
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Yangyang He | + 1921 word(s) | 1921 | 2021-06-03 10:19:27 | | | |
| 2 | Peter Tang | Meta information modification | 1921 | 2021-06-10 02:59:01 | | |
Video Upload Options
Osteoporosis is characterized by low bone mass and damage to the bone tissue’s microarchitecture, leading to increased fracture risk. Extracellular vesicles (EVs) are intercellular communicators, transfer substances encapsulated in them, modify the phenotype and function of target cells, mediate cell-cell communication, and, therefore, have critical applications in disease progression and clinical diagnosis and therapy.
1. Introduction
Osteoporosis is an age-related bone disease characterized by reduced bone mass and bone microarchitecture destruction, resulting in decreased bone strength, increased bone fragility, and fracture risk [1]. Sustained stress can inhibit osteoblast activity and enhance osteoclast-mediated bone resorption, thus possibly leading to a decrease in bone mass in the long term [2]. However, cell-cell communications that exacerbate these processes are not well understood to date. In recent years, extracellular vesicles (EVs) have emerged as critical modulators of cell-cell communication in health and disease [3], and can regulate the function of osteoblasts and osteoclasts, and consequently have a potential impact on osteoporosis [4].
2. The Characteristics of Extracellular Vesicles
EVs is a general term for numerous vesicles with a lipid bilayer membrane structure released by cells into the extracellular environment [5]. Based on their subcellular origin and biogenesis, EVs divide into three main categories: small EVs (also known as exosomes), medium/large EVs (also known as microvesicles), and apoptotic bodies [6]. Exosomes are vesicles with a ≈40–200 nm diameter and uniform size, which are released from intracellular multivesicular bodies (MVBs) fused with the cytoplasmic membrane [7][8][9]. In contrast, microvesicles are non-uniform particles ranging from 200–2000 nm in diameter that are formed and released from the cytoplasmic membrane in a budding manner. Apoptotic cells undergo programmed cell death and release apoptotic bodies (800–5000 nm in diameter), which share certain characteristics with microvesicles [10]. EVs carry multiple biomolecules, including DNA, RNA, proteins, glycans, lipids, and metabolites [11][12]. Thus, they can be used as cargoes to deliver information and alter the signaling pathways and biochemical composition of receptor cells. EVs can be derived from a variety of cells, such as mesenchymal stem cells (MSCs) [13], immune cells [14], tumor cells [15], platelets [16], and cardiomyocytes [17]. Furthermore, they can be detected in most body fluids, such as peripheral blood, breast milk, semen, urine, and saliva [18]. Thus, EVs have been recognized increasingly as promising biomarkers for the diagnosis and prognosis of several diseases.
The composition of EVs has a crucial influence on their biological functions; as transmitters, EVs can activate cell surface receptor binding on target cells through proteins and bioactive lipid ligands, thereby inducing intracellular signaling and regulating the biological activity of the target cells. Besides, EVs can deliver their contents to target cells by fusing with the plasma membrane [4][19]. Figure 1 shows the biogenesis and secretion of EVs and their effects on target cells. Studies on EVs show that they have a complex composition, including lipids, proteins, nucleic acids, and other metabolites. These components play an essential role in the function of EVs. Nucleic acids carried by EVs can be potential biomarkers because of their genetic characteristics [20]. Current research is more focused on microRNA (miRNA, miR). MiRNAs are 17–24 nucleotide endogenous, non-coding RNAs, which post-transcriptionally silence target genes’ expression by binding to the 3’-untranslated region (UTR) open reading frame region of target messenger RNAs [21][22], thus playing a vital regulatory role in the organism. Because of the potential relevance of miRNAs as disease markers and therapeutic tools, it is of great importance to further our understanding of their biological properties and functions [23][24]. The roles of EVs in human tissues are listed in Table 1.
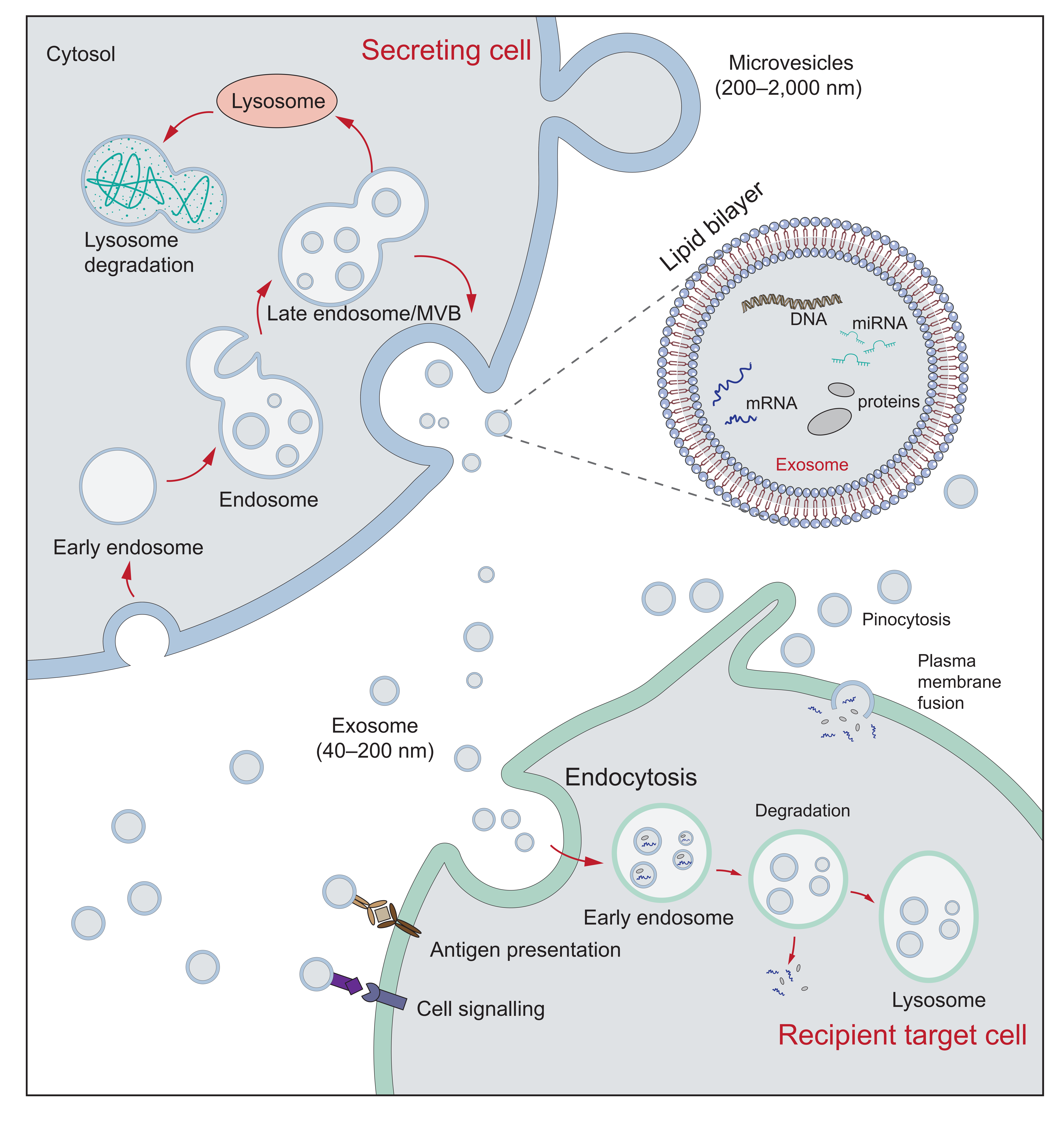
Figure 1. The biogenesis and secretion of EVs and their effects on target cells. The formation of exosomes begins with the endocytosis of the cell membrane. The endosome membrane sprouts inward to form vesicles, which transform into MVB. MVB can be sent to lysosomes for degradation or secreted into the exosomes (40–200 nm) by fusion with the plasma membrane. Microvesicles (200–2000 nm) are vesicles formed through a process of membrane budding or exocytosis. EVs can interact with target cells through receptor-mediated binding. Additionally, target cells can internalize EVs by target cells through endocytosis, pinocytosis, and plasma membrane fusion [25], where EVs can release their cargoes to affect target cells, or be degraded by lysosomes.
Table 1. Role of EVs in human tissues
|
Tissue |
Functions |
Reference |
|
Tumor |
Biomarker Alters tumor microenvironment Regulates tumor immune response Involved in tumor angiogenesis |
|
|
Bone |
Biomarker Regulates osteogenic differentiation of mesenchymal stem cells Regulates osteoblast proliferation and activity Affects osteoblast differentiation Regulates osteoclast function and induces osteoclast differentiation |
|
|
Heart |
Biomarker Promotes angiogenesis Cardioprotection and regeneration |
|
|
Brain |
Biomarker Influences inflammatory and regulatory pathways in the brain Neuroprotective effect |
|
|
Kidney |
Biomarker Involved in the development of renal fibrosis Contributing to kidney repair |
[36] |
|
Gastro-intestinal tract |
Immunomodulation Response of anti-apoptotic, antioxidant stress Regulates the homeostasis of gut microbiota |
3. The Role of EVs in Osteoporosis
3.1. Overview of Osteoporosis and Bone Remodeling
As one of the human body’s essential tissues, bone needs sufficient stiffness and toughness to maintain bone strength to avoid fractures. In terms of the body’s natural processes, the positive balance between bone formation (by osteoblasts) and bone resorption (by osteoclasts) before adulthood increases bone mass and reaches its peak (typically achieved at different skeletal sites from 25 to 35 age years [39]), and bone remodeling balance maintains bone mass in adulthood. However, with increasing age, most bone loss occurs during and after menopause.
Bone remodeling, a lifelong process, refers to bone formation (form new bone tissue) and bone resorption (remove mature bone from the skeleton). This process involves skeletal-related cells, such as osteoclasts, osteoblasts, osteocytes, and several immune cells, such as T cells, B cells, and megakaryocytes [40]. Bone remodeling occurs in the basic multicellular unit, consisting of osteoblasts, osteoclasts, and osteocytes within the bone-remodeling cavities [41]. The process begins with bone-resorbing osteoclasts, followed by bone-forming osteoblasts, and in normal bone, the remodeling cycle results in complete filling of the resorption cavity with new bone [41][42]. Osteocytes, the most abundant cells in bone tissues, can sense and respond to environmental mechanical stimuli and regulate bone formation and bone resorption [43]. Thus, osteocytes are the central coordinator of bone reconstruction and mineral homeostasis. In the bone remodeling process, runt-related transcription factor 2 (Runx2) and Osterix plays an essential role for osteoblast differentiation [44][45], and the osteoclast differentiation is mainly regulated by the receptor activator of nuclear factor κ-Β ligand (RANKL)/receptor activator of nuclear factor κ-Β(RANK)/osteoprotegerin pathway. Namely, osteoblasts can produce RANKL, which can bind to RANK on osteoclasts’ precursor, thus promoting osteoclast differentiation. To tightly regulate osteoclastogenesis, osteoblasts also secrete osteoprotegerin to compete with RANK to bind RANKL, thus inhibiting osteoclast differentiation [46].
3.2. EVs Regulate Osteoclasts Differentiation and Activity
MiRNAs, as one of the cargoes carried by EVs, have a vital role in bone homeostasis. For example, the highly expressed miR-503-3p in EVs released by osteoblasts can inhibit osteoclastogenesis by inactivating the RANK/RANKL signaling pathway [47][48]. Besides, blood vessels play an essential role in bone repair and regeneration [49]. A study by Song et al. [50] demonstrated that EVs derived from the vascular endothelial cell have more effective bone targeting than those derived from osteoblast or bone marrow mesenchymal stem cells (BMSCs) and can inhibit the activity and differentiation of osteoclasts through miR-155. Thus, the miR-155-containing EVs may be a potential target against osteoporosis. Interestingly, some tumor cells can affect osteoclast function by secreting EVs. Increased expression of miR-21 was observed in EVs derived from lung adenocarcinoma cells, which promoted osteoclastogenesis by targeting programmed cell death protein 4 [51]. Similarly, breast cancer cells secrete miR-20a-5p-containing EVs, which promote the proliferation and differentiation of osteoclasts [52].
EVs can affect bone remodeling by directly regulating osteoclast differentiation and activity. Huynh et al. [53] found that the EVs derived from osteoclast precursors stimulate the formation of vitamin D-dependent osteoclasts. However, EVs from osteoclast-enriched cultures inhibited osteoclastogenesis. The results of this experimental study show that the EVs from mature osteoclasts contain RANK, which could competitively inhibit the stimulation of RANK on the osteoclast surface, similar to the role of osteoprotegerin mentioned above. Besides, the RANK-containing EVs can use the RANK/RANKL interaction to target RANKL-expressing cells to transfer regulatory molecules [53]. Moreover, osteoblasts can affect osteoclasts by secreting EVs. The RANKL-containing EVs released by osteoblasts are transferred to the precursors of osteoclasts, thus stimulating RANKL/RANK signal transduction and promoting the formation of osteoclasts [54]. To better understand the role of EVs in osteoblast-osteoclast communication, researchers loaded osteoblast-derived EVs with osteoclast-inhibiting drugs (zoledronate and dasatinib). They found that osteoblast EVs internalized and shuttled osteoclast-inhibiting drugs to inhibit osteoclasts’ activity in vivo and in vitro [55], which opens up an avenue for the use of EVs in the treatment of bone diseases. The above studies show that EVs from a variety of cells can regulate osteoclasts.
3.3. EVs Affect Osteoblasts and Osteogenic Function
Osteoblasts are the bone-forming cells of remodeling units and are crucial for skeletal growth and maintenance [56]. As mentioned above, osteoblasts can secrete EVs to influence osteoclast function. In turn, osteoclasts can secrete EVs that modulate osteoblast activity. Sun et al. [57] found that osteoclasts secrete miR-214-containing EVs, specifically recognizing osteoblasts through the ephrina2/ephrin type-A receptor 2 interaction. Moreover, miR-214 directly targets activating transcription factor 4 to inhibit bone formation [58]. The osteoclast-derived EVs exist not only in the bone microenvironment but they can also enter the blood. Researchers found upregulated levels of miR-214 in serum EVs of osteoporotic patients, which means that miR-214 in EVs serve as a potential biomarker of bone loss [57]. Likewise, osteoclasts-derived miR-23a-5p-containing EVs inhibit the activity of osteoblasts by targeting Runx2 [59]. Therefore, the EV-mediated intercellular communication between osteoblasts and osteoclasts may be a new direction for the study of bone remodeling mechanisms.
MSCs are known to stimulate tissue regeneration. Furthermore, EVs released from MSCs have attracted much attention in bone research. A recent study showed that BMSCs-derived EVs could regulate osteoblast differentiation and osteogenic gene expression in vitro, thus improving osteogenic function [60]. Additionally, MSCs-derived EVs induce osteogenic differentiation and mineralization during the late stages of osteogenic differentiation. Furthermore, target prediction of differentially expressed miRNAs in EVs suggests a significant enrichment of signaling pathways regulating osteogenic differentiation [61]. Some researchers have explored the possible clinical applications of BMSCs based on previous literature. For example, Fang et al. [62] found that BMSCs-derived EVs significantly reverse the decreased osteogenic differentiation of BMSCs in steroid-induced femoral head necrosis, thus serving as a potential therapeutic strategy for steroid-induced femoral head necrosis. These studies reveal the potential application of MSCs-derived EVs in bone regeneration therapy. Many studies support the role of EVs in bone remodeling, shown in Table 2, but it is not discussed in detail.
Table 2. A summary of EVs associated with bone remodeling.
|
Source |
Bioactive Factors Containing |
Target |
Function |
References |
|
Osteoclasts |
RANK |
Osteoclasts |
Inhibits osteoclast formation |
[53] |
|
Osteoclasts |
miR-214 |
Osteoblasts |
Inhibits the activity of osteoblasts through ephrina2/ephrin type-A receptor 2 interaction and targets activating transcription factor 4 to inhibit bone formation |
|
|
Osteoclasts |
miR-23a-5p |
Osteoblasts |
Inhibits the activity of osteoblasts by targeting Runx2 |
[59] |
|
Osteoclasts |
miR-214-3p |
Osteoblasts |
Inhibits osteoblastic bone formation |
[63] |
|
Osteoblasts |
RANKL |
Osteoclast precursors |
Facilitates osteoclast formation by binding RANK on the osteoclast precursor surface |
[54] |
|
Osteoblasts |
RANKL |
Osteoclasts |
Induces the apoptosis of osteoclasts |
[55] |
|
Preosteoblasts |
TRIP-1 |
The extracellular matrix of bone |
Promotes mineralization |
[64] |
|
BMSCs |
miR-196a |
Osteoblasts |
Improves osteogenic function |
[60] |
|
BMSCs |
miR-885-5p |
BMSCs |
Inhibits osteogenic differentiation by repressing Runx2 |
[65] |
|
BMSCs |
miR-151-5p |
BMSCs |
Promotes osteogenic differentiation |
[66] |
|
Endothelial cells |
miR-155 |
Osteoclasts |
Inhibits the activity and differentiation of osteoclasts |
[50] |
|
Endothelial cells |
miR-31 |
MSCs |
Inhibits osteogenic differentiation by repressing Frizzled-3 |
[67] |
(BMSCs: Bone marrow mesenchymal stem cells; MSCs: Mesenchymal stem cells; RANK: Receptor activator of nuclear factor κ-B; RANKL: Receptor activator of nuclear factor κ-Β ligand; TRIP-1: Transforming growth factor beta receptor II interacting protein-1; Runx2: Runt-related transcription factor 2).
References
- NIH Consensus Development Panel on Osteoporosis Prevention, Diagnosis, and Therapy Osteoporosis Prevention, Diagnosis, and Therapy. JAMA 2001, 285, 785–795, doi:10.1001/jama.285.6.785.
- Riancho, J.A.; Brennan-Olsen, S.L. The Epigenome at the Crossroad Between Social Factors, Inflammation, and Osteoporosis Risk. Clin. Rev. Bone Miner. Metab. 2017, 15, 59–68, doi:10.1007/s12018-017-9229-5.
- Gieseler, F.; Ender, F. Extracellular Vesicles and Cell–Cell Communication: New Insights and New Therapeutic Strategies Not Only in Oncology. Int. J. Mol. Sci. 2020, 21, 4331, doi:10.3390/ijms21124331.
- Xie, X.; Xiong, Y.; Panayi, A.C.; Hu, L.; Zhou, W.; Xue, H.; Lin, Z.; Chen, L.; Yan, C.; Mi, B.; et al. Exosomes as a Novel Approach to Reverse Osteoporosis: A Review of the Literature. Front. Bioeng. Biotechnol. 2020, 8, doi:10.3389/fbioe.2020.594247.
- Colombo, M.; Raposo, G.; Théry, C. Biogenesis, Secretion, and Intercellular Interactions of Exosomes and Other Extracellular Vesicles. Annu. Rev. Cell Dev. Biol. 2014, 30, 255–289, doi:10.1146/annurev-cellbio-101512-122326.
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal Information for Studies of Extracellular Vesicles 2018 (MISEV2018): A Position Statement of the International Society for Extracellular Vesicles and Update of the MISEV2014 Guidelines. J. Extracell. Vesicles 2018, 7, 1535750, doi:10.1080/20013078.2018.1535750.
- van Niel, G.; D’Angelo, G.; Raposo, G. Shedding Light on the Cell Biology of Extracellular Vesicles. Nat. Rev. Mol. Cell Biol. 2018, 19, 213–228, doi:10.1038/nrm.2017.125.
- Shao, H.; Im, H.; Castro, C.M.; Breakefield, X.; Weissleder, R.; Lee, H. New Technologies for Analysis of Extracellular Vesicles. Chem. Rev. 2018, 118, 1917–1950, doi:10.1021/acs.chemrev.7b00534.
- Alzhrani, G.N.; Alanazi, S.T.; Alsharif, S.Y.; Albalawi, A.M.; Alsharif, A.A.; Abdel-Maksoud, M.S.; Elsherbiny, N. Exosomes: Isolation, Characterization, and Biomedical Applications. Cell Biol. Int. 2021, 1–25, doi:https://doi.org/10.1002/cbin.11620.
- Crescitelli, R.; Lässer, C.; Szabó, T.G.; Kittel, A.; Eldh, M.; Dianzani, I.; Buzás, E.I.; Lötvall, J. Distinct RNA Profiles in Subpopulations of Extracellular Vesicles: Apoptotic Bodies, Microvesicles and Exosomes. J. Extracell. Vesicles 2013, 2, doi:10.3402/jev.v2i0.20677.
- Mathieu, M.; Martin-Jaular, L.; Lavieu, G.; Théry, C. Specificities of Secretion and Uptake of Exosomes and Other Extracellular Vesicles for Cell-to-Cell Communication. Nat. Cell Biol. 2019, 21, 9–17, doi:10.1038/s41556-018-0250-9.
- Pegtel, D.M.; Gould, S.J. Exosomes. Annu. Rev. Biochem. 2019, 88, 487–514, doi:10.1146/annurev-biochem-013118-111902.
- Gong, M.; Yu, B.; Wang, J.; Wang, Y.; Liu, M.; Paul, C.; Millard, R.W.; Xiao, D.-S.; Ashraf, M.; Xu, M. Mesenchymal Stem Cells Release Exosomes That Transfer MiRNAs to Endothelial Cells and Promote Angiogenesis. Oncotarget 2017, 8, 45200–45212, doi:10.18632/oncotarget.16778.
- Wu, R.; Gao, W.; Yao, K.; Ge, J. Roles of Exosomes Derived From Immune Cells in Cardiovascular Diseases. Front. Immunol. 2019, 10, doi:10.3389/fimmu.2019.00648.
- Ahmadi, M.; Rezaie, J. Tumor Cells Derived-Exosomes as Angiogenenic Agents: Possible Therapeutic Implications. J. Transl. Med. 2020, 18, 249, doi:10.1186/s12967-020-02426-5.
- Goetzl, E.J.; Goetzl, L.; Karliner, J.S.; Tang, N.; Pulliam, L. Human Plasma Platelet-Derived Exosomes: Effects of Aspirin. FASEB J. 2016, 30, 2058–2063, doi:https://doi.org/10.1096/fj.201500150R.
- Yu, H.; Wang, Z. Cardiomyocyte-Derived Exosomes: Biological Functions and Potential Therapeutic Implications. Front. Physiol. 2019, 10, doi:10.3389/fphys.2019.01049.
- Wang, J.; Zheng, Y.; Zhao, M. Exosome-Based Cancer Therapy: Implication for Targeting Cancer Stem Cells. Front. Pharmacol. 2017, 7, doi:10.3389/fphar.2016.00533.
- Kalluri, R. The Biology and Function of Exosomes in Cancer. J. Clin. Invest. 2016, 126, 1208–1215, doi:10.1172/JCI81135.
- Sanz-Rubio, D.; Martin-Burriel, I.; Gil, A.; Cubero, P.; Forner, M.; Khalyfa, A.; Marin, J.M. Stability of Circulating Exosomal MiRNAs in Healthy Subjects. Sci. Rep. 2018, 8, 10306, doi:10.1038/s41598-018-28748-5.
- Bartel, D.P. MicroRNAs: Genomics, Biogenesis, Mechanism, and Function. Cell 2004, 116, 281–297, doi:10.1016/S0092-8674(04)00045-5.
- Zhang, J.; Li, S.; Li, L.; Li, M.; Guo, C.; Yao, J.; Mi, S. Exosome and Exosomal MicroRNA: Trafficking, Sorting, and Function. Genomics Proteomics Bioinformatics 2015, 13, 17–24, doi:10.1016/j.gpb.2015.02.001.
- Bellavia, D.; De Luca, A.; Carina, V.; Costa, V.; Raimondi, L.; Salamanna, F.; Alessandro, R.; Fini, M.; Giavaresi, G. Deregulated MiRNAs in Bone Health: Epigenetic Roles in Osteoporosis. Bone 2019, 122, 52–75, doi:10.1016/j.bone.2019.02.013.
- Pethő, A.; Chen, Y.; George, A. Exosomes in Extracellular Matrix Bone Biology. Curr. Osteoporos. Rep. 2018, 16, 58–64, doi:10.1007/s11914-018-0419-y.
- Bellavia, D.; Raimondi, L.; Costa, V.; De Luca, A.; Carina, V.; Maglio, M.; Fini, M.; Alessandro, R.; Giavaresi, G. Engineered Exosomes: A New Promise for the Management of Musculoskeletal Diseases. Biochim. Biophys. Acta BBA - Gen. Subj. 2018, 1862, 1893–1901, doi:10.1016/j.bbagen.2018.06.003.
- Wang, J.; Veirman, K.D.; Faict, S.; Frassanito, M.A.; Ribatti, D.; Vacca, A.; Menu, E. Multiple Myeloma Exosomes Establish a Favourable Bone Marrow Microenvironment with Enhanced Angiogenesis and Immunosuppression. J. Pathol. 2016, 239, 162–173, doi:https://doi.org/10.1002/path.4712.
- Aslan, C.; Maralbashi, S.; Salari, F.; Kahroba, H.; Sigaroodi, F.; Kazemi, T.; Kharaziha, P. Tumor-Derived Exosomes: Implication in Angiogenesis and Antiangiogenesis Cancer Therapy. J. Cell. Physiol. 2019, 234, 16885–16903, doi:https://doi.org/10.1002/jcp.28374.
- Foessl, I.; Kotzbeck, P.; Obermayer-Pietsch, B. MiRNAs as Novel Biomarkers for Bone Related Diseases. J. Lab. Precis. Med. 2019, 4.
- Gao, M.; Gao, W.; Papadimitriou, J.M.; Zhang, C.; Gao, J.; Zheng, M. Exosomes—the Enigmatic Regulators of Bone Homeostasis. Bone Res. 2018, 6, 1–13, doi:10.1038/s41413-018-0039-2.
- Raimondi, L.; De Luca, A.; Fontana, S.; Amodio, N.; Costa, V.; Carina, V.; Bellavia, D.; Raimondo, S.; Siragusa, S.; Monteleone, F.; et al. Multiple Myeloma-Derived Extracellular Vesicles Induce Osteoclastogenesis through the Activation of the XBP1/IRE1α Axis. Cancers 2020, 12, doi:10.3390/cancers12082167.
- Patil, M.; Henderson, J.; Luong, H.; Annamalai, D.; Sreejit, G.; Krishnamurthy, P. The Art of Intercellular Wireless Communications: Exosomes in Heart Disease and Therapy. Front. Cell Dev. Biol. 2019, 7, doi:10.3389/fcell.2019.00315.
- Milano, G.; Biemmi, V.; Lazzarini, E.; Balbi, C.; Ciullo, A.; Bolis, S.; Ameri, P.; Di Silvestre, D.; Mauri, P.; Barile, L.; et al. Intravenous Administration of Cardiac Progenitor Cell-Derived Exosomes Protects against Doxorubicin/Trastuzumab-Induced Cardiac Toxicity. Cardiovasc. Res. 2020, 116, 383–392, doi:10.1093/cvr/cvz108.
- Patel, N.A.; Moss, L.D.; Lee, J.-Y.; Tajiri, N.; Acosta, S.; Hudson, C.; Parag, S.; Cooper, D.R.; Borlongan, C.V.; Bickford, P.C. Long Noncoding RNA MALAT1 in Exosomes Drives Regenerative Function and Modulates Inflammation-Linked Networks Following Traumatic Brain Injury. J. Neuroinflammation 2018, 15, 204, doi:10.1186/s12974-018-1240-3.
- Song, Y.; Li, Z.; He, T.; Qu, M.; Jiang, L.; Li, W.; Shi, X.; Pan, J.; Zhang, L.; Wang, Y.; et al. M2 Microglia-Derived Exosomes Protect the Mouse Brain from Ischemia-Reperfusion Injury via Exosomal MiR-124. Theranostics 2019, 9, 2910–2923, doi:10.7150/thno.30879.
- Goetzl, L.; Merabova, N.; Darbinian, N.; Martirosyan, D.; Poletto, E.; Fugarolas, K.; Menkiti, O. Diagnostic Potential of Neural Exosome Cargo as Biomarkers for Acute Brain Injury. Ann. Clin. Transl. Neurol. 2018, 5, 4–10, doi:https://doi.org/10.1002/acn3.499.
- Lv, L.-L.; Feng, Y.; Tang, T.-T.; Liu, B.-C. New Insight into the Role of Extracellular Vesicles in Kidney Disease. J. Cell. Mol. Med. 2019, 23, 731–739, doi:https://doi.org/10.1111/jcmm.14101.
- Baghaei, K.; Tokhanbigli, S.; Asadzadeh, H.; Nmaki, S.; Zali, M.R.; Hashemi, S.M. Exosomes as a Novel Cell-Free Therapeutic Approach in Gastrointestinal Diseases. J. Cell. Physiol. 2019, 234, 9910–9926, doi:https://doi.org/10.1002/jcp.27934.
- Hu G.; Gong A.-Y.; Roth A.L.; Huang B.Q.; Ward H.D.; Zhu G.; LaRusso N.F.; Hanson N.D.; Chen X.-M. Release of Luminal Exosomes Contributes to TLR4-Mediated Epithelial Antimicrobial Defense. PLOS Pathog. 2013, 9, e1003261, doi:10.1371/journal.ppat.1003261.
- Chew, C.K.; Clarke, B.L. Causes of Low Peak Bone Mass in Women. Maturitas 2018, 111, 61–68, doi:10.1016/j.maturitas.2017.12.010.
- Raggatt, L.J.; Partridge, N.C. Cellular and Molecular Mechanisms of Bone Remodeling. J. Biol. Chem. 2010, 285, 25103–25108, doi:10.1074/jbc.R109.041087.
- Eriksen, E.F. Cellular Mechanisms of Bone Remodeling. Rev. Endocr. Metab. Disord. 2010, 11, 219–227, doi:10.1007/s11154-010-9153-1.
- Behera, J.; Tyagi, N. Exosomes: Mediators of Bone Diseases, Protection, and Therapeutics Potential. Oncoscience 2018, 5, 181–195, doi:10.18632/oncoscience.421.
- Chen, H.; Senda, T.; Kubo, K. The Osteocyte Plays Multiple Roles in Bone Remodeling and Mineral Homeostasis. Med. Mol. Morphol. 2015, 48, 61–68, doi:10.1007/s00795-015-0099-y.
- Han, Y.; Kim, Y.-M.; Kim, H.S.; Lee, K.Y. Melatonin Promotes Osteoblast Differentiation by Regulating Osterix Protein Stability and Expression. Sci. Rep. 2017, 7, 5716, doi:10.1038/s41598-017-06304-x.
- Komori, T. Runx2, an Inducer of Osteoblast and Chondrocyte Differentiation. Histochem. Cell Biol. 2018, 149, 313–323, doi:10.1007/s00418-018-1640-6.
- Martin, T.J.; Sims, N.A. RANKL/OPG; Critical Role in Bone Physiology. Rev. Endocr. Metab. Disord. 2015, 16, 131–139, doi:10.1007/s11154-014-9308-6.
- Cui, Y.; Luan, J.; Li, H.; Zhou, X.; Han, J. Exosomes Derived from Mineralizing Osteoblasts Promote ST2 Cell Osteogenic Differentiation by Alteration of MicroRNA Expression. FEBS Lett. 2016, 590, 185–192, doi:https://doi.org/10.1002/1873-3468.12024.
- Chen, C.; Cheng, P.; Xie, H.; Zhou, H.-D.; Wu, X.-P.; Liao, E.-Y.; Luo, X.-H. MiR-503 Regulates Osteoclastogenesis via Targeting RANK. J. Bone Miner. Res. 2014, 29, 338–347, doi:https://doi.org/10.1002/jbmr.2032.
- Sivan, U.; De Angelis, J.; Kusumbe, A.P. Role of Angiocrine Signals in Bone Development, Homeostasis and Disease. Open Biol. 9, 190144, doi:10.1098/rsob.190144.
- Song, H.; Li, X.; Zhao, Z.; Qian, J.; Wang, Y.; Cui, J.; Weng, W.; Cao, L.; Chen, X.; Hu, Y.; et al. Reversal of Osteoporotic Activity by Endothelial Cell-Secreted Bone Targeting and Biocompatible Exosomes. Nano Lett. 2019, 19, 3040–3048, doi:10.1021/acs.nanolett.9b00287.
- Xu, Z.; Liu, X.; Wang, H.; Li, J.; Dai, L.; Li, J.; Dong, C. Lung Adenocarcinoma Cell-Derived Exosomal MiR-21 Facilitates Osteoclastogenesis. Gene 2018, 666, 116–122, doi:10.1016/j.gene.2018.05.008.
- Guo, L.; Zhu, Y.; Li, L.; Zhou, S.; Yin, G.; Yu, G.; Cui, H. Breast Cancer Cell-Derived Exosomal MiR-20a-5p Promotes the Proliferation and Differentiation of Osteoclasts by Targeting SRCIN1. Cancer Med. 2019, 8, 5687–5701, doi:https://doi.org/10.1002/cam4.2454.
- Huynh, N.; VonMoss, L.; Smith, D.; Rahman, I.; Felemban, M.F.; Zuo, J.; Rody, W.J.; McHugh, K.P.; Holliday, L.S. Characterization of Regulatory Extracellular Vesicles from Osteoclasts. J. Dent. Res. 2016, 95, 673–679, doi:10.1177/0022034516633189.
- Deng, L.; Wang, Y.; Peng, Y.; Wu, Y.; Ding, Y.; Jiang, Y.; Shen, Z.; Fu, Q. Osteoblast-Derived Microvesicles: A Novel Mechanism for Communication between Osteoblasts and Osteoclasts. Bone 2015, 79, 37–42, doi:10.1016/j.bone.2015.05.022.
- Cappariello, A.; Loftus, A.; Muraca, M.; Maurizi, A.; Rucci, N.; Teti, A. Osteoblast-Derived Extracellular Vesicles Are Biological Tools for the Delivery of Active Molecules to Bone. J. Bone Miner. Res. 2018, 33, 517–533, doi:https://doi.org/10.1002/jbmr.3332.
- Lee, W.-C.; Guntur, A.R.; Long, F.; Rosen, C.J. Energy Metabolism of the Osteoblast: Implications for Osteoporosis. Endocr. Rev. 2017, 38, 255–266, doi:10.1210/er.2017-00064.
- Sun, W.; Zhao, C.; Li, Y.; Wang, L.; Nie, G.; Peng, J.; Wang, A.; Zhang, P.; Tian, W.; Li, Q.; et al. Osteoclast-Derived MicroRNA-Containing Exosomes Selectively Inhibit Osteoblast Activity. Cell Discov. 2016, 2, 1–23, doi:10.1038/celldisc.2016.15.
- Wang, X.; Guo, B.; Li, Q.; Peng, J.; Yang, Z.; Wang, A.; Li, D.; Hou, Z.; Lv, K.; Kan, G.; et al. MiR-214 Targets ATF4 to Inhibit Bone Formation. Nat. Med. 2013, 19, 93–100, doi:10.1038/nm.3026.
- Yang, J.-X.; Xie, P.; Li, Y.-S.; Wen, T.; Yang, X.-C. Osteoclast-Derived MiR-23a-5p-Containing Exosomes Inhibit Osteogenic Differentiation by Regulating Runx2. Cell. Signal. 2020, 70, 109504, doi:10.1016/j.cellsig.2019.109504.
- Qin, Y.; Wang, L.; Gao, Z.; Chen, G.; Zhang, C. Bone Marrow Stromal/Stem Cell-Derived Extracellular Vesicles Regulate Osteoblast Activity and Differentiation in Vitro and Promote Bone Regeneration in Vivo. Sci. Rep. 2016, 6, 21961, doi:10.1038/srep21961.
- Wang, X.; Omar, O.; Vazirisani, F.; Thomsen, P.; Ekström, K. Mesenchymal Stem Cell-Derived Exosomes Have Altered MicroRNA Profiles and Induce Osteogenic Differentiation Depending on the Stage of Differentiation. PLOS ONE 2018, 13, e0193059, doi:10.1371/journal.pone.0193059.
- Fang, S.; Li, Y.; Chen, P. Osteogenic Effect of Bone Marrow Mesenchymal Stem Cell-Derived Exosomes on Steroid-Induced Osteonecrosis of the Femoral Head. Drug Des. Devel. Ther. 2018, 13, 45–55, doi:10.2147/DDDT.S178698.
- Li, D.; Liu, J.; Guo, B.; Liang, C.; Dang, L.; Lu, C.; He, X.; Cheung, H.Y.-S.; Xu, L.; Lu, C.; et al. Osteoclast-Derived Exosomal MiR-214-3p Inhibits Osteoblastic Bone Formation. Nat. Commun. 2016, 7, 10872, doi:10.1038/ncomms10872.
- Ramachandran, A.; Ravindran, S.; Huang, C.-C.; George, A. TGF Beta Receptor II Interacting Protein-1, an Intracellular Protein Has an Extracellular Role as a Modulator of Matrix Mineralization. Sci. Rep. 2016, 6, 37885, doi:10.1038/srep37885.
- Xu, J.-F.; Yang, G.; Pan, X.-H.; Zhang, S.-J.; Zhao, C.; Qiu, B.-S.; Gu, H.-F.; Hong, J.-F.; Cao, L.; Chen, Y.; et al. Altered MicroRNA Expression Profile in Exosomes during Osteogenic Differentiation of Human Bone Marrow-Derived Mesenchymal Stem Cells. PLOS ONE 2014, 9, e114627, doi:10.1371/journal.pone.0114627.
- Chen, C.; Wang, D.; Moshaverinia, A.; Liu, D.; Kou, X.; Yu, W.; Yang, R.; Sun, L.; Shi, S. Mesenchymal Stem Cell Transplantation in Tight-Skin Mice Identifies MiR-151-5p as a Therapeutic Target for Systemic Sclerosis. Cell Res. 2017, 27, 559–577, doi:10.1038/cr.2017.11.
- Weilner, S.; Schraml, E.; Wieser, M.; Messner, P.; Schneider, K.; Wassermann, K.; Micutkova, L.; Fortschegger, K.; Maier, A.B.; Westendorp, R.; et al. Secreted Microvesicular MiR-31 Inhibits Osteogenic Differentiation of Mesenchymal Stem Cells. Aging Cell 2016, 15, 744–754, doi:https://doi.org/10.1111/acel.12484.




