly heterogeneous [16][17]. The phytocannabinoids (Δ9-THC, Δ8-THC, cannabinol) and associated analogs (i.e., synthetic) form the classical group. O-arachidonoylethanolamine (virodhamine), arachidonoylethanolamide (anandamide), 2-Arachidonoyl Glycerol (2-AG), and other anandamide associated synthetic analogs/derivatives are present in the eicosanoid group. A cellular system of an organism usually produces the majority of ECs found in the eicosanoid group. Non-classical and AAIs groups contain synthetic cannabinoids. Both CB1 and CB2 receptors are the chief receptors of the ECS, and each of them possesses a different affinity of endocannabinoid agonists. For instance, the CB1 receptor has a greater affinity of Δ9-THC, and without selective marking of CB1/CB2 receptor(s), the phytocannabinoid cannabinol possesses partial affinity (Figure 1) [24].
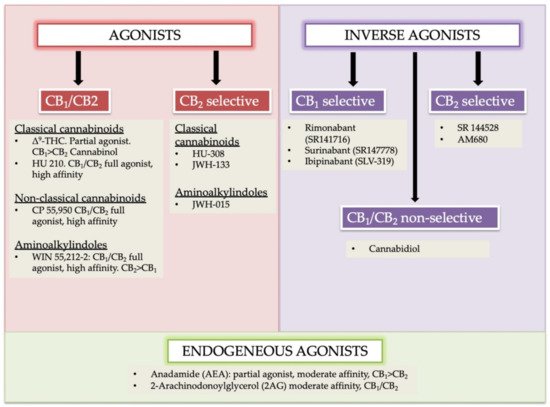
Figure 1. Agonists, antagonists, and endogenous agonist cannabinoids and their sub-types. Endogenous agonists: AEA and 2-AG; Exogenous agonist Δ9-tetrahydrocannabinol (Δ9-THC). The key psychoactive cannabis module, synthetic derivatives, HU 210, HU 308, CP 55,940 are identified. These compounds are commonly used as pharmacologic tools. Modified and reprinted from Ref. [24] with permission from Elsevier. License Number: 5066110366934. Abbreviation: CB1 (cannabinoid receptor 1); CB2 (cannabinoid receptor 2); HU-210, (highly potent cannabinoid receptor agonist); JWH-015 (a selective CB2 agonist); JWH-133 (a potent selective CB2 agonist); SR141716 (Rimonabant, a selective CB1 receptor antagonist or an inverse agonist); SR141716 (Rimonabant, a selective CB1 receptor antagonist or an inverse agonist); HU-308 (cannabidiol (CBD)-derivative drug); SLV 319 (a potent and selective CB1 receptor antagonist); CP 55,950 (a synthetic cannabinoid).
2.2. Cannabinoids Receptors CB1 and CB2 and Functional Pathway
Recognized CBR CB1 and CB2 belong to the structural membrane receptors and family of G protein-coupled receptors. They also have seven transmembrane spanning domains. Limiting cellular response towards the specific cannabinoid receptor ligands, the effect of partial agonism is variable from its binding, and thereby inverse agonism to its functional selectivity plays a crucial role [25]. The functional influence of both CB1 and CB2 receptors is acquired when heterotrimeric Gi/o (a subunit of G protein) proteins are coupled. However, the activated CB1 receptors perform their functions due to G alpha i/o activation [26]. The inhibition of the adenylate cyclase enzyme synthesis is due to the attachment of CB1 to its agonist. The binding of CB1 to its ligand decreases the levels of cAMP and the elevated level of mitogen-activated protein kinase (MAPKs). Moreover, in few cases, the activated CB1 receptor corresponding to Gs proteins may accelerate adenylate cyclase cAMP [25][26][27][28].
In the cell membrane, both receptors (i.e., CB1 and CB2) and various ion channels, such as the potassium and calcium channels, are influenced positively because of independent activity. These ionic channels are activated when the cAMP-dependent interaction takes place between the molecules and the receptor. These molecules are recognized as protein kinase C, protein kinase A (PKA), p38, Raf-1, extracellular regulated kinase (ERK), N-terminal kinase (JNK), and c-fos, c-Jun [27][28]. The CB1 activation causes the lowering of the Ca2+ ions entry in the cell, which is the key factor for releasing neurotransmitters without cAMP association and results in reduced secretions of neurotransmitters. Therefore, a pre-synaptic receptor (CB1 receptor), when activated in a dose-dependent manner, leads to neurotransmitter release [29][30][31]. The regulation of the phosphorylation process and activation of various entities of the family of MAPKs, MAP kinase p38, and c-Jun can be performed through using both receptors. Consequently, JNK MAPK directs cell adhesion, proliferation, motility, and apoptosis. Glucose metabolism is also linked to gene expression [30][32][33][34]. Both receptors CB1 and CB2 that also respond via the stimulation of their agonists (exogenous/endogenous/synthetic). Agonist molecules are instantly deactivated when transported/uptake into cells, followed by their release and metabolic function. Both anandamide and 2-AG perform metabolism due to their enzymatic hydrolysis characteristics, and this process is carried out in combined activity of fatty acid amide hydrolase enzyme (FAAH) [35][36]. Furthermore, additional metabolic activities require monoglyceride lipase for the hydrolysis of 2-AG [37][38]. Figure 2 illustrates the summary of potential mechanisms of action [24].
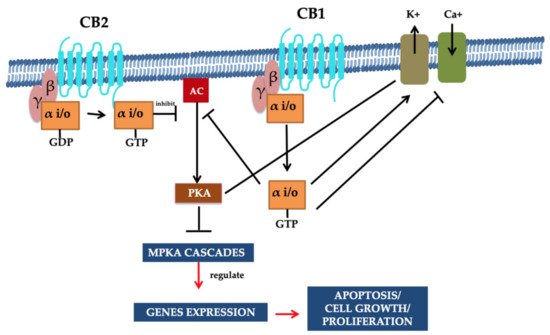
Figure 2. Signaling pathways of CB1 and CB2 receptors. G proteins are associated with CBR (i.e., CB1 and CB2). The inhibition of adenylyl cyclase activity and the stimulation of the variant MAPK cascades were demonstrated through these receptors’ activation. Moreover, the CB1 cannabinoid receptor facilitates the regulation of the voltage gated Ca2+ channels as they are negatively regulated, and inwardly resolving K+ channels are positively regulated. Intracellular free Ca2+ increase is prompted through phospholipase C (PLC) activation. The inhibition of gene expression is facilitated by the PKA, and reduction in cAMP directs cannabinoid-mediated inhibition. It is resulting in the MAPK cascade activation associated with cell survival or apoptosis. Such signaling pathways/mechanisms are associated with the multiple functions of the cells that are regulated through CBR. Reprinted from Ref. [24] with permission from Elsevier. License Number: 5066110366934.
In addition to a few non-neuronal cells, central and peripheral neurons defined CB1 receptors [8][39]. The heterogeneous distribution of CB1 receptors is found among the CNS that aid the functional activities. Mainly all the functional activities controlling parts of the brain such as the cerebral cortex, entopeduncular nucleus, substantia nigrapars reticulate, hippocampus, caudate putamen, globuspallidus, cerebellum, and other areas of the brain and spinal cord possess the dense distribution CB1 receptors. The presence of CB1 receptors helps in processing or controlling the nociceptive information. Agonists’ ability associated with the CB1 receptor could be categorized using the distribution pattern of CB1 receptor among the CNS to impair cognition memory. Moreover, their potential role in changing the motor function and the development of an anti-nociception are also studied [40][41][42][43][44]. The terminal end of the central and peripheral nerves contains few CB1 receptors and is involved in controlling the release of excitatory and inhibitory neurotransmitters [8][45]. Primarily CB2 receptors found on immune cells are well-characterized and play a key role in immunomodulation [37][46][47]. Chemical messengers releasing capability is shared between CB1 and CB2 receptors. Initially, at the CNS, cannabinoids act with multiple neurotransmitters and participate in the modulation of their release through the function of the CB1 receptor [48][49] (Figure 3). Secondly, inflammatory cytokines release, and the immune system regulation require the prospective activity of CB2 receptors. In the following section, we have highlighted other types of CBR belonging to the ECS.
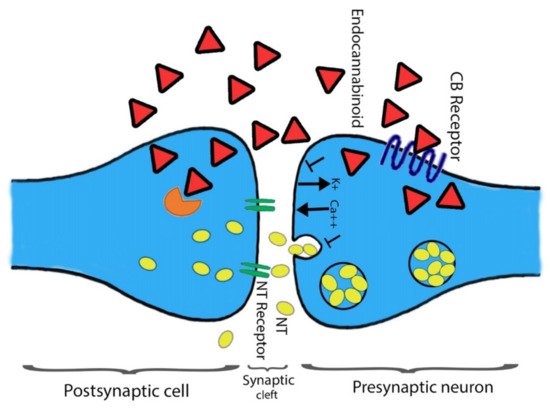
Figure 3. The action(s) of cannabinoids at the site of the presynaptic terminal. Agonists of a cannabinoid such as THC, 2-AG, and AEA attach to the CB1 receptor. The attachment is prompting alteration in intracellular levels of Ca2+ and K+ ions. Consequently, direct towards secretion of neurotransmitter blockage at the site of presynaptic neurons. At the postsynaptic neuron site, cannabinoids are devastated through the FAAH enzyme, and the respective metabolites are reused. Shapes representation: Red triangle for endocannabinoid; yellow oval spots for neurotransmitters; Orange color shape represents FAAH.
References
- Morales, P.; Goya, P.; Jagerovic, N.; Hernandez-Folgado, L. Allosteric modulators of the CB1 cannabinoid receptor: A structural update review. Cannabis Cannabinoid Res. 2016, 1, 22–30.
- Glass, M.; Govindpani, K.; Furkert, D.P.; Hurst, D.P.; Reggio, P.H.; Flanagan, J.U. One for the price of two… are bivalent ligands targeting cannabinoid receptor dimers capable of simultaneously binding to both receptors? Trends Pharmacol. Sci. 2016, 37, 353–363.
- Khan, M.I.; Soboci, A.; Czarnecka, A.; Król, M.; Botta, B.; Szczylik, C. The therapeutic aspects of the endocannabinoid system (ECS) for cancer and their development: From nature to laboratory. Curr. Pharm. Des. 2016, 22, 1756–1766.
- Tomko, A.M.; Whynot, E.G.; Ellis, L.D.; Dupré, D.J. Anti-cancer potential of cannabinoids, terpenes, and flavonoids present in cannabis. Cancers 2020, 12, 1985.
- Iannotti, F.A.; Di Marzo, V.; Petrosino, S. Endocannabinoids and endocannabinoid-related mediators: Targets, metabolism and role in neurological disorders. Prog. Lipid Res. 2016, 62, 107–128.
- Popp, J.R.; Petrakis, E.A.; Angelis, A.; Halabalaki, M.; Bonn, G.K.; Stuppner, H.; Skaltsounis, L.A. Rapid isolation of acidic cannabinoids from Cannabis sativa L. using pH-zone-refining centrifugal partition chromatography. J. Chromatogr. A 2019, 1599, 196–202.
- Atakan, Z. Cannabis, a complex plant: Different compounds and different effects on individuals. Ther. Adv. Psychopharmacol. 2012, 2, 241–254.
- Zou, S.; Kumar, U. Cannabinoid Receptors and the Endocannabinoid System: Signaling and Function in the Central Nervous System. Int. J. Mol. Sci. 2018, 19, 833.
- Kalueff, A.V.; Stewart, A.M.; Song, C.; Berridge, K.C.; Graybiel, A.M.; Fentress, J.C. Neurobiology of rodent self-grooming and its value for translational neuroscience. Nat. Rev. Neurosci. 2016, 17, 45.
- Palmer, S.L.; Thakur, G.A.; Makriyannis, A. Cannabinergic ligands. Chem. Phys. Lipids 2002, 121, 3–19.
- Console-Bram, L.; Marcu, J.; Abood, M.E. Cannabinoid receptors: Nomenclature and pharmacological principles. Prog. Neuro Psychopharmacol. Biol. Psychiatry 2012, 38, 4–15.
- Moreno, E.; Cavic, M.; Krivokuca, A.; Casadó, V.; Canela, E. The Endocannabinoid System as a Target in Cancer Diseases: Are We There Yet? Front. Pharmacol. 2019, 10, 339.
- Boorman, E.; Zajkowska, Z.; Ahmed, R.; Pariante, C.M.; Zunszain, P.A. Crosstalk between endocannabinoid and immune systems: A potential dysregulation in depression? Psychopharmacology 2016, 233, 1591–1604.
- Bydalek, S. The Synthesis and Characterization of 2-Arachidonoyl Glycerol. Master’s Thesis, University of Northern Colorado, Greeley, CO, USA, 2019.
- Stensson, N. Endocannabinoids and Related Lipids in Chronic Pain: Analytical and Clinical Aspects. Ph.D. Thesis, Linköping University Electronic Press, Linköping, Sweden, 2018.
- Morell, C.; Bort, A.; Vara, D.; Ramos-Torres, A.; Rodriguez-Henche, N.; Diaz-Laviada, I. The cannabinoid WIN 55,212-2 prevents neuroendocrine differentiation of LNCaP prostate cancer cells. Prostate Cancer Prostatic Dis. 2016, 19, 248.
- Boroughs, L.K.; DeBerardinis, R.J. Metabolic pathways promoting cancer cell survival and growth. Nat. Cell Biol. 2015, 17, 351–359.
- Hansen, H.; Schmid, P.; Bittigau, P.; Lastres-Becker, I.; Berrendero, F.; Manzanares, J.; Ikonomidou, C.; Schmid, H.; Fernández-Ruiz, J.; Hansen, H. Anandamide, but not 2-arachidonylglycerol, accumulates during in vivo neurodegeneration. J. Neurochem. 2001, 78, 1415–1427.
- Spruston, N.; Stuart, G.; Häusser, M. Principles of dendritic integration. Dendrites 2016, 351, 597.
- Di Marzo, V.; Stella, N.; Zimmer, A. Endocannabinoid signalling and the deteriorating brain. Nat. Rev. Neurosci. 2015, 16, 30–42.
- Soderstrom, K.; Soliman, E.; van Dross, R. Cannabinoids Modulate Neuronal Activity and Cancer by CB1 and CB2 Receptor-Independent Mechanisms. Front. Pharmacol. 2019, 8, 20.
- Buser, G.L.; Gerona, R.R.; Horowitz, B.Z.; Vian, K.P.; Troxell, M.L.; Hendrickson, R.G.; Houghton, D.C.; Rozansky, D.; Su, S.W.; Leman, R.F. Acute kidney injury associated with smoking synthetic cannabinoid. Clin. Toxicol. 2014, 52, 664–673.
- Gatch, M.B.; Forster, M.J. Δ9-Tetrahydrocannabinol-like effects of novel synthetic cannabinoids found on the gray market. Behav. Pharmacol. 2015, 26, 460.
- Katchan, V.; David, P.; Shoenfeld, Y. Cannabinoids and autoimmune diseases: A systematic review. Autoimmun. Rev. 2016, 15, 513–528.
- Maroteaux, L.; Béchade, C.; Roumier, A. Dimers of serotonin receptors: Impact on ligand affinity and signaling. Biochimie 2019, 161, 23–33.
- de Oliveira, P.G.; Ramos, M.L.S.; Amaro, A.J.; Dias, R.A.; Vieira, S.I. Gi/o-Protein Coupled Receptors in the Aging Brain. Front. Aging Neurosci. 2019, 11.
- Slominski, A.T.; Li, W.; Kim, T.-K.; Semak, I.; Wang, J.; Zjawiony, J.K.; Tuckey, R.C. Novel activities of CYP11A1 and their potential physiological significance. J. Steroid Biochem. Mol. Biol. 2015, 151, 25–37.
- Lee, J.; Jung, M.; Park, H.; Kim, K.; Cho, D. Erdr1 suppresses murine melanoma growth via regulation of apoptosis. Int. J. Mol. Sci. 2016, 17, 107.
- Mecha, M.; Feliú, A.; Carrillo-Salinas, F.J.; Rueda-Zubiaurre, A.; Ortega-Gutiérrez, S.; de Sola, R.G.; Guaza, C. Endocannabinoids drive the acquisition of an alternative phenotype in microglia. Brain. Behav. Immun. 2015, 49, 233–245.
- Fraga, D.; Zanoni, C.I.S.; Zampronio, A.R.; Parada, C.A.; Rae, G.A.; Souza, G.E.P. Endocannabinoids, through opioids and prostaglandins, contribute to fever induced by key pyrogenic mediators. Brain. Behav. Immun. 2016, 51, 204–211.
- Li, X.; Peng, X.-Q.; Jordan, C.J.; Li, J.; Bi, G.-H.; He, Y.; Yang, H.-J.; Zhang, H.-Y.; Gardner, E.L.; Xi, Z.-X. mGluR5 antagonism inhibits cocaine reinforcement and relapse by elevation of extracellular glutamate in the nucleus accumbens via a CB1 receptor mechanism. Sci. Rep. 2018, 8.
- Abrams, D.I.; Guzman, M. Cannabis in cancer care. Clin. Pharmacol. Ther. 2015, 97, 575–586.
- Moranta Mesquida, D.; Esteban Valdés, S.C.; Garcia-Sevilla, J.A. Acute, chronic and withdrawal effects of the cannabinoid receptor agonist WIN55212-2 on the sequential activation of MAPK/Raf-MEK-ERK signaling in the rat cerebral frontal cortex: Short-term regulation by intrinsic and extrinsic pathways. J. Neurosci. Res. 2018, 85, 656–667.
- Alexandre, J.; Carmo, H.; Carvalho, F.; Silva, J.P. Synthetic cannabinoids and their impact on neurodevelopmental processes. Addict. Biol. 2019, 25, e12824.
- Kaya, M.C.; Bulut, M.; Kaplan, İ.; Gunes, M. Levels of endocannabinoid metabolizing enzymes are not related with BDNF levels in patients with schizophrenia: A case-controlled study. Psychiatry Clin. Psychopharmacol. 2018, 29, 441–445.
- Dempsey, S.K.; Gesseck, A.M.; Ahmad, A.; Daneva, Z.; Ritter, J.K.; Poklis, J.L. Formation of HETE-EAs and dihydroxy derivatives in mouse kidney tissue and analysis by high-performance liquid chromatography tandem mass spectrometry. J. Chromatogr. B 2019, 1126, 121748.
- Iuvone, T.; Affaitati, G.; de Filippis, D.; Lopopolo, M.; Grassia, G.; Lapenna, D.; Negro, L.; Costantini, R.; Vaia, M.; Cipollone, F. Ultramicronized palmitoylethanolamide reduces viscerovisceral hyperalgesia in a rat model of endometriosis plus ureteral calculosis: Role of mast cells. Pain 2016, 157, 80–91.
- Gil-Ordóñez, A.; Martín-Fontecha, M.; Ortega-Gutiérrez, S.; López-Rodríguez, M.L. Monoacylglycerol lipase (MAGL) as a promising therapeutic target. Biochem. Pharmacol. 2018, 157, 18–32.
- Uhelski, M.L.; Khasabova, I.; Simone, D.A. Modulation of Pain by Endocannabinoids in the Periphery. In Recent Advances in Cannabinoid Research; IntechOpen: London, UK, 2018; Volume 13.
- Iversen, L. Cannabis and the brain. Brain 2003, 126, 1252–1270.
- Pertwee, R.G. Pharmacology of cannabinoid CB 1 and CB 2 receptors. Pharmacol. Ther. 1997, 74, 129–180.
- Pertwee, R.G. Pharmacological actions of cannabinoids. In Cannabinoids; Springer: Berlin/Heidelberg, Germany, 2005; pp. 1–51.
- Pertwee, R.G.; Gibson, T.M.; Stevenson, L.A.; Ross, R.A.; Banner, W.K.; Saha, B.; Razdan, R.K.; Martin, B.R. O-1057, a potent water-soluble cannabinoid receptor agonist with antinociceptive properties. Br. J. Pharmacol. 2000, 129, 1577–1584.
- Zhang, M.W.; Ho, R. The cannabis dilemma: A review of its associated risks and clinical efficacy. J. Addict. 2015, 2015, 707596.
- Lötsch, J.; Weyer-Menkhoff, I.; Tegeder, I. Current evidence of cannabinoid-based analgesia obtained in preclinical and human experimental settings. Eur. J. Pain 2018, 22, 471–484.
- Lomazzo, E.; Bindila, L.; Remmers, F.; Lerner, R.; Schwitter, C.; Hoheisel, U.; Lutz, B. Therapeutic potential of inhibitors of endocannabinoid degradation for the treatment of stress-related hyperalgesia in an animal model of chronic pain. Neuropsychopharmacology 2015, 40, 488.
- Martín-Fontecha, M.; Angelina, A.; Rückert, B.; Rueda-Zubiaurre, A.; Martín-Cruz, L.; van de Veen, W.; Akdis, M.; Ortega-Gutiérrez, S.; López-Rodríguez, M.L.; Akdis, C.A.; et al. A Fluorescent Probe to Unravel Functional Features of Cannabinoid Receptor CB1 in Human Blood and Tonsil Immune System Cells. Bioconjugate Chem. 2019, 29, 382–389.
- Marsicano, G.; Moosmann, B.; Hermann, H.; Lutz, B.; Behl, C. Neuroprotective properties of cannabinoids against oxidative stress: Role of the cannabinoid receptor CB1. J. Neurochem. 2002, 80, 448–456.
- Puri, B.; Hall, A.; Ho, R. Revision Notes in Psychiatry; CRC Press: Boca Raton, FL, USA, 2013; ISBN 1444170147.
More
Information
Subjects:
Medicine, Research & Experimental
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.2K
Revisions:
2 times
(View History)
Update Date:
10 Jun 2021
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





