Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Luminita Duma | + 2581 word(s) | 2581 | 2021-06-04 11:20:30 | | | |
| 2 | Dean Liu | -10 word(s) | 2571 | 2021-06-10 08:23:46 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Duma, L.; Gonzato, C. Molecularly Imprinted Polymers. Encyclopedia. Available online: https://encyclopedia.pub/entry/10695 (accessed on 05 March 2026).
Duma L, Gonzato C. Molecularly Imprinted Polymers. Encyclopedia. Available at: https://encyclopedia.pub/entry/10695. Accessed March 05, 2026.
Duma, Luminita, Carlo Gonzato. "Molecularly Imprinted Polymers" Encyclopedia, https://encyclopedia.pub/entry/10695 (accessed March 05, 2026).
Duma, L., & Gonzato, C. (2021, June 09). Molecularly Imprinted Polymers. In Encyclopedia. https://encyclopedia.pub/entry/10695
Duma, Luminita and Carlo Gonzato. "Molecularly Imprinted Polymers." Encyclopedia. Web. 09 June, 2021.
Copy Citation
Molecularly imprinted polymers (MIPs) are synthetic recognition materials obtained by the polymerisation of functional and cross-linking monomers in the presence of a template. MIPs are attractive not only for their recognition properties that are close to those of natural receptors and their availability for a wide range of targets but also for their superior chemical and physical stability compared to biological receptors.
molecularly imprinted polymers
chemosensors
sensors
1. Introduction
Molecularly imprinted polymers (MIPs) have seen a continuous development as sensing elements in bio-/chemo-sensors since the late 1990s [1][2][3][4][5][6]. MIPs are attractive not only for their recognition properties that are close to those of natural receptors and their availability for a wide range of targets but also for their superior chemical and physical stability compared to biological receptors. These advantages have led to the application of MIPs in fields as various as immunoassays, separation, depollution, sensing, cell imaging, and therapy [7][8][9]. The imprinting process consists of polymerising functional and cross-linking monomers in the presence of a template. The removal of the latter leaves cavities complementary in shape, size, and chemical functionality, therefore allowing for the template to rebind specifically.
According to the International Union of Pure and Applied Chemistry (IUPAC), a chemical sensor is “a device that transforms chemical information, ranging from the concentration of a specific sample component to total composition analysis, into an analytically useful signal. The chemical information, mentioned above, may originate from a chemical reaction of the analyte or from a physical property of the investigated system” [10]. Therefore, a suitable sensor has to fulfil specific criteria such as high sensitivity and selectivity, stability, low sample consumption, reliability, and reproducibility. Overall, an ideal sensor should also be inexpensive, portable, foolproof, able to instantaneously respond to the analyte of interest in any desired medium, and capable of generating a measurable signal in the required concentration range. Unfortunately, current sensors are far from being “ideal”, and chemical sensors in particular are generally narrowly optimised for a given application [11][12].
A chemical sensor consists of two main elements (see Scheme 1): a chemical recognition element, called the receptor, and a physico-chemical transducer. The receptor transforms the binding event of the target into a form of energy that can be measured by the transducer. For chemical sensors, this binding event involves chemical species and generation of a signal upon a change of a physico-chemical parameter (e.g., the formation/breaking of a bond, exchange of electrons, and mass change or refractive index modification). Subsequently, the transducer transforms the chemical information received from the receptor into a useful analytical signal. The transduction process can be based on several physical phenomena, such as optical or thermal changes, electrochemical reactions, and mass variations.
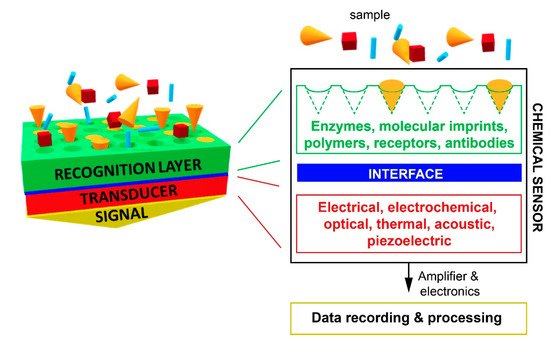
Scheme 1. Scheme of a chemical sensor consisting of a recognition layer, an interface, a transducer, and a signal detection element.
As a recent trend, the miniaturisation of sensors and their readout deserves a special attention due to the increasing number of applications available for smartphones [13][14][15][16][17]. From a general point of view, receptor design is perhaps the crucial step in the process of sensor development because it has to match the requirements of high selectivity and sensitivity for a target with the particular characteristics of a given readout. Thus, the straightforward engineering, fine-tuning, and easiness of integration into the standard industrial process of MIPs makes them ideal candidates for recognition elements.
2. Electrochemical MIP Sensors
Electrochemical sensors are defined as devices wherein a sensing layer is coupled to an electrochemical transducer [18] and represent one of the most successfully applied MIP-based sensors [19][20][21][22][23]. Depending on the electrical phenomenon used to transduce the binding event, different families of electrochemical sensors can be distinguished: potentiometric (sensing a change in the membrane potential), conductometric (conductance variation), impedimetric (impedance fluctuation), and voltammetric or amperometric (overall current variation induced either by an electrochemical reaction upon a voltage switch or a time-dependent evolution over a constant potential, respectively) [24].
In the early 1990s, Mosbach and coworkers reported on the first use of MIPs for the capacitive [25] or amperometric [19] detection of L-phenylalanine and morphine. Already in these early works, it became clear that the different transduction methods dictated the requirements for the suitable interfacing of the MIP. A major drawback of the most commonly used (meth)acrylate-based MIPs was their nonconductive nature; therefore, they were combined with electrically conductive polymers that remarkably improved the transduction [20]. Many valuable reviews have covered the wide range of applications of electrochemical MIP sensors [24][26][27][28].
Electrochemical devices are classified depending on the specific reaction (amperometric, potentiometric and conductometric sensors) or on the detection technique (impedimetric or field-effect sensors) [29]. As a result, different readouts are available: (i) a measurable current for amperometric sensors, (ii) a measurable potential or a charge accumulation for potentiometric sensors, and (iii) a measurable alteration of the conductive properties for conductometric sensors. Impedimetric sensors measure the impedance based on both resistance and reactance effects [30], whereas field-effect devices quantify the current induced by a potentiometric effect at a gate electrode in a transistor [18].
2.1. Potentiometric Sensors
Among the different chemical sensors, potentiometric sensors are the most popular due to their low cost and easy handling. This type of sensor works in the near-zero current flow, measuring the difference in potential between a working electrode (WE) and a reference electrode (RE), which generates an analytical signal. Ion selective electrodes (ISEs), which use a permselective membrane able to produce a potential in contact with solutions of a given analyte, are a prominent example of this class of transducers. The most known examples are glass electrodes for pH measurements. The membrane, which consists of a lipophilic-complexing agent that selectively binds the analyte of interest, is the key component.
When moving to MIP-based systems, potentiometric sensors represent a well-established class. For these sensors, low conductivity, hydrophobicity, and high selectivity are all requirements that (meth)acrylate-based MIPs fit perfectly. Two different kinds of potentiometric sensors can be distinguished: faradaic coupled devices (with a conventional inner-reference ISE; see Figure 1a) together with capacitive coupled devices (with solid inner contacts, coated wire ISE; see Figure 1b), and ion-sensitive field-effect transistors (ISFETs).
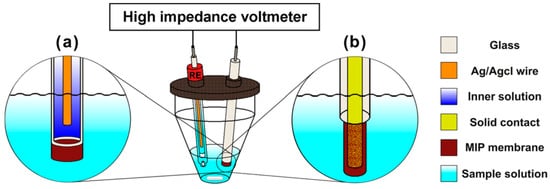
Figure 1. Typical setup for potentiometric measurements: with either an inner-reference ion-selective electrode (ISE) (a) or coated wire ISE (b) and a reference electrode (RE). Reprinted with permission from [24].
The classical setup is a faradaic coupled device in which the permselective membrane separates two compartments with solutions of different activity for a given ion (the sample and the internal electrolyte solution). Approaches similar to those of conventional ISEs are used to incorporate MIPs into the membranes as selective ionophores by embedding imprinted particles into an inert polymer matrix, typically polyvinylchloride (PVC). These have been referred to as “imprinted polymer inclusion membranes” [31]. Such membranes have been reported for the detection of dysprosium (III) and other lanthanides [32], as well as for the pesticide atrazine [33] and melamine in milk [20]. Their response greatly depends on how the membrane is tailored, e.g., the presence of ionic additives and the type and amount of the added plasticizer and MIP.
In addition to faradaic potentiometric sensors, capacitive sensors are also well-established. In contrast to the former, capacitive sensors do not use an inner solution, as the ion-selective membrane is directly in contact with a solid phase and the sample. The solid contact consists of conductors, semi-conductors, or insulators. This type of sensor can be further split into two subgroups, with either the membrane parallel to the coated wire electrodes or with a field-effect transistor device. For the development of solid-contact potentiometric sensors, conductive polymers are a clear advantage. A polymer coating is usually achieved by either the electropolymerization of MIPs (e.g., polypyrrole) or by the drop-casting of pre-synthesized acrylic MIPs embedded in plasticized PVC matrixes (as described before) on noble metals (such as Pt and Au) or carbon material (such as glassy carbon and graphite) for coated wire electrodes. The capacitive readout is based on the ionic charge-transfer occurring on the membrane in the presence of an analyte. Such coated electrodes have been used for sensing various compounds: nitrates [34], pesticides [35], antibiotics [36], and β-blockers [37]. Table 1 summarises the type of MIP, analyte, limit of detection (LOD), and, when available, the response time and stability of the sensors for representative examples. Another important way to obtain a selective electrode is by mixing a MIP-based membrane with carbon paste (e.g., graphite powder) [38] or relying on self-assembled monolayers (SAMs) [39]. For the second subgroup, ion-sensitive field-effect transistors are the most common examples. In this case, the ion-selective membrane is deposited onto the input dielectric of the field-effect transistor. Examples of target analytes for these are chloroaromatic acids [40], nucleotides and various acids [41][42], thiophenols [42], and NAD(P)+/NAD(P)H cofactors [43] (see Table 1). The development of such potentiometric sensors led to the fabrication of a platform consisting of the sensor and the readout circuit in one device called a microelectromechanical system (MEMS) [44], which is particularly interesting for the miniaturization and, thus, for mass-production of commercial sensing devices. An emerging field in the development of MIP-based potentiometric sensors is the replacement of expensive gold or glassy carbon electrode substrates by commercially-available, low-cost chromatography paper [45]. A paper sensor selective for bisphenol A showed a comparable analytical performance to a classical glassy carbon-based sensor. The detection limit was 0.15 µM (see Table 1), with good selectivity over other phenols.
Table 1. MIP-based electrochemical sensors.
| Transducer | Receptor | Analyte | LOD (mol/L) | Response Time (s) | Stability (Weeks) | Ref |
|---|---|---|---|---|---|---|
| Potentiometric | MIPs | Nitrates | 0.2 × 10−6 | 24 | - | [34] |
| MIPs into membranes | Dy(III) | 2 × 10−6 | 10 | - | [32] | |
| Atrazine | 0.5 × 10−6 | 120 | - | [33] | ||
| Melamine | 5 × 10−6 | 16 | - | [20] | ||
| NAD(P)+/NAD(P)H | 2 × 10−7 | 60 | 4 | [43] | ||
| Thiophenols | 2 × 10−6 | 45 | 2 | [42] | ||
| MIP-covered electrode | Amoxicillin | 0.3 × 10−6 | 20 | - | [36] | |
| Metoprolol | 1.3 × 10−7 | 14 | - | [37] | ||
| MIP TiO2 thin films | Acids | 5 × 10−4 | 300 | - | [40] | |
| 5 × 10−5 | 300 | - | [41] | |||
| AMP 1 GMP CMP |
1.5 × 10−5 1.5 × 10−5 8 × 10−7 |
60 | - | [41] | ||
| Impedimetric | MIP layer on Au electrode | Phenylalanine | 3 × 10−3 | 900 | - | [30] |
| Glucose | 50 × 10−6 | - | <1 | [46] | ||
| MIP film | Nicotine | 0.5 × 10−6 | 600 | 12 | [23] | |
| MIP film | Theophylline | 1 × 10−6 | 600 | - | [47] | |
| MIP on Au electrode | Resorcinol | 0.1 × 10−6 | - | 5 | [48] | |
| MIP particles on Au electrode | Imidacloprid | 4.61 × 10−6 | - | - | [49] | |
| Multiplex MIP on gold electrodes | AMP 2 NFA 3 BMK 4 |
50 × 10−6 20 × 10−6 20 × 10−6 |
- | - | [50] | |
| Conductometric | MIPs into membranes | Atrazine | 0.5 × 10−6 | 1800 | 16 | [51] |
| MIPs | Haloacetic acids | 3 × 10−9 | 30 | 12 | [52] | |
| Voltametric | MIP NPs | Morphine | 0.3 × 10−3 | - | - | [53] |
| MIP films | Ephedrine | 0.5 × 10−3 | - | - | [54] | |
| MIP | Paracetamol | 7.9 × 10−7 | - | - | [55] | |
| MIP | Dopamine | 1.98 × 10−9 | 4 | 1 | [56] | |
| MIP | Atrazine | 1 × 10−6 | 600 | - | [57] | |
| MIP Sol–gel film |
Creatinine | 1.23 × 10−3 | 120 | <1 | [58] | |
| MIP-based electrode | Melamine | 0.83 × 10−9 | - | 4 | [59] | |
| TATP 5 | 27 × 10−6 | - | - | [22] | ||
| MIp(DA) 6 films | TNT 7 RDX 8 |
0.1 × 10−9 10 × 10−9 |
- | - | [60] | |
| MIP-covered electrode | Cholestanol | 1 × 10−12 | - | 7 | [61] |
1 AMP: adenosine monophosphate; GMP: guanosine monophosphate; CMP: cytidine monophosphate; 2 AMP: amphetamine; 3 NFA: N-formyl amphetamine; 4 BMK: benzyl methyl ketone; 5 TATP: triacetone triperoxide; 6 MIp(DA): molecularly imprinted polydopamine; 7 TNT: 2,4,6-trinitrotoluene; 8 RDX: Research Department eXplosive or 1,3,5-trinitroperhydro-1,3,5-triazine.
2.2. Conductometric and Impedimetric Sensors
Conductometric and impedimetric sensors were also adapted to include MIPs as a recognition layer, which also ensures the selectivity. Conductometric sensors are based on a time-dependent change in conductivity triggered by the binding event and are less popular than potentiometric ones. In their design, two main setups can be distinguished. The first approach was reported by Mosbach et al. [62]. In their work, a membrane selective for benzyltriphenylphosphonium ions was pressing the bare polymer particles onto a polished surface connected to two Pt-wires and fixed on a filter paper. An alternative approach was proposed by Piletsky et al. [51], where a MIP membrane was placed between two Pt-electrodes in a conventional electrochemical cell with separated compartments. Since conductometric devices are in general poorly selective, especially in buffers due to the high ionic strength, reference NIP (non-imprinted polymers)-based sensors are always needed. This type of device can be upgraded by using interdigitated electrodes, which allow for a more reliable comparison between MIPs and NIPs [52].
Impedimetric sensors are based on the measurement of an alternated current generated by the binding interaction towards the selective membrane compared to the applied alternating current on the system. The measurement relies on the change of the total electrical resistance and reactance to the flow of the applied current through the given medium. Depending on the sensing principle, impedimetric sensors can be classified into two major subclasses: resistive and capacitive sensors [63]. Capacitive sensors are nowadays widely used, especially those that provide a near-ideal capacitor behaviour, with a phase angle close to 90°. To fulfil this condition, the imprinted polymer layer has to be electrically insulated and the binding event has to take place very close to the transducer. The insulation condition can be easily achieved with (meth)acrylic MIPs. For the second condition, thin films are preferred because they provide better performance than thick films. Indeed, thin films concentrate all the available binding sites close to the transducer and thus improve the sensitivity. It has also been reported that electropolymerised thin layers (around 10–50 nm) do not usually provide a convenient insulation because of the presence of local defects in the polymer matrix. Hence, special treatments with “blocking agents” such as alkanethiols on gold transducers have been shown to conveniently enhance insulation, thus improving sensor performance [30]. A wide variety of molecules has been detected using impedimetric sensors: phenylalanine [30], nicotine [23], glucose [46], theophylline [47], melamine [59], resorcinol [48], imidacloprid [49], and amphetamine [50] (see Table 1 for the characteristics of these sensors).
MIP interfacing with a transducer can be made either with SAMs [64], as previously mentioned, or by grafting photopolymerization [65]. The trend of miniaturization and mass production led to the development of interdigitated capacitance sensors, e.g., for amino acids [66] and protein allergens [67].
2.3. Voltametric and Amperometric Sensors
The last type of electrochemical sensors that we describe is represented by voltammetric and amperometric sensors, which are currently extensively used. The measured signal corresponds to the current flow as primary transduction phenomenon, so these sensors are considered to be faradaic coupled devices. A potential is applied between the sensor and a reference electrode in a three-electrode setup. The flow through the counter electrode and the sensor is continuously measured to produce an analytical signal. Electroactive targets are required for both types of sensors. Non-electroactive analytes can nevertheless be detected, but an electroactive probe such as ferro-ferricyanide is required in that case. The detection requires the analyte to diffuse close enough to the transducer in order to undergo an electron transfer. Since there is no physical contact between the analyte and the transducer, the electron transfer has to be mediated by the sensing element, and conductive MIPs are particularly suited for such sensors.
The correct integration of MIPs into an electrode is crucial, and various strategies, such as embedding nanoparticles into polymer matrices, deposition by sol–gel chemistry, and mixing with graphite as a paste to form electrodes, have been explored. Electropolymerised films have been used to embed particles into electrodes. The direct grafting or electropolymerisation onto an electrode also represents a convenient approach. To increase conductivity and obtain a better signal, graphite powder [30] and nanoparticles [68][69][70] have been included in these sensors. Morphine [19][53], ephedrine [54], paracetamol [55], dopamine [56][68], homovanillic acid [71], atrazine [57], creatinine [58], cholestanol [61], triacetone triperoxide [22], 2,4,6-trinitrotoluene, and 1,3,5-trinitroperhydro-1,3,5-triazine explosives [60] (see Table 1 for the characteristics of these sensors) are representative analytes. The analytic results obtained for the detection of creatinine using a MIP-based sensor showed comparable precision and accuracy with results obtained by HPLC [58]. We should note that voltammetry is the most selective electrochemical technique because it can specifically detect the analyte thanks to its intrinsic properties such as specific oxidation and reduction peaks. Kröger et al. developed one of the first imprinted polymer-based sensor using screen-printed electrodes and differential pulse voltammetry [72]. Several types of voltammetric sensors can be distinguished based on the shape of their applied potential function. The most commonly used are linear sweep voltammetry and cyclic voltammetry sensors. The applied potential shape varies either linearly with time (for linear sweep voltammetry and cyclic voltametry) or as constant increments on a linear ramp (for differential pulse voltammetry) or as square wave function (for square wave voltammetry), respectively. These techniques generally improve the signal-to-noise ratio and therefore the sensitivity.
References
- Ye, L.; Haupt, K. Molecularly imprinted polymers as antibody and receptor mimics for assays, sensors and drug discovery. Anal. Bioanal. Chem. 2004, 378, 1887–1897.
- Hayden, O.; Lieberzeit, P.A.; Blaas, D.; Dickert, F.L. Artificial antibodies for bioanalyte detection—Sensing viruses and proteins. Adv. Funct. Mater. 2006, 16, 1269–1278.
- Hayden, O. One binder to bind them all. Sensors 2016, 16, 1665.
- Uzun, L.; Turner, A.P.F. Molecularly-imprinted polymer sensors: Realising their potential. Biosens. Bioelectron. 2016, 76, 131–144.
- Dickert, F.L. Molecular imprinting and functional polymers for all transducers and applications. Sensors 2018, 18, 327.
- Lowdon, J.W.; Diliën, H.; Singla, P.; Peeters, M.; Cleij, T.J.; van Grinsven, B.; Eersels, K. MIPs for commercial application in low-cost sensors and assays – An overview of the current status quo. Sens. Actuators B Chem. 2020, 325.
- Chen, L.; Wang, X.; Lu, W.; Wu, X.; Li, J. Molecular imprinting: Perspectives and applications. Chem. Soc. Rev. 2016, 45, 2137–2211.
- Haupt, K.; Rangel, P.X.M.; Tse, B.; Bui, S. Molecularly imprinted polymers: Antibody mimics for bioimaging and therapy. Chem. Rev. 2020.
- Bedwell, T.S.; Whitcombe, M.J. Analytical applications of MIPs in diagnostic assays: Future perspectives. Anal. Bioanal. Chem. 2016, 408, 1735–1751.
- Hulanicki, A.; Glab, S.; Ingman, F. Chemical sensors definitions and classification. Pure Appl. Chem. 1991, 63, 1247–1250.
- Lu, W.; Xue, M.; Xu, Z.; Dong, X.; Xue, F.; Wang, F.; Wang, Q.; Meng, Z. Molecularly imprinted polymers for the sensing of explosives and chemical warfare agents. Curr. Org. Chem. 2015, 19, 62–71.
- Awino, J.K.; Zhao, Y. Molecularly imprinted nanoparticles as tailor-made sensors for small fluorescent molecules. Chem. Commun. 2014, 50, 5752–5755.
- Xiao, W.; Xiao, M.; Fu, Q.; Yu, S.; Shen, H.; Bian, H.; Tang, Y. A portable smart-phone readout device for the detection of mercury contamination based on an aptamer-assay nanosensor. Sensors 2016, 16, 1871.
- Baş, D. Sensitive and reliable paper-based glucose sensing mechanisms with smartphone readout using the: L ∗ a ∗ b ∗ color space. Anal. Methods 2017, 9, 6698–6704.
- Gao, X.; Wu, N. Smartphone-Based Sensors, Electrochem. Soc. Interface. Available online: (accessed on 29 April 2021).
- Broeders, J.; Croux, D.; Peeters, M.; Beyens, T.; Duchateau, S.; Cleij, T.J.; Wagner, P.; Thoelen, R.; De Ceuninck, W. Mobile application for impedance-based biomimetic sensor readout. IEEE Sens. J. 2013, 13, 2659–2665.
- Guembe-García, M.; Santaolalla-García, V.; Moradillo-Renuncio, N.; Ibeas, S.; Reglero, J.A.; García, F.C.; Pacheco, J.; Casado, S.; García, J.M.; Vallejos, S. Monitoring of the evolution of human chronic wounds using a ninhydrin-based sensory polymer and a smartphone. Sens. Actuators B Chem. 2021, 335, 129688.
- Thévenot, D.R.; Toth, K.; Durst, R.A.; Wilson, G.S. Electrochemical biosensors: Recommended definitions and classification. Anal. Lett. 2001, 34, 635–659.
- Kriz, D.; Mosbach, K. Competitive amperometric morphine sensor based on an agarose immobilised molecularly imprinted polymer. Anal. Chim. Acta 1995, 300, 71–75.
- Liang, R.; Zhang, R.; Qin, W. Potentiometric sensor based on molecularly imprinted polymer for determination of melamine in milk. Sens. Actuators B Chem. 2009, 141, 544–550.
- Goud, K.Y.; M, S.; Reddy, K.K.; Gobi, K.V. Development of highly selective electrochemical impedance sensor for detection of sub-micromolar concentrations of 5-Chloro-2,4-dinitrotoluene. J. Chem. Sci. 2016, 128, 763–770.
- Mamo, S.K.; Gonzalez-Rodriguez, J. Development of a molecularly imprinted polymer-based sensor for the electrochemical determination of triacetone triperoxide (TATP). Sensors 2014, 14, 23269–23282.
- Liu, K.; Wei, W.Z.; Zeng, J.X.; Liu, X.Y.; Gao, Y.P. Application of a novel electrosynthesized polydopamine-imprinted film to the capacitive sensing of nicotine. Anal. Bioanal. Chem. 2006, 385, 724–729.
- Antuña-Jiménez, D.; Díaz-Díaz, G.; Blanco-López, M.C.; Lobo-Castañón, M.J.; Miranda-Ordieres, A.J.; Tuñón-Blanco, P. Molecularly Imprinted Electrochemical Sensors: Past, Present, and Future; Li, S., Piletsky, S.A., Ge, Y., Lunec, J., Eds.; ScienceDirect: Amsterdam, The Netherlands, 2012; ISBN 9780444563316.
- Hedborg, E.; Winquist, F.; Lundström, I.; Andersson, L.I.; Mosbach, K. Some studies of molecularly-imprinted polymer membranes in combination with field-effect devices. Sens. Actuators A. Phys. 1993, 37–38, 796–799.
- Piletsky, S.A.; Turner, A.P.F. Electrochemical sensors based on molecularly imprinted polymers. Electroanalysis 2002, 14, 317–323.
- Sharma, P.S.; Pietrzyk-Le, A.; D’Souza, F.; Kutner, W. Electrochemically synthesized polymers in molecular imprinting for chemical sensing. Anal. Bioanal. Chem. 2012, 402, 3177–3204.
- Blanco-López, M.C.; Lobo-Castañón, M.J.; Miranda-Ordieres, A.J.; Tuñón-Blanco, P. Electrochemical sensors based on molecularly imprinted polymers. TrAC Trends Anal. Chem. 2004, 23, 36–48.
- Grieshaber, D.; MacKenzie, R.; Vörös, J.; Reimhult, E. Electrochemical biosensors - Sensor principles and architectures. Sensors 2008, 8, 1400–1458.
- Panasyuk, T.L.; Mirsky, V.M.; Piletsky, S.A.; Wolfbeis, O.S. Electropolymerized molecularly imprinted polymers as receptor layers in capacitive chemical sensors. Anal. Chem. 1999, 71, 4609–4613.
- Prasada Rao, T.; Kala, R. Potentiometric transducer based biomimetic sensors for priority envirotoxic markers-An overview. Talanta 2008, 76, 485–496.
- Prasad, K.; Kala, R.; Prasada Rao, T.; Naidu, G.R.K. Ion imprinted polymer based ion-selective electrode for the trace determination of dysprosium(III) ions. Anal. Chim. Acta 2006, 566, 69–74.
- Prasad, K.; Prathish, K.P.; Gladis, J.M.; Naidu, G.R.K.; Rao, T.P. Molecularly imprinted polymer (biomimetic) based potentiometric sensor for atrazine. Sens. Actuators B Chem. 2007, 123, 65–70.
- Hutchins, R.S.; Bachas, L. Nitrate-selective electrode developed by electrochemically mediated imprinting/doping of polypyrrole. Anal. Chem. 1995, 67, 1654–1660.
- Kamel, A.H.; Moreira, F.T.C.; Almeida, S.A.A.; Sales, M.G.F. Novel potentiometric sensors of molecular imprinted polymers for specific binding of chlormequat. Electroanalysis 2008, 20, 194–202.
- Guerreiro, J.R.L.; Sales, M.G.F.; Moreira, F.T.C.; Rebelo, T.S.R. Selective recognition in potentiometric transduction of amoxicillin by molecularly imprinted materials. Eur. Food Res. Technol. 2011, 232, 39–50.
- Tehrani, M.S.; Vardini, M.T.; Azar, P.A.; Husain, S.W. Molecularly imprinted polymer based PVC-membrane-coated graphite electrode for the determination of metoprolol. J. Iran. Chem. Soc. 2010, 7, 759–769.
- Javanbakht, M.; Fard, S.E.; Abdouss, M.; Mohammadi, A.; Ganjali, M.R.; Norouzi, P.; Safaraliee, L. A biomimetic potentiometric sensor using molecularly imprinted polymer for the cetirizine assay in tablets and biological fluids. Electroanalysis 2008, 20, 2023–2030.
- Wang, Y.; Zhou, Y.; Sokolov, J.; Rigas, B.; Levon, K.; Rafailovich, M. A potentiometric protein sensor built with surface molecular imprinting method. Biosens. Bioelectron. 2008, 24, 162–166.
- Lahav, M.; Kharitonov, A.B.; Katz, O.; Kunitake, T.; Willner, I. Tailored chemosensors for chloroaromatic acids using molecular imprinted TiO2 thin films on ion-sensitive field-effect transistors. Anal. Chem. 2001, 73, 720–723.
- Zayats, M.; Lahav, M.; Kharitonov, A.B.; Willner, I. Imprinting of specific molecular recognition sites in inorganic and organic thin layer membranes associated with ion-sensitive field-effect transistors. Tetrahedron 2002, 58, 815–824.
- Pogorelova, S.P.; Kharitonov, A.B.; Willner, I.; Sukenik, C.N.; Pizem, H.; Bayer, T. Development of ion-sensitive field-effect transistor-based sensors for benzylphosphonic acids and thiophenols using molecularly imprinted TiO 2 films. Anal. Chim. Acta 2004, 504, 113–122.
- Pogorelova, S.P.; Zayats, M.; Bourenko, T.; Kharitonov, A.B.; Lioubashevski, O.; Katz, E.; Willner, I. Analysis of NAD(P)+/NAD(P)H cofactors by imprinted polymer membranes associated with ion-sensitive field-effect transistor devices and Au-quartz crystals. Anal. Chem. 2003, 75, 509–517.
- Tsai, H.H.; Lin, C.F.; Juang, Y.Z.; Wang, I.L.; Lin, Y.C.; Wang, R.L.; Lin, H.Y. Multiple type biosensors fabricated using the CMOS BioMEMS platform. Sens. Actuators B Chem. 2010, 144, 407–412.
- Kamel, A.H.; Jiang, X.; Li, P.; Liang, R. A paper-based potentiometric sensing platform based on molecularly imprinted nanobeads for determination of bisphenol A. Anal. Methods 2018, 10, 3890–3895.
- Cheng, Z.; Wang, E.; Yang, X. Capacitive detection of glucose using molecularly imprinted polymers. Biosens. Bioelectron. 2001, 16, 179–185.
- Wang, Z.; Kang, J.; Liu, X.; Ma, Y. Capacitive detection of theophylline based on electropolymerized molecularly imprinted polymer. Int. J. Polym. Anal. Charact. 2007, 12, 131–142.
- Kumar Prusty, A.; Bhand, S. Molecularly imprinted polyresorcinol based capacitive sensor for sulphanilamide detection. Electroanalysis 2019, 31, 1797–1808.
- El-Akaad, S.; Mohamed, M.A.; Abdelwahab, N.S.; Abdelaleem, E.A.; De Saeger, S.; Beloglazova, N. Capacitive sensor based on molecularly imprinted polymers for detection of the insecticide imidacloprid in water. Sci. Rep. 2020, 10, 14479.
- De Rycke, E.; Leman, O.; Dubruel, P.; Hedström, M.; Völker, M.; Beloglazova, N.; De Saeger, S. Novel multiplex capacitive sensor based on molecularly imprinted polymers: A promising tool for tracing specific amphetamine synthesis markers in sewage water. Biosens. Bioelectron. 2021, 178.
- Piletsky, S.A.; Piletskaya, E.V.; Elgersma, A.V.; Yano, K.; Karube, I.; Parhometz, Y.P.; El’skaya, A.V. Atrazine sensing by molecularly imprinted membranes. Biosens. Bioelectron. 1995, 10, 959–964.
- Suedee, R.; Intakong, W.; Dickert, F.L. Molecularly imprinted polymer-modified electrode for on-line conductometric monitoring of haloacetic acids in chlorinated water. Anal. Chim. Acta 2006, 569, 66–75.
- Ho, K.C.; Yeh, W.M.; Tung, T.S.; Liao, J.Y. Amperometric detection of morphine based on poly(3,4-ethylenedioxythiophene) immobilized molecularly imprinted polymer particles prepared by precipitation polymerization. Anal. Chim. Acta 2005, 542, 90–96.
- Mazzotta, E.; Picca, R.A.; Malitesta, C.; Piletsky, S.A.; Piletska, E.V. Development of a sensor prepared by entrapment of MIP particles in electrosynthesised polymer films for electrochemical detection of ephedrine. Biosens. Bioelectron. 2008, 23, 1152–1156.
- Özcan, L.; Şahin, Y. Determination of paracetamol based on electropolymerized-molecularly imprinted polypyrrole modified pencil graphite electrode. Sens. Actuators B Chem. 2007, 127, 362–369.
- Li, J.; Zhao, J.; Wei, X. A sensitive and selective sensor for dopamine determination based on a molecularly imprinted electropolymer of o-aminophenol. Sens. Actuators B Chem. 2009, 140, 663–669.
- Shoji, R.; Takeuchi, T.; Kubo, I. Atrazine sensor based on molecularly imprinted polymer-modified gold electrode. Anal. Chem. 2003, 75, 4882–4886.
- Patel, A.K.; Sharma, P.S.; Prasad, B.B. Development of a creatinine sensor based on a molecularly imprinted polymer-modified sol-gel film on graphite electrode. Electroanalysis 2008, 20, 2102–2112.
- Shamsipur, M.; Moradi, N.; Pashabadi, A. Coupled electrochemical-chemical procedure used in construction of molecularly imprinted polymer-based electrode: A highly sensitive impedimetric melamine sensor. J. Solid State Electrochem. 2018, 22, 169–180.
- Leibl, N.; Duma, L.; Gonzato, C.; Haupt, K. Polydopamine-based molecularly imprinted thin films for electro-chemical sensing of nitro-explosives in aqueous solutions. Bioelectrochemistry 2020, 135.
- Jalalvand, A.R.; Zangeneh, M.M.; Jalili, F.; Soleimani, S.; Díaz-Cruz, J.M. An elegant technology for ultrasensitive impedimetric and voltammetric determination of cholestanol based on a novel molecularly imprinted electrochemical sensor. Chem. Phys. Lipids 2020, 229, 104895.
- Kriz, D.; Kempe, M.; Mosbach, K. Introduction of molecularly imprinted polymers as recognition elements in conductometric chemical sensors. Sens. Actuators B 1996, 33, 178–181.
- Carminati, M. Advances in high-resolution microscale impedance sensors. J. Sens. 2017, 2017.
- Yang, L.; Wei, W.; Xia, J.; Tao, H.; Yang, P. Capacitive biosensor for glutathione detection based on electropolymerized molecularly imprinted polymer and kinetic investigation of the recognition process. Electroanalysis 2005, 17, 969–977.
- Delaney, T.L.; Zimin, D.; Rahm, M.; Weiss, D.; Wolfbeis, O.S.; Mirsky, V.M. Capacitive detection in ultrathin chemosensors prepared by molecularly imprinted grafting photopolymerization. Anal. Chem. 2007, 79, 3220–3225.
- Belbruno, J.J.; Zhang, G.; Gibson, U.J. Capacitive sensing of amino acids in molecularly imprinted nylon films. Sens. Actuators B Chem. 2011, 155, 915–918.
- Sontimuang, C.; Suedee, R.; Dickert, F. Interdigitated capacitive biosensor based on molecularly imprinted polymer for rapid detection of Hev b1 latex allergen. Anal. Biochem. 2011, 410, 224–233.
- Kan, X.; Zhao, Y.; Geng, Z.; Wang, Z.; Zhu, J.J. Composites of multiwalled carbon nanotubes and molecularly imprinted polymers for dopamine recognition. J. Phys. Chem. C 2008, 112, 4849–4854.
- Zhang, Z.; Hu, Y.; Zhang, H.; Yao, S. Novel layer-by-layer assembly molecularly imprinted sol-gel sensor for selective recognition of clindamycin based on Au electrode decorated by multi-wall carbon nanotube. J. Colloid Interface Sci. 2010, 344, 158–164.
- Huang, J.; Xing, X.; Zhang, X.; He, X.; Lin, Q.; Lian, W.; Zhu, H. A molecularly imprinted electrochemical sensor based on multiwalled carbon nanotube-gold nanoparticle composites and chitosan for the detection of tyramine. Food Res. Int. 2011, 44, 276–281.
- Diñeiro, Y.; Menéndez, M.I.; Blanco-López, M.C.; Lobo-Castañón, M.J.; Miranda-Ordieres, A.J.; Tuñón-Blanco, P. Computational predictions and experimental affinity distributions for a homovanillic acid molecularly imprinted polymer. Biosens. Bioelectron. 2006, 22, 364–371.
- Kröger, S.; Turner, A.P.F.; Mosbach, K.; Haupt, K. Imprinted polymer-based sensor system for herbicides using differential-pulse voltammetry on screen-printed electrodes. Anal. Chem. 1999, 71, 3698–3702.
More
Information
Subjects:
Polymer Science
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
2.2K
Revisions:
2 times
(View History)
Update Date:
10 Jun 2021
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





