Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Franziska Hanschen | + 5239 word(s) | 5239 | 2021-06-02 08:47:08 | | | |
| 2 | Vivi Li | Meta information modification | 5239 | 2021-06-09 08:56:06 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Hanschen, F.; Winkelmann, T. Biofumigation for Fighting Replant Disease. Encyclopedia. Available online: https://encyclopedia.pub/entry/10657 (accessed on 07 February 2026).
Hanschen F, Winkelmann T. Biofumigation for Fighting Replant Disease. Encyclopedia. Available at: https://encyclopedia.pub/entry/10657. Accessed February 07, 2026.
Hanschen, Franziska, Traud Winkelmann. "Biofumigation for Fighting Replant Disease" Encyclopedia, https://encyclopedia.pub/entry/10657 (accessed February 07, 2026).
Hanschen, F., & Winkelmann, T. (2021, June 09). Biofumigation for Fighting Replant Disease. In Encyclopedia. https://encyclopedia.pub/entry/10657
Hanschen, Franziska and Traud Winkelmann. "Biofumigation for Fighting Replant Disease." Encyclopedia. Web. 09 June, 2021.
Copy Citation
Replant disease is a soil (micro-) biome-based, harmfully-disturbed physiological and morphological reaction of plants to replanting similar cultures on the same sites by demonstrating growth retardation and leading to economic losses especially in Rosaceae plant production. Commonly, replant disease is overcome by soil fumigation with toxic chemicals. With chemical soil fumigation being restricted in many countries, other strategies are needed. Biofumigation, which is characterized by the incorporation of Brassicaceae plant materials into soil, is a promising method.
Brassicaceae
glucosinolates
isothiocyanate
microbiome
Rosaceae
replant problems
soil-borne pathogens
1. The Replant Disease Syndrome
After replanting similar crop species at the same site, severe plant growth depression can be observed. This phenomenon has been termed as “replant problems” (then including soil structural and chemical problems [1]), “soil sickness”, “soil decline”, “soil fatigue”, or “replant disease”. Since partial or full soil disinfection can restore plant growth in most cases, biological living agents are the most likely cause of replant problems. According to Winkelmann et al. [2], replant disease is “the harmfully disturbed physiological and morphological reaction of plants to soils that faced alterations in their (micro-) biome due to the previous cultures of the same or related species.” This definition implies the previous culture as the starting point of replant disease, which affects the soil microbial and mesofauna communities by root exudates or rhizodeposits and decomposition of plant parts. Thus, replant disease can be classified as negative plant-soil feedback [3] and is mainly due to microbial dysbiosis [2].
Replant disease has been reported for many horticultural and forestry crops, being especially pronounced in fruit orchards with apple [2][4], peach [5][6] and cherry [7][8], but also affecting roses [9][10], grapevine [11][12], asparagus [13], medicinal plants like Rehmannia glutinosa [14], and several forestry tree species [15].
The etiology and definite causes of replant disease are still not fully understood. It is considered as a disease complex and is strongly influenced by the plant species and genotype as well as by soil properties including soil texture, pH, organic matter content, and aeration or water saturation [2]. The plant as the initiator of replant disease suggests that autotoxicity is involved. This is caused by the release of chemicals, often phenolic secondary metabolites, which are toxic to the same and related plant species [3][16]. These autotoxins were shown to be rapidly degraded by rhizosphere and soil microbes [17], resulting in shifts in microbial community composition. In consequence, the accumulation of pathogenic microorganisms as well as the absence of beneficial, plant growth-promoting microorganisms have been reported as associations of replant diseases of several plant species (reviewed by [2][15]). Frequently mentioned pathogenic fungi and oomycetes include species of the genera Pythium, Fusarium, Ilyonectria (and other Cylindrocarpon-like fungi), and Rhizoctonia (e.g., [13][18][19][20]). Bacterial genera that have been associated with replant situations comprise amongst others, Bacillus and Pseudomonas [3]. With the arrival of new sequencing technologies, replanted soils have recently been subjected to concise microbial community analyses revealing pronounced changes in their structure as well as functions (e.g., [21][22][23][24]). Nematodes can contribute to apple replant disease either as phytopathogenic nematodes or free-living nematodes shaping microbial communities [25][26]. Studies on other soil organisms, especially of the mesofauna, are needed to better understand the complex changes in soil biota in replant situations.
The most obvious counteraction against replant disease is crop rotation or changing of sites. This is, however, no longer possible in many cases, especially in central production areas for fruit or wine in which large investments are taken to set up modern orchards with irrigation systems and nets for protection against hail, for example. Intercropping can help to mitigate replant disease by repelling nematodes or by increasing the diversity of soil biota [15][27]. Similarly, the biodiversity in the soil can be increased by soil amendments, typically with compost [28][29][30]. Anaerobic soil disinfection, i.e., the incorporation of organic carbon under water saturation and sealing with plastic foils that leads to oxygen depletion by facultative anaerobes, was found to be an effective countermeasure against replant disease for instance in apple and cherry [31][32]. Another strategy would be breeding for replant disease tolerance [33][34], but this is time-consuming and difficult as long as the causes and etiology are not resolved. Soil disinfection by heat or chemical means is effective, but ecologically harmful and expensive. Chemicals used for soil fumigation are toxic and nowadays include mainly dazomet or metam sodium (both releasing methyl isothiocyanate) as well as 1,3-dichloropropene/chloropicrine [23][35][36][37]. Interest is shown in developing sustainable management options to potentially replace chemical soil fumigation. Biofumigation that is based on the release of toxic metabolites from biological material of members of the Brassicaceae plant family, is one of the management options for mitigating replant disease.
2. Biofumigation
Upon biofumigation practice, fresh glucosinolate-rich Brassicaceae crops, are chopped and incorporated into the soil in order to achieve natural isothiocyanate formation. Alternatively, Brassicaceae seed meals can be applied [38]. Typical biofumigation crops are mustards such as Brassica juncea, Sinapis alba, Eruca sativa or Raphanus sativus varieties [39][40]. Indian mustard (Brassica juncea), which is rich in allyl glucosinolate, a precursor to allyl isothiocyanate, was most effective in bioassay screenings of Brassicaceae cultivars [41][42][43].
The term “biofumigation” was coined by J. A. Kirkegaard in 1993 [44]. In the mid-nineties of the 20th century, the first studies on biofumigation were performed where some antiherbicidal potential by formation of volatile glucosinolate hydrolysis products in soil was observed [45]. By incorporating Brassicaceae plants into the soil, isothiocyanates and other compounds are released by enzymatic hydrolysis from glucosinolates, secondary plant metabolites occurring in Brassicales plants [46]. So far, around 137 glucosinolates have been identified and according to their variable side chain they can be classified into aliphatic, aryl(aliphatic), and indole glucosinolates [47]. In Brassicaceae plants, glucosinolates are present in all plants parts but their profile and levels differ enormously within plant organs, ontogenetic stages, species, and varieties [48][49][50]. For example, ripe seeds of Indian mustard had a glucosinolate content of 61 µmol/g dry weight (DW), while at flowering stage, mustard stems, roots, and leaves had only around 5, 5, and 4 µmol/g DW, respectively. However, the contents in leaves and roots increased to the “green seeds in pods” stage to approximately 14 and 8 µmol/g DW [48].
The hydrolysis of glucosinolates is initiated when glucosinolates come into contact with myrosinase upon tissue disruption. This β-D-thioglucosidase cleaves β-D-glucose and the intermediary-formed aglucon spontaneously degrades to isothiocyanate or nitrile. Depending on the glucosinolate structure and the presence of specifier proteins, epithionitriles or thiocyanates can also be released [51][52] (Figure 1a). In plants with no or low specifier proteins activity, isothiocyanates usually are the main products [53]. Thiocyanate ions (SCN-, demonstrating weed suppressive effects) can also be released from instable isothiocyanates such as 4-hydroxybenzyl isothiocyanate (released from 4-hydroxybenzyl glucosinolate in Sinapis alba) [54][55] (Figure 1b). Isothiocyanates on the other hand have antimicrobial [56][57][58], antifungal [58][59], and antinematicidal [40][60] properties.
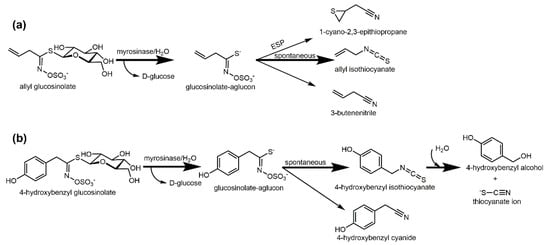
Figure 1. Enzymatic hydrolysis of glucosinolates during biofumigation. (a) Hydrolysis of allyl glucosinolate from Brassica juncea to the corresponding isothiocyanate, nitrile, or, if epithiospecifier proteins (ESP) are present, to the corresponding epithionitrile; (b) Hydrolysis of 4-hydroxybenzyl glucosinolate from Sinapis alba and hydrolysis of the released 4-hydroxybenzyl isothiocyanate to the corresponding alcohol and thiocyanate ions. Figure originally published in Agronomy 2020, 10(3), 425; https://doi.org/10.3390/agronomy10030425
Several factors affect the isothiocyanate formation in the soils: Next to the initial glucosinolate concentration of the plant material, which is usually highest before flowering, the amount of plant material, the myrosinase activity of both plant material and soil, the extent of tissue disruption, the soil temperature, and the water content affect the hydrolysis [38][50][61][62]. Therefore, isothiocyanate levels in soils after biofumigation can range widely from 1 to 100 nmol isothiocyanate/g soil [38]. Calculated effective values for soil sterilization with methyl isothiocyanate range from 517 to 1294 nmol/g soil [63]. For Verticillium dahliae control, a necessary allyl isothiocyanate concentration of 150 nmol/g soil was estimated [42]. Therefore, for soil disinfection via biofumigation, high isothiocyanate levels are needed. Biofumigation with Brassicaceae green manure in this respect is often not efficient in reaching adequately high isothiocyanate levels in the soil, as the isothiocyanate-release efficiency of Brassicaceae biomass is typical below 5% due to insufficient cell disruption [38][62][64]. Moreover, the conversion rate can vary between glucosinolates, cover crops and years of cultivation [65]. Thus, the crucial factor is the release of isothiocyanates into the soil and not the glucosinolate levels of the plants themselves [62]. By optimizing the preparation of soil and tissue disruption, higher conversion rates can be achieved. For example, a total isothiocyanate concentration of 91 nmol/g field soil was reached by irrigating the soil 2 days prior to biofumigation (30 mm), then grinding the above-ground high-glucosinolate Brassica juncea tissue (by using a rotating flail mulcher running at high revolutions and low ground speed and grinding the plant material to a maximal size of 3 × 3 cm), followed by immediately incorporating it into the first 10 cm with a rotary hoe and consolidating the soil with two passes of a rubber-tyre roller. Finally, the soil again was irrigated (18 mm) within 3 h [44][62][66]. Nevertheless, often a more practical approach is the use of seed meal to overcome these obstacles: Brassicaceae seeds have higher levels of glucosinolates compared to fresh plant materials [48]. Thus, in Brassicaceae seed meals optimized for biofumigation glucosinolates range from 170 µmol up to 303 µmol/g seed meal [67][68].
Due to the tissue already being homogenized, the hydrolysis of glucosinolates from seed meals after water addition is more efficient than in fresh material. More finely ground B. juncea cv. Pacific Gold seed meal (≤ 1mm) released allyl isothiocyanates at a higher rate compared to coarse seed meal (2–4 mm) (5–7 nmol/g soil compared to 4–5 nmol/g soil). Nevertheless, the conversion rate detected in that study was still low (303 µmol glucosinolates/g seed meal, 3 mg seed meal/g soil applied = 909 nmol glucosinolates/g soil applied; = conversion rate of 7.7%). However, it has to be kept in mind that in that study, only the head space above the treated soil was sampled and not the soil itself [69]. Further, waterlogging to enhance isothiocyanate release [62] and soil tarping with plastic foils to keep the volatile compounds in the soil [67] are recommended. Usually, the release of glucosinolate hydrolysis products is fast and in many studies the highest isothiocyanate levels were detected in the first few hours after the biofumigation treatment [53][62][63][65][70][71]. Release of thiocyanate ions (SCN-) is slower, and SCN- is more persistent in the soil [63].
Not only their formation but also isothiocyanate degradation has to be considered for successful results. Especially lipophilic isothiocyanate levels can decline due to sorption to soil particles and soils rich in organic matter absorb more isothiocyanates [38][72][73][74]. The sorption to soil organic matter also seems to influence the vapor concentration of isothiocyanates in soil (or in the headspace) [75][76], which also reduced the disinfestation efficacy of isothiocyanates in volatile toxicity assays where only isothiocyanates in the gaseous phase contacted the test organism [77]. Therefore, sandy soils with usually low organic matter content will reach higher isothiocyanate peak concentrations compared to soils with high organic matter (for example peat). The disinfestation efficacy of isothiocyanates also depends on the temperature: Using volatile toxicity assays, isothiocyanates were more toxic at higher temperatures compared to lower temperatures (5–20 °C tested, in vitro and with soil [moisture content 75% of field capacity; sand (pH 7.2, 3.26% organic matter), loam (pH 4.9, 6.77% organic matter), and peat (pH 5.69, 31.55% organic matter) tested]) [77]. Again, due to lower organic matter content, assays in sandy soils were more effectively compared to peat soils [77]. Moreover, some of the non-sorbed isothiocyanates may escape due to evaporation [38], but probably most isothiocyanates are degraded due to biodegradation [73][78][79] with chemical degradation playing a minor role [53]. Repeated treatment of soils with Brassicaceae crops (or the same compounds) can stimulate biodegradation [44][79]. Biodegradation increases with elevated pH combined with elevated calcium levels in the soils [44]. Thus, for successful biofumigation, optimal plant material, pretreatment; dosage; weather; and also the soils and their preparation, determine the outcome.
However, several studies could not directly correlate the effects of Brassicaceae biofumigation with glucosinolate or isothiocyanate contents in the treated soils [80][81][82][83]. These studies imply that shifts in the microbial community structure are responsible for the effects of biofumigation resulting in disease suppression [80][81][82][84]. In addition, Brassica green manure crops effectively incorporated soil mineral nitrogen that may otherwise leach to the groundwater. Thus, when later incorporated into the soil, Brassica materials can provide a source of organic nitrogen [85].
Other compounds formed during Brassicaceae biomass decomposition may also contribute to biofumigation effects. Brassicaceae plants were shown also to release other volatile sulfur-containing compounds. In addition to methanthiol, carbondisulfide, dimethylsulfide and dimethyldisulfide were generated after soil incorporation of Brassicaceae plants [86][87][88]. Toxic effects have been shown for these compounds on soil microorganisms [89][90]. Dimethyldisulfide is also the active component of an approved chemical fumigant in the USA [91]. Probably, these volatile compounds are degradation products of sulfur containing amino acids such as S-methyl-L-cysteine sulfoxide inherently formed in Brassicaceae plants [92][93].
One important aspect for the implementation and economic consideration of biofumigation for the agricultural and horticultural practice is the availability of the plant material that needs to be incorporated into the soil. If Brassicaceaes are grown as a rotation crop, farmers lose time for their cash crops. Thus, and also due to the fact that seed meal or oil-less seed cakes contain high amounts of glucosinolates, the use of these by-products of the biofuel production can enable the provisioning of the required biomass [94][95].
3. Effects of Biofumigation on the Soil Biota
Biofumigation treatments affect organisms in the soil. Next to intended effects on plant pathogens, beneficial soil invertebrates can also be affected. 2-Phenylethyl isothiocyanate showed acute toxicity on the soil arthropods Folsomia candida and Protaphorura fimata [96], the isopod Porcellio scaber [97], and the earthworm Eisenia andrei [98][99].
One of the most investigated effects of biofumigation is the nematicidal effect, which was recently reviewed by Dutta et al. [100]. Especially Brassicaceae plants releasing the aliphatic allyl isothiocyanate as well as aromatic isothiocyanates such as 2-phenylethyl and benzyl isothiocyanate were promising against these nematodes, but not all stages of the pest are equally susceptible to the treatment [100]. Dutta et al. concluded that biofumigation with Brassicaceae tissues is helpful in plant parasitic nematode control, but that it is unlikely that biofumigation alone will eliminate plant parasitic nematodes in soil. However, in combination with other techniques such as soil solarization or minimal use of nematicides, biofumigation may enable acceptable plant parasitic nematode control [100].
The soil microbial community is important for plant health. Both pathogenic and beneficial strains affect plant growth and health [101]. A shift in microbial community composition with the accumulation of pathogenic microorganisms and the absence of plant growth-promoting microorganisms are linked with replant diseases of several plant species [2][15]. Biofumigation can alter the soil microbial community. Isothiocyanates were reported to inhibit nitrifying bacteria in in vitro bioassays at a dose of 10 µg isothiocyanate/g soil (= 101 nmol allyl isothiocyanate/g soil) depending on the soil type (using sandy- and clay-loam soil, pH 5.9 and pH 7.5, respectively; soils moistened to −480 kPa, incubation at 15 °C for up to 42 days) [102]. Interestingly, in laboratory experiments, 0.32 µmol allyl glucosinolate/g soil [slightly loamy sand and sandy soil (pH 4.8–5.3), water holding capacity 100%] affected the soil microbial communities even stronger than in combination with myrosinase (0.16 µmol/g soil + 0.02 units of myrosinase/g soil), which released the isothiocyanate (room temperature, sampling after 7 days) [53]. Moreover, Siebers et al. reported a decline in soil microbial diversity as accessed by next generation sequencing (sampling after 7–28 days) in a laboratory experiment after soil (loamy sand, pH 6.1) treatment with a rapeseed extract (RSE) rich in glucosinolate hydrolysis products (33 µL RSE/g soil (incubation at 21 °C, moisture less than 18%, RSE addition every 3 days for up to 28 days; in sum ~575 nmol goitrin and ~366 nmol sinapic acid choline ester/g soil added). However, when cultivating surviving fungi and bacteria from treated soils, many of these strains could mobilize phosphate from insoluble sources and had growth-promoting properties on Arabidopsis thaliana [103]. Therefore, one important role of glucosinolate hydrolysis products in the efficiency of biofumigation seems to be the potential to favor beneficial microbiota. While metham sodium treatment reduced soil microbial activity in pot experiments (300 µg/g sandy loam soil, pH 7.2, water holding capacity set to 45%, sampling after 3, 15, and 60 days at 23 °C), an increase in soil microbial activity and specific changes in ascomycetes strain abundance were reported after biofumigation with broccoli leaves in a laboratory experiment (15 mg homogenized broccoli leaves/g dry soil, water holding capacity set to 45%) [82]. This effect was probably due to microbial responses to C-substrates, as the response to myrosinase treated broccoli was less pronounced [82]. Organic amendments such as (defatted) seed meals add organic carbon and nitrogen into the soil that are easily available for soil microbial degradation [94]. Moreover, biofumigation with rapeseed meal increased soil content of NO3−, available P and available K [104]. Thus, increased soil respiration rates as well as enzymatic activities (for example β-glucosidase) were observed in the first month after biofumigation with Brassica carinata seed meal or sunflower seed meals, both obtained from a biofuel byproduct (3 t/ha applied on clay soil, tillage of soil) [105]. Four weeks after biofumigation in field experiments using Indian mustard and radish, there was a shift in soil bacterial community and even more so in fungal community composition: some strains vanished while other strains were promoted due to biofumigation (sandy soil and sandy loamy sand, biofumigation at full flowering of cover crops) [71]. In another field experiment, biofumigation with mustard (3.5 kg/m2 of cut material) increased the biodiversity in bacteria and fungi compared to control and fumigated soils, as observed by denaturing gradient gel electrophoresis (DGGE) [106]. Here, treatment with mustard having a glucosinolate content of 38.5 µmol/g DW (being mainly 3-butenyl glucosinolate) was similarly effective in the control of Fusarium oxysporum compared to soil fumigation with hymexazol [106]. Biofumigation with rapeseed meal reduced disease incidence of Phytophthora blight and significantly increased yield in pepper in a field experiment (loam clay soil, pH 7.2, 0.4% w/w of rapeseed meal incorporated, irrigated after incorporation, covered with plastic foil), although no reductions in Phytophthora capsici counts were observed. However, the biofumigation increased richness and bacterial diversity, while it decreased fungal diversity. Thus, changes in soil microbial community structure were hypothesized to be responsible for the disease suppression. The group further reported a negative correlation between soil bacterial diversity and disease incidence of Phytophthora blight [107]. In this experiment, biofumigation of soil pots with rapeseed meal (soil pH 7.2, 4 g rapeseed meal/kg dry soil; water 50% of water holding capacity, soil covered with plastic film after incorporation, incubation at 25 °C for 20 days) increased soil bacterial diversity, bacterial populations including Bacillus and Actinobacteria, and reduced Phytophthora capsici and disease incidence [107]. The use of integrated biofumigation with an antagonistic strain (Bacillus amyloliquefaciens) (application of strain after biofumigation) further increased disease suppression effectiveness of biofumigation [107]. Repeated biofumigation with B. carinata pellets (Biofence®) and Sinapis alba green manure (clay loam, pH 6.4, treatments over three growth periods) showed the highest increase in total bacteria, actinomycetes and Pseudomonas ssp. in treated soils compared to soils treated with other non-Brassica-based organic amendments [108]. Further, Pseudomonas ssp abundance was negatively correlated with the growth of the plant pathogen Rhizoctonia solani [108]. Mowlick et al. suggested that Clostridia, members of the Firmicutes, play an important role in the control of spinach wilt. Clostridia-induced organic acid release was discussed as a possible mode of action to explain the effects of biofumigation (B. juncea) and Avena sativa green manure treatment [109].
Several of the bacterial genera that were observed to be favored due to biofumigation, such as Pseudomonas, are known to have beneficial properties. Pseudomonas spp. are beneficial bacteria for plant growth as they act as antagonists against soil pathogenic fungi and enhance sulfate uptake [30][110][111]. Moreover, several members of the phylum Actinobacteria with plant growth-promoting properties are involved in soil-borne disease suppression [30][112]. Therefore, biofumigation-induced increase in plant growth-promoting and disease-suppressing bacteria seems to be an important mechanism in biofumigation efficiency.
4. Efficacy of Biofumigation on Replant Disease
Measuring the effectiveness of a management strategy against replant disease is not a trivial task. Up to now, a comparison of plant growth in replant soil and disinfected replant soil seems to be the most reliable measure (e.g. [23]). Early diagnostic tools were suggested using microscopic preparations of apple roots [113], however, they might not allow to quantify the severity of the disease. A possible approach is to develop early genetic markers on the transcriptional level.
Table 1 summarizes the literature investigating biofumigation for fighting replant diseases of different plant species. Due to the worldwide economic relevance, most studies published so far addressed apple replant disease. Generally, it has been shown that plant growth and fruit yields were significantly improved by biofumigation treatments, especially if Brassicaceae seed meal was used. Nevertheless, due to these treatments, considerable amounts of organic material are added to the soils, which also may contribute to the positive treatment effects due to improved soil structure and provision of nutrients [44]. For example, soil aggregate stability and water infiltration in sandy soils were described to be improved after biofumigation [44].
Table 1. Overview of studies using biofumigation as counteraction of replant disease. Ref.-Reference. Table originally published in Agronomy 2020, 10(3), 425; https://doi.org/10.3390/agronomy10030425
| Kind of Replant Disease | Biofumigation Treatment | Environmental Conditions | Measurement of Efficacy by | Efficacy | Observations | Ref. |
|---|---|---|---|---|---|---|
| Apple replant disease | Brassica napus as cover crop | No information provided | Field trials, counts of Pratylenchus penetrans and recovery of Pythium from soil | No positive effects | No reduction, but rather an increase in Pratylenchus penetrans and Pythium abundance | [114] |
| Apple replant disease | B. napus seed meal 0.1–2.0% | Incubation in the greenhouse (20 °C), no information on soil moisture etc. | Greenhouse pot trials | Increased plant growth, but toxic effects at high concentration. | No consistent reduction in Pythium infections, suppression of Rhizoctonia and Pratylenchus penetrans at 0.1% and increased abundance of fluorescent Pseudomonas spp. at 0.1 and 1.0% | [81] |
| Apple replant disease | B. napus seed meal 8.5 t ha−1 and green manure (for one-three years) | Seed meal incorporation in May 2001, some variants covered by plastic foil (no information on soil temperature/moisture) | Field trial with tree growth and yield measurements | Growth and yield improvement by both, B. napus green manure and seed meal treatments, especially when combined with fungicide treatment. | Reduction of ARD associated pathogens, i.e., Pratylenchus penetrans, Pythium, Cylindrocarpon, Rhizoctonia, but not Fusarium by the combined treatment of B. napus seed meal and fungicide, not by green manure. | [115] |
| Apple replant disease | Brassica juncea plant material (1–3 years) and B. napus seed meal combined with other treatments | No information provided | Field test and greenhouse bio-test of plant growth and yield (field) | Cumulative yield increase in a site-dependent way, mainly by seed meal treatments | Control of Cylindrocarpon, Rhizoctonia and Pythium ultimum by seed meal treatments, best in combination with a fungicide treatment, lower effect on Pratylenchus penetrans | [116] |
| Straw-berry replant disease | B. juncea cover crop incorporated into the soil | Incorporation of plant residues in April 2002, no further information on soil temperature or moisture | Pot trial and field experiment | Fruit yield as well as vegetative growth parameters increased in the pot and the field trial | Rhizoctonia abundance was reduced by mustard treatment, but causes for this kind of replant disease is not clear. | [117] |
| Apple replant disease | Seed meals of B. juncea, Sinapis alba and B. napus; 0.5% (wt/wt) | Eight weeks of incubation at 22 ± 3 °C, no information on soil moisture | Greenhouse bio-test in pots | Seed meal improved apple seedling growth, seed meal reduced Rhizoctonia solani infection in native but not in pasteurized soil, while Streptomyces ssp. increased it | B. juncea seed meal was most effective in Pratylenchus penetrans suppression and the only seed meal that did not increase Pythium populations | [70] |
| Apple replant disease | Seed meals of B. juncea, S. alba and B. napus; 0.5% (vol/vol) | Blending and sieving (< 1 mm) of seed meals, 8 weeks of incubation at 22 ± 3 °C, no information on soil moisture | Greenhouse bio-test in pots | Seed meal-specific effects on Pythium and Pratylenchus penetrans numbers and infections. | B. juncea seed meal suppressed Pythium and P. penetrans populations. | [118] |
| Apple replant disease | Seed meal of B. juncea; 0.3% (wt/wt) = 4.5 t ha−1 | Fine (<1 mm) and coarse (2–4 mm) seed meal particles incorporated, no further information on soil temperature or moisture | Bio-test in greenhouse, variation of particle sizes of seed meal | Suppression of Rhizoctonia solani SG5 (for fine seed meal), Pratylenchus penetrans and Pythium spp. infections | Biological and chemical effects of the seed meal, increased population densities of Streptomyces and more free-living nematodes | [69] |
| Apple replant disease | Seed meals of B. juncea, S. alba and B. napus; 4.5 kg m−1 tree row | Incorporation in April 2005, May 2006, April 2007, respectively, tarped with plastic foil for 1 week, no further information on soil temperature or moisture | Field trial with measures of tree diameter and cumulative yield | Significant improvement of tree growth and cumulative fruit yield when seed meals (except for B. napus) were combined with fungicide soil drench | Seed meal specific effects, B. napus resulted in increased Pythium and Pratylenchus penetrans densities, whereas B. juncea reduced both pathogens as well as Cylindrocarpon infections but only when combined with fungicide drench. Without fungicide treatment, B. napus and S. alba seed meal amendments caused Pythium and B. juncea caused Phytophtora infections. | [68] |
| Apple replant disease | Seed meals of B. juncea, S. alba and B. napus; 0.3% (wt/wt) | Blending and sieving (<1 mm) of seed meals, 48 h incubation in plastic bags, no information on soil temperature or moisture | Bio-test in greenhouse | Reduction of apple seedling mortality after B. juncea seed meal application in one soil. | Soil-dependent and seed meal-dependent shifts in Pythium communities, S. alba led to increased P. ultimum levels. | [119] |
| Apple replant disease | Seed meal of B. juncea; 0.3% (wt/wt) = 4.5 t ha−1 | Fine (<1 mm) and coarse (2–4 mm) seed meal particles incorporated, bagged or non-bagged incubation for 48 h, no further information on soil temperature or moisture | Bio-test in greenhouse | Reduction of Pythium abappressorium infections, especially in the bagged variants | Suppressiveness of soil was achieved, possibly due to long-term changes in fungal communities, especially promotion of Trichoderma spp. | [120] |
| Apple replant disease | Seed meal blends of B. juncea, S. alba and B. napus; 6.7 t ha−1 | Incorporation of seed meals once in March 2010 or twice in September 2009 and April 2010, tarped for 1 week, no further information on soil temperature or moisture | Field test of plant growth and yield | Significant increase in tree growth of B. juncea + S. alba, positive long-term effect (4 years), but mortality if applied few weeks prior to planting. Efficacy superior to chemical fumigation | Effective reduction of Pratylenchus penetrans mainly in the first year, Pythium infections enduringly reduced. Resilient changes in rhizosphere microbial communities. | [121] |
| Peach replant disease | B. juncea plant biomass and canola seed meal cake in a field experiment | Watering before incorporation in June 20, 1 day later tarping, recording of soil temperature during the 2-months treatment (26–34 °C) | Field test of plant growth | Significantly improved tree growth | Better plant health, lower mortality | [122] |
| Apple replant disease | Incorporation of plant material of B. juncea and Raphanus sativus in the field | Incorporation in May and August 2012 and 2013, no further information on soil temperature or moisture | Greenhouse bio-test of plant growth and field test | Site specific increase in biomass production after biofumigation. | Nutrient effect and stronger shifts in fungal than in bacterial community composition | [71] |
| Apple replant disease | Incorporation of plant material of B. juncea and R. sativus in the field | Incorporation in May and August 2012 and 2013, no further information on soil temperature or moisture | Field test of plant growth | Site specific effects (only in the tested sandy soil about 150% increase in growth, no significant change in the second soil). | Bacterial genera with increased abundance: Arthrobacter (R. sativus), Ferruginibacter (B. juncea, R. sativus). Fungal genera of higher abundance: Podospora, Monographella and Mucor (B. juncea, R. sativus) | [27] |
| Apple replant disease | Incorporation of seed meal formulation of B. juncea and S. alba 1:1 in the field, 2.2, 4.4, 6.6 t ha−1 | Incorporation in April 2016, tarping for 2 weeks, soil temperature: 12−14 °C, no information on soil moisture | Field test of plant growth | Significantly improved tree growth, 4.4 t ha−1 was optimal | Soil fumigation and seed meal amendments suppressed Pythium infection in rootstook-specific way. Long-term effect on soil microbial communities. Beneficial microbes increased due to biofumigation | [67] |
| Apple replant disease | Incorporation of seed meal formulation of B. juncea and S. alba 1:1, dosage, 2.2, 4.4, 6.6 t ha−1 | Incorporation into moist soil (−63 to −92 hPa), incubation in bags for 48 h under greenhouse conditions | Greenhouse bio-test in pots | Significantly improved tree growth at all dosages; no difference between 4.4 and 6.6 t ha−1, high efficacy in P. penetrans and Pythium ssp. control | Geneva rootstocks had less colonization by Pythium ssp. or P. penetrans compared to Mailling rootstocks; both rootstock genotype and soil treatment affected soil microbiom | [123] |
The efficacy was demonstrated to depend on the soil and site and its prevalent pathogenic organisms. A focus of many studies was on the effects of biofumigation on reducing major causal agents of apple (or strawberry) replant disease, mainly Pythium, Rhizoctonia, Cylindrocarpon, and Pratylenchus penetrans [70][115][116][117][118][121]. This reduction in several pathogens was attributed to chemical and nutritional effects of the treatments but also to biological effects, i.e., changes in microbial communities and increased abundance of disease-suppressive microbes, such as fluorescent pseudomonads [81], Streptomyces spp. [69] or Trichoderma spp. [120]. Sometimes, however, and depending on the amount of amended seed meal and especially on the Brassicaceae plant species and its glucosinolate content and composition, even increased populations of Pythium and the nematode Pratylenchus penetrans or toxic effects on the cultivated plants have been reported [81]. The latter was probably due to thiocyanate ions, which have phytotoxic activity [54]. In a recent study, apple replant disease incidence declined in soils biofumigated with Raphanus sativus or B. juncea covering crops for 2 years in a site-dependent manner (field plot experiment, biofumigation twice a year at full bloom into moist soil, mechanical cutting and chopping of plants with a flail mulcher, immediate incorporation with a rotary cultivator; soil layering with rolls of a sowing machine, no soil tarping). In sandy soil (pH 5.2) K, the effect was superior to that of slightly loamy sand soil A with pH 4.8. Biofumigation in slightly loamy sand soil M with pH 5.7 did not increase the growth of indicator plants [71]. This correlated to the shifts in the bacterial and fungal communities (analyzed 30 days after second biofumigation in each year), which were strongest at soil K [71], and thus, again point to the role of the soil microbial community in the cure of replant disease. Later, using amplicon sequencing, bacterial genera (for example Arthrobacter or Ferruginibacter) and fungal genera (for example Podospora) were identified that were increased due to biofumigation with the cover crops and were positively linked with growth of apple M106 plants. In contrast, the bacterial genera Flavitalea and the fungal genera unclassified Pleosporales, Cryptococcus, and Mucor were negatively correlated with the growth of M106 plants [27].
Brassicaceae seed meals from a single species so far failed to achieve a similar control of replant disease compared to chemical fumigation (rotovated soil, application of 2.23 kg/m2 of seed meal, coverage with plastic film for 1 week after rotovation) [68]. Appropriately implemented biofumigations with particularly formulated seed meals of different Brassicaceae species performed in soil disinfestation comparably to chemical fumigation treatments [67][68][121]. When comparing seed meal formulations, formulations of Brassica juncea [rich in allyl glucosinolate (sinigrin), Figure 1a] in combination with Sinapis alba [rich in 4-hydroxybenzyl glucosinolate (sinalbin), Figure 1b] were superiorly compared to B. juncea-Brassica napus seed meal [121]. In order to reach effective concentrations of glucosinolate breakdown products, high amounts of Brassicaceae seed meal have to be incorporated into the soils, ranging from 1.5 t ha−1 (= 0.1% wt/wt) [81] up to 8.5 t ha−1 [115], with toxic effects at 30 t ha−1 (= 2.0% wt/wt) [81]. However, the glucosinolate content is not necessarily linked to the efficacy of the biofumigation on replant disease, and the latest studies observed best effects at reduced seed meal levels of 4.4 t ha−1 (seed meal formulation of 1:1 B. juncea and S. alba with 173 µmol glucosinolates/g, tillage applied on soil (Burch loam, pH 6.8) before and after biofumigation, plastic foil cover after incorporation, temperature 12–14 °C) [67][123]. Part of the observed variability in the efficacy of biofumigation especially in field trials may also be due to other environmental factors influencing plant growth in addition to replant disease, which also differs in severity. From the data presented in Table 1, it becomes obvious that environmental factors, such as soil temperature and moisture were not considered in most of the studies. The important question of tarping in the case of field application and bagging or sealing in the case of laboratory and greenhouse application and its duration was shown to have a dramatic effect on efficacy in terms of pathogen suppressiveness [117]. Moreover, the apple genotype affects the efficacy of disease control, and usage of more tolerant apple rootstocks is recommended [67][123].
To summarize, so far, the best results against replant disease have been achieved using a seed meal formulation of Brassica juncea and Sinapis alba (1:1) at an application rate of 4.4 t/ha in combination with tolerant apple rootstocks. Here, mainly the effect of biofumigation on the soil microbiome and nematodes is linked to the plant health improvement. However, more research is needed in order to optimize its efficacy, which is also site-dependent. Future studies should address the optimal timing as well as amount and type of incorporated plant material in dependence of the soil physical and chemical characteristics. In-depth studies should unravel the effects of Brassicaceae biomass but also glucosinolates and glucosinolate breakdown products on different key soil organisms in order to come to designed mixtures of plant materials that can be used in effective biofumigation treatments.
References
- Utkhede, R.S. Soil sickness, replant problem or replant disease and its integrated control. Allelopath. J. 2006, 18, 23–38.
- Winkelmann, T.; Smalla, K.; Amelung, W.; Baab, G.; Grunewaldt-Stöcker, G.; Kanfra, X.; Meyhöfer, R.; Reim, S.; Schmitz, M.; Vetterlein, D. Apple replant disease: Causes and mitigation strategies. Curr. Issues Mol. Biol. 2019, 30, 89–106.
- Huang, L.-F.; Song, L.-X.; Xia, X.-J.; Mao, W.-H.; Shi, K.; Zhou, Y.-H.; Yu, J.-Q. Plant-soil feedbacks and soil sickness: From mechanisms to application in agriculture. J. Chem. Ecol. 2013, 39, 232–242.
- Mazzola, M.; Manici, L.M. Apple replant disease: Role of microbial ecology in cause and control. Annu. Rev. Phytopathol. 2012, 50, 45–65.
- Israel, D.W.; Giddens, J.E.; Powell, W.W. The toxicity of peach tree roots. Plant Soil 1973, 39, 103–112.
- Benizri, E.; Piutti, S.; Verger, S.; Pagès, L.; Vercambre, G.; Poessel, J.L.; Michelot, P. Replant diseases: Bacterial community structure and diversity in peach rhizosphere as determined by metabolic and genetic fingerprinting. Soil Biol. Biochem. 2005, 37, 1738–1746.
- Mai, W.F.; Abawi, G.S. Determining the cause and extent of apple, cherry and pear replant diseases under controlled conditions. Phytopathology 1978, 68, 1540–1544.
- Reginato, G.; Córdova, C.; Mauro, C. Evaluation of rootstock and management practices to avoid cherry replant diesease in Chile. Acta Hortic. 2008, 795, 357–362.
- Spethmann, W.; Otto, G. Replant problems and soil sickness. In Encyclopedia of Rose Science; Roberts, A.V., Debener, T., Gudin, S., Eds.; Elsevier: Oxford, UK, 2003; pp. 169–180.
- Baumann, A.; Yim, B.; Grunewaldt-Stöcker, G.; Liu, B.; Beerhues, L.; Sapp, M.; Nesme, J.; Sørensen, S.J.; Smalla, K.; Winkelmann, T. Rose replant disease: Detailed analyses of plant reactions, root endophytes and rhizosphere microbial communities. Acta Hortic. 2020, in press.
- Deal, D.; Mail, W.; Boothroyd, C. A survey of biotic relationships in grape replant situations. Phytopathology 1972, 62, 503–507.
- Westphal, A.; Browne, G.T.; Schneider, S. Evidence for biological nature of the grape replant problem in California. Plant Soil 2002, 242, 197–203.
- Elmer, W. Asparagus decline and replant problem: A look back and a look forward at strategies for mitigating losses. Acta Hortic. 2018, 1223, 195–204.
- Wu, L.; Wang, J.; Huang, W.; Wu, H.; Chen, J.; Yang, Y.; Zhang, Z.; Lin, W. Plant-microbe rhizosphere interactions mediated by Rehmannia glutinosa root exudates under consecutive monoculture. Sci. Rep. 2015, 5, 15871.
- Long, F.; Lin, Y.M.; Hong, T.; Wu, C.Z.; Li, J. Soil sickness in horticulture and forestry: A review. Allelopathy J. 2019, 47, 57–72.
- Singh, H.P.; Batish, D.R.; Kohli, R.K. Autotoxicity: Concept, organisms, and ecological significance. Crit. Rev. Plant Sci. 1999, 18, 757–772.
- Kaur, H.; Kaur, R.; Kaur, S.; Baldwin, I.T.; Inderjit. Taking ecological function seriously: Soil microbial communities can obviate allelopathic effects of released metabolites. PLoS ONE 2009, 4, e4700.
- Mazzola, M. Identification and pathogenicity of Rhizoctonia spp. isolated from apple roots and orchard soils. Phytopathology 1997, 87, 582–587.
- Mazzola, M. Elucidation of the microbial complex having a causal role in the development of apple replant disease in Washington. Phytopathology 1998, 88, 930–938.
- Manici, L.M.; Kelderer, M.; Caputo, F.; Saccà, M.L.; Nicoletti, F.; Topp, A.R.; Mazzola, M. Involvement of Dactylonectria and Ilyonectria spp. in tree decline affecting multi-generation apple orchards. Plant Soil 2018, 425, 217–230.
- Tewoldemedhin, Y.T.; Mazzola, M.; Labuschagne, I.; McLeod, A. A multi-phasic approach reveals that apple replant disease is caused by multiple biological agents, with some agents acting synergistically. Soil Biol. Biochem. 2011, 43, 1917–1927.
- Franke-Whittle, I.H.; Manici, L.M.; Insam, H.; Stres, B. Rhizosphere bacteria and fungi associated with plant growth in soils of three replanted apple orchards. Plant Soil 2015, 395, 317–333.
- Yim, B.; Winkelmann, T.; Ding, G.-C.; Smalla, K. Different bacterial communities in heat and gamma irradiation treated replant disease soils revealed by 16S rRNA gene analysis—Contribution to improved aboveground apple plant growth? Front. Microbiol. 2015, 6.
- Radl, V.; Winkler, J.B.; Kublik, S.; Yang, L.; Winkelmann, T.; Vestergaard, G.; Schröder, P.; Schloter, M. Reduced microbial potential for the degradation of phenolic compounds in the rhizosphere of apple plantlets grown in soils affected by replant disease. Environ. Microbiome 2019, 14, 8.
- Jaffee, B.A.; Abawi, G.S.; Mai, W.F. Role of soil microflora and Pratylenchus penetrans in an apple replant disease. Phytopathology 1982, 72, 247–251.
- Kanfra, X.; Liu, B.; Beerhues, L.; Sørensen, S.J.; Heuer, H. Free-living nematodes together with associated microbes play an essential role in apple replant disease. Front. Plant. Sci. 2018, 9, 1666.
- Yim, B.; Nitt, H.; Wrede, A.; Jacquiod, S.; Sørensen, S.J.; Winkelmann, T.; Smalla, K. Effects of soil pre-teatment with Basamid® ganules, Brassica juncea, Raphanus sativus, and Tagetes patula on bacterial and fungal communities at two apple replant disease sites. Front. Microbiol. 2017, 8.
- Forge, T.; Neilsen, G.; Neilsen, D. Organically acceptable practices to improve replant success of temperate tree-fruit crops. Sci. Hort. 2016, 200, 205–214.
- Franke-Whittle, I.H.; Juárez, M.F.-D.; Insam, H.; Schweizer, S.; Naef, A.; Topp, A.-R.; Kelderer, M.; Rühmer, T.; Baab, G.; Henfrey, J.; et al. Performance evaluation of locally available composts to reduce replant disease in apple orchards of central Europe. Renew. Agric. Food Syst. 2018, 34, 543–557.
- De Corato, U. Disease-suppressive compost enhances natural soil suppressiveness against soil-borne plant pathogens: A critical review. Rhizosphere 2020, 13, 100192.
- Hewavitharana, S.S.; Mazzola, M. Carbon source-dependent efects of aaerobic soil disinfestation on soil microbiome and suppression of Rhizoctonia solani AG-5 and Pratylenchus penetrans. Phytopathology 2016, 106, 1015–1028.
- Browne, G.; Ott, N.; Poret-Peterson, A.; Gouran, H.; Lampinen, B. Efficacy of anaerobic soil disinfestation for control of Prunus replant disease. Plant Dis. 2018, 102, 209–219.
- Dorcas, K.I.; Ian, A.M. Malus germplasm varies in resistance or tolerance to apple replant disease in a mixture of New York orchard soils. HortScience 2000, 35, 262–268.
- Reim, S.; Siewert, C.; Winkelmann, T.; Wöhner, T.; Hanke, M.-V.; Flachowsky, H. Evaluation of Malus genetic resources for tolerance to apple replant disease (ARD). Sci. Hort. 2019, 256, 108517.
- Nicola, L.; Turco, E.; Albanese, D.; Donati, C.; Thalheimer, M.; Pindo, M.; Insam, H.; Cavalieri, D.; Pertot, I. Fumigation with dazomet modifies soil microbiota in apple orchards affected by replant disease. Appl. Soil Ecol. 2017, 113, 71–79.
- Nyoni, M.; Mazzola, M.; Wessels, J.P.B.; McLeod, A. The efficacy of semiselective chemicals and chloropicrin/1,3-dichloropropene–containing fumigants in managing apple replant disease in South Africa. Plant Dis. 2019, 103, 1363–1373.
- Rudolph, R.E.; Zasada, I.A.; Hesse, C.; DeVetter, L.W. Brassicaceous seed meal, root removal, and chemical fumigation vary in their effects on soil quality parameters and Pratylenchus penetrans in a replanted floricane raspberry production system. Appl. Soil Ecol. 2019, 133, 44–51.
- Gimsing, A.; Kirkegaard, J. Glucosinolates and biofumigation: Fate of glucosinolates and their hydrolysis products in soil. Phytochem. Rev. 2009, 8, 299–310.
- Edwards, S.; Ploeg, A. Evaluation of 31 potential biofumigant Brassicaceous plants as hosts for three meloiodogyne species. J. Nematol. 2014, 46, 287–295.
- Ntalli, N.; Caboni, P. A review of isothiocyanates biofumigation activity on plant parasitic nematodes. Phytochem. Rev. 2017, 16, 827–834.
- Kruger, D.H.M.; Fourie, J.C.; Malan, A.P. Control potential of Brassicaceae cover crops as green manure and their host status for Meloidogyne javanica and Criconemoides xenoplax. South Afr. J. Enol. Vitic. 2015, 36, 165–174.
- Neubauer, C.; Heitmann, B.; Müller, C. Biofumigation potential of Brassicaceae cultivars to Verticillium dahliae. Eur. J. Plant Pathol. 2014, 140, 341–352.
- Ríos, P.; Obregón, S.; González, M.; de Haro, A.; Sánchez, M.E. Screening brassicaceous plants as biofumigants for management of Phytophthora cinnamomi oak disease. For. Pathol. 2016, 46, 652–659.
- Matthiessen, J.N.; Kirkegaard, J.A. Biofumigation and enhanced biodegradation: Opportunity and challenge in soilborne pest and disease management. Crit. Rev. Plant Sci. 2006, 25, 235–265.
- Brown, P.D.; Morra, M.J. Glucosinolate-containing plant tissues as bioherbicides. J. Agric. Food Chem. 1995, 43, 3070–3074.
- Hanschen, F.S.; Lamy, E.; Schreiner, M.; Rohn, S. Reactivity and stability of glucosinolates and their breakdown products in foods. Angew. Chem. Int. Ed. 2014, 53, 11430–11450.
- Blažević, I.; Montaut, S.; Burčul, F.; Olsen, C.E.; Burow, M.; Rollin, P.; Agerbirk, N. Glucosinolate structural diversity, identification, chemical synthesis and metabolism in plants. Phytochemistry 2020, 169, 112100.
- Bellostas, N.; Sørensen, J.C.; Sørensen, H. Profiling glucosinolates in vegetative and reproductive tissues of four Brassica species of the U-triangle for their biofumigation potential. J. Sci. Food Agric. 2007, 87, 1586–1594.
- Kirkegaard, J.A.; Sarwar, M. Biofumigation potential of brassicas I. Plant Soil 1998, 201, 71–89.
- Sarwar, M.; Kirkegaard, J.A. Biofumigation potential of brassicas II. Plant Soil 1998, 201, 91–101.
- Witzel, K.; Abu Risha, M.; Albers, P.; Börnke, F.; Hanschen, F.S. Identification and characterization of three epithiospecifier protein isoforms in Brassica oleracea. Front. Plant. Sci. 2019, 10.
- Wittstock, U.; Burow, M. Glucosinolate breakdown in Arabidopsis: Mechanism, regulation and biological significance. Arab. Book 2010, 8, e0134.
- Hanschen, F.S.; Yim, B.; Winkelmann, T.; Smalla, K.; Schreiner, M. Degradation of biofumigant isothiocyanates and allyl glucosinolate in soil and their effects on the microbial community composition. PLoS ONE 2015, 10, e0132931.
- Hansson, D.; Morra, M.J.; Borek, V.; Snyder, A.J.; Johnson-Maynard, J.L.; Thill, D.C. Ionic thiocyanate (SCN−) production, fate, and phytotoxicity in soil amended with Brassicaceae seed meals. J. Agric. Food Chem. 2008, 56, 3912–3917.
- Borek, V.; Morra, M.J. Ionic thiocyanate (SCN-) production from 4-hydroxybenzyl glucosinolate contained in Sinapis alba seed meal. J. Agric. Food Chem. 2005, 53, 8650–8654.
- Dufour, V.; Stahl, M.; Baysse, C. The antibacterial properties of isothiocyanates. Microbiology 2015, 161, 229–243.
- Fahey, J.W.; Haristoy, X.; Dolan, P.M.; Kensler, T.W.; Scholtus, I.; Stephenson, K.K.; Talalay, P.; Lozniewski, A. Sulforaphane inhibits extracellular, intracellular, and antibiotic-resistant strains of Helicobacter pylori and prevents benzo[a]pyrene-induced stomach tumors. Proc. Natl. Acad. Sci. USA 2002, 99, 7610–7615.
- Hu, P.; Hollister, E.B.; Somenahally, A.C.; Hons, F.M.; Gentry, T.J. Soil bacterial and fungal communities respond differently to various isothiocyanates added for biofumigation. Front. Microbiol. 2015, 5.
- Azaiez, I.; Meca, G.; Manyes, L.; Fernández-Franzón, M. Antifungal activity of gaseous allyl, benzyl and phenyl isothiocyanate in vitro and their use for fumonisins reduction in bread. Food Control 2013, 32, 428–434.
- Aissani, N.; Tedeschi, P.; Maietti, A.; Brandolini, V.; Garau, V.L.; Caboni, P. Nematicidal activity of allylisothiocyanate from horseradish (Armoracia rusticana) roots against Meloidogyne incognita. J. Agric. Food Chem. 2013, 61, 4723–4727.
- Gabler, F.M.; Fassel, R.; Mercier, J.; Smilanick, J.L. Influence of temperature, inoculation interval, and dosage on biofumigation with muscodor albus to control postharvest gray mold on grapes. Plant Dis. 2006, 90, 1019–1025.
- Morra, M.J.; Kirkegaard, J.A. Isothiocyanate release from soil-incorporated Brassica tissues. Soil Biol. Biochem. 2002, 34, 1683–1690.
- Brown, P.D.; Morra, M.J.; McCaffrey, J.P.; Auld, D.L.; Williams, L. Allelochemicals produced during glucosinolate degradation in soil. J. Chem. Ecol. 1991, 17, 2021–2034.
- Gardiner, J.B.; Morra, M.J.; Eberlein, C.V.; Brown, P.D.; Borek, V. Allelochemicals released in soil following incorporation of rapeseed (Brassica napus) green manures. J. Agric. Food Chem. 1999, 47, 3837–3842.
- Bangarwa, S.K.; Norsworthy, J.K.; Mattice, J.D.; Gbur, E.E. Glucosinolate and isothiocyanate production from Brassicaceae cover crops in a plasticulture production system. Weed Sci. 2011, 59, 247–254.
- Gimsing, A.L.; Kirkegaard, J.A. Glucosinolate and isothiocyanate concentration in soil following incorporation of Brassica biofumigants. Soil Biol. Biochem. 2006, 38, 2255–2264.
- Wang, L.; Mazzola, M. Field evaluation of reduced rate Brassicaceae seed meal amendment and rootstock genotype on the microbiome and control of apple replant disease. Phytopathology 2019, 109, 1378–1391.
- Mazzola, M.; Brown, J. Efficacy of brassicaceous seed meal formulations for the control of apple replant disease in conventional and organic production systems. Plant Dis. 2010, 94, 835–842.
- Mazzola, M.; Zhao, X. Brassica juncea seed meal particle size influences chemistry but not soil biology-based suppression of individual agents inciting apple replant disease. Plant Soil 2010, 337, 313–324.
- Mazzola, M.; Brown, J.; Izzo, A.D.; Cohen, M.F. Mechanism of action and efficacy of seed meal-induced pathogen suppression differ in a Brassicaceae species and time-dependent manner. Phytopathology 2007, 97, 454–460.
- Yim, B.; Hanschen, F.S.; Wrede, A.; Nitt, H.; Schreiner, M.; Smalla, K.; Winkelmann, T. Effects of biofumigation using Brassica juncea and Raphanus sativus in comparison to disinfection using Basamid on apple plant growth and soil microbial communities at three field sites with replant disease. Plant Soil 2016, 406, 389–408.
- Gimsing, A.L.; Sørensen, J.C.; Strobel, B.W.; Hansen, H.C.B. Adsorption of glucosinolates to metal oxides, clay minerals and humic acid. Appl. Clay Sci. 2007, 35, 212–217.
- Gimsing, A.L.; Strobel, B.W.; Hansen, H.C.B. Degradation and sorption of 2-propenyl and benzyl isothiocyanate in soil. Environ. Toxicol. Chem. 2009, 28, 1178–1184.
- Borek, V.; Morra, M.J.; Brown, P.D.; McCaffrey, J.P. Transformation of the glucosinolate-derived allelochemicals allyl isothiocyanate and allylnitrile in soil. J. Agric. Food Chem. 1995, 43, 1935–1940.
- Price, A.J.; Charron, C.S.; Saxton, A.M.; Sams, C.E. Allyl isothiocyanate and carbon dioxide produced during degradation of Brassica juncea tissue in different soil conditions. HortScience 2005, 40, 1734–1739.
- Matthiessen, J.N.; Desmarchelier, J.M.; Vu, L.T.; Shackleton, M.A. Comparative efficacy of fumigants against hatchling whitefringed beetle (Coleoptera: Curculionidae) larvae and their sorption by soil. J. Econ. Entomol. 1996, 89, 1372–1378.
- Matthiessen, J.N.; Shackleton, M.A. Biofumigation: Environmental impacts on the biological activity of diverse pure and plant-derived isothiocyanates. Pest Manag. Sci. 2005, 61, 1043–1051.
- Dungan, R.S.; Gan, J.; Yates, S.R. Accelerated degradation of methyl isothiocyanate in soil. Water, Air, Soil Pollut. 2003, 142, 299–310.
- Warton, B.; Matthiessen, J.N.; Shackleton, M.A. Cross-enhancement: Enhanced biodegradation of isothiocyanates in soils previously treated with metham sodium. Soil Biol. Biochem. 2003, 35, 1123–1127.
- Cohen, M.F.; Yamasaki, H.; Mazzola, M. Brassica napus seed meal soil amendment modifies microbial community structure, nitric oxide production and incidence of Rhizoctonia root rot. Soil Biol. Biochem. 2005, 37, 1215–1227.
- Mazzola, M.; Granatstein, D.M.; Elfving, D.C.; Mullinix, K. Suppression of specific apple root pathogens by Brassica napus seed meal amendment regardless of glucosinolate content. Phytopathology 2001, 91, 673–679.
- Omirou, M.; Rousidou, C.; Bekris, F.; Papadopoulou, K.; Menkissoglou-Spiroudi, U.; Ehaliotis, C.; Karpouzas, D. The impact of biofumigation and chemical fumigation methods on the structure and function of the soil microbial community. Microb. Ecol. 2011, 61, 201–213.
- Vervoort, M.T.W.; Vonk, J.A.; Brolsma, K.M.; Schütze, W.; Quist, C.W.; de Goede, R.G.M.; Hoffland, E.; Bakker, J.; Mulder, C.; Hallmann, J.; et al. Release of isothiocyanates does not explain the effects of biofumigation with Indian mustard cultivars on nematode assemblages. Soil Biol. Biochem. 2014, 68, 200–207.
- Cohen, M.F.; Mazzola, M. Resident bacteria, nitric oxide emission and particle size modulate the effect of Brassica napus seed meal on disease incited by Rhizoctonia solani and Pythium spp. Plant Soil 2006, 286, 75–86.
- Thorup-Kristensen, K.; Magid, J.; Jensen, L.S. Catch crops and green manures as biological tools in nitrogen management in temperate zones. In Advances in Agronomy; Academic Press: Cambridge, MA, USA, 2003; Volume 79, pp. 227–302.
- Wang, D.; Rosen, C.; Kinkel, L.; Cao, A.; Tharayil, N.; Gerik, J. Production of methyl sulfide and dimethyl disulfide from soil-incorporated plant materials and implications for controlling soilborne pathogens. Plant Soil 2009, 324, 185–197.
- Bending, G.D.; Lincoln, S.D. Characterisation of volatile sulphur-containing compounds produced during decomposition of Brassica juncea tissues in soil. Soil Biol. Biochem. 1999, 31, 695–703.
- Lewis, J.; Papavizas, G. Evolution of volatile sulfur-containing compounds from decomposition of crucifers in soil. Soil Biol. Biochem. 1970, 2, 239–246.
- Bremner, J.; Bundy, L. Inhibition of nitrification in soils by volatile sulfur compounds. Soil Biol. Biochem. 1974, 6, 161–165.
- Canessa, E.F.; Morrell, J.J. Effect of mixtures of carbon disulfide and methylisothiocyanate on survival of wood-colonizing fungi. Wood Fiber Sci. 1995, 27, 207–224.
- Gurtler, J.B. Pathogen decontamination of food crop soil: A review. J. Food Prot. 2017, 80, 1461–1470.
- Marks, H.S.; Hilson, J.A.; Leichtweis, H.C.; Stoewsand, G.S. S-Methylcysteine sulfoxide in Brassica vegetables and formation of methyl methanethiosulfinate from Brussels sprouts. J. Agric. Food Chem. 1992, 40, 2098–2101.
- Morris, C.J.; Thompson, J.F. The identification of (+)S-methyl-L-cysteine sulfoxide in plants. J. Am. Chem. Soc. 1956, 78, 1605–1608.
- De Corato, U.; Pane, C.; Bruno, G.L.; Cancellara, F.A.; Zaccardelli, M. Co-products from a biofuel production chain in crop disease management: A review. Crop. Prot. 2015, 68, 12–26.
- De Corato, U.; De Bari, I.; Viola, E.; Pugliese, M. Assessing the main opportunities of integrated biorefining from agro-bioenergy co/by-products and agroindustrial residues into high-value added products associated to some emerging markets: A review. Renew. Sustain. Energy Rev. 2018, 88, 326–346.
- Van Ommen Kloeke, A.E.E.; van Gestel, C.M.; Styrishave, B.; Hansen, M.; Ellers, J.; Roelofs, D. Molecular and life-history effects of a natural toxin on herbivorous and non-target soil arthropods. Ecotoxicology 2012, 21, 1084–1093.
- Van Ommen Kloeke, A.E.E.; Jager, T.; van Gestel, C.A.M.; Ellers, J.; Pomeren, M.v.; Krommenhoek, T.; Styrishave, B.; Hansen, M.; Roelofs, D. Time-related survival effects of two gluconasturtiin hydrolysis products on the terrestrial isopod Porcellio Scaber. Chemosphere 2012, 89, 1084–1090.
- Van Ommen Kloeke, A.E.E.; Gong, P.; Ellers, J.; Roelofs, D. Effects of a natural toxin on life history and gene expression of Eisenia andrei. Environ. Toxicol. Chem. 2014, 33, 412–420.
- Fouché, T.; Maboeta, M.; Claassens, S. Effect of biofumigants on soil microbial communities and ecotoxicology of earthworms (Eisenia andrei). Water Air Soil Pollut. 2016, 227, 256.
- Dutta, T.K.; Khan, M.R.; Phani, V. Plant-parasitic nematode management via biofumigation using brassica and non-brassica plants: Current status and future prospects. Curr. Plant Biol. 2019, 17, 17–32.
- Vukicevich, E.; Lowery, T.; Bowen, P.; Úrbez-Torres, J.R.; Hart, M. Cover crops to increase soil microbial diversity and mitigate decline in perennial agriculture. A review. Agron. Sustain. Dev. 2016, 36, 48.
- Bending, G.D.; Lincoln, S.D. Inhibition of soil nitrifying bacteria communities and their activities by glucosinolate hydrolysis products. Soil Biol. Biochem. 2000, 32, 1261–1269.
- Siebers, M.; Rohr, T.; Ventura, M.; Schütz, V.; Thies, S.; Kovacic, F.; Jaeger, K.-E.; Berg, M.; Dörmann, P.; Schulz, M. Disruption of microbial community composition and identification of plant growth promoting microorganisms after exposure of soil to rapeseed-derived glucosinolates. PLoS ONE 2018, 13, e0200160.
- Wang, Q.; Ma, Y.; Yang, H.; Chang, Z. Effect of biofumigation and chemical fumigation on soil microbial community structure and control of pepper Phytophthora blight. World J. Microbiol. Biotechnol. 2014, 30, 507–518.
- Zaccardelli, M.; Villecco, D.; Celano, G.; Scotti, R. Soil amendment with seed meals: Short term effects on soil respiration and biochemical properties. Appl. Soil Ecol. 2013, 72, 225–231.
- Meng, L.; Yao, X.; Yang, Z.; Zhang, R.; Zhang, C.; Wang, X.; Xu, N.; Li, S.; Liu, T.; Zheng, C. Changes in soil microbial diversity and control of Fusarium oxysporum in continuous cropping cucumber greenhouses following biofumigation. Emir. J. Food Agric. 2018, 30, 644–653.
- Wang, Q.; Ma, Y.; Wang, G.; Gu, Z.; Sun, D.; An, X.; Chang, Z. Integration of biofumigation with antagonistic microorganism can control Phytophthora blight of pepper plants by regulating soil bacterial community structure. Eur. J. Soil Biol. 2014, 61, 58–67.
- Nuñez-Zofío, M.; Larregla del Palacio, S.; Garbisu, C. Repeated biodisinfection controls the incidence of Phytophthora root and crown rot of pepper while improving soil quality. Span. J. Agric. Res. 2012, 10, 12.
- Mowlick, S.; Yasukawa, H.; Inoue, T.; Takehara, T.; Kaku, N.; Ueki, K.; Ueki, A. Suppression of spinach wilt disease by biological soil disinfestation incorporated with Brassica juncea plants in association with changes in soil bacterial communities. Crop. Prot. 2013, 54, 185–193.
- Behera, B.C.; Patra, M.; Dutta, S.K.; Thatoi, H.N. Isolation and characterisation of sulphur oxidising Bacteria from mangrove soil of Mahanadi river delta and their sulphur oxidising ability. J. Appl. Environ. Microbiol. 2014, 2, 1–5.
- Zaccardelli, M.; De Nicola, F.; Villecco, D.; Scotti, R. The development and suppressive activity of soil microbial communities under compost amendment. J. Soil Sci. Plant Nutr. 2013, 13, 730–742.
- Palaniyandi, S.A.; Yang, S.H.; Zhang, L.; Suh, J.-W. Effects of actinobacteria on plant disease suppression and growth promotion. Appl. Microbiol. Biotechnol. 2013, 97, 9621–9636.
- Grunewaldt-Stöcker, G.; Mahnkopp, F.; Popp, C.; Maiss, E.; Winkelmann, T. Diagnosis of apple replant disease (ARD): Microscopic evidence of early symptoms in fine roots of different apple rootstock genotypes. Sci. Hort. 2019, 243, 583–594.
- Edwards, L.; Utkhede, R.S.; Vrain, T. Effect of antagonistic plants on apple replant disease. Acta Hortic. 1994, 363, 135–140.
- Mazzola, M.; Mullinix, K. Comparative field efficacy of management strategies containing Brassica napus seed meal or green manure for the control of apple replant disease. Plant Dis. 2005, 89, 1207–1213.
- Mazzola, M.; Brown, J.; Izzo, A.; Ghanem, R.A.; Cohen, M. Progress towards development of biologically-based strategies for the management of apple replant disease. Phytopathol. Pol. 2006, 39, 11–18.
- Seigies, A.T.; Pritts, M. Cover crop rotations alter soil microbiology and reduce replant disorders in strawberry. HortScience 2006, 41, 1303.
- Mazzola, M.; Brown, J.; Zhao, X.; Izzo, A.D.; Fazio, G. Interaction of brassicaceous seed meal and apple rootstock on recovery of Pythium spp. and Pratylenchus penetrans from roots grown in replant soils. Plant Dis. 2009, 93, 51–57.
- Mazzola, M.; Reardon, C.L.; Brown, J. Initial Pythium species composition and Brassicaceae seed meal type influence extent of Pythium-induced plant growth suppression in soil. Soil Biol. Biochem. 2012, 48, 20–27.
- Weerakoon, D.M.N.; Reardon, C.L.; Paulitz, T.C.; Izzo, A.D.; Mazzola, M. Long-term suppression of Pythium abappressorium induced by Brassica juncea seed meal amendment is biologically mediated. Soil Biol. Biochem. 2012, 51, 44–52.
- Mazzola, M.; Hewavitharana, S.S.; Strauss, S.L. Brassica seed meal soil amendments transform the rhizosphere microbiome and improve apple production through resistance to pathogen reinfestation. Phytopathology 2015, 105, 460–469.
- Pokharel, R.R.; Reighard, G.L. Evaluation of biofumigation, soil solarization and rootstock on peach replant disease. Acta Hortic. 2015, 1084, 577–584.
- Wang, L.; Mazzola, M. Interaction of Brassicaceae seed meal soil amendment and apple rootstock genotype on microbiome structure and replant disease suppression. Phytopathology 2019, 109, 607–614.
More
Information
Subjects:
Agronomy
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.3K
Revisions:
2 times
(View History)
Update Date:
09 Jun 2021
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




