
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Andrea Schlegel | + 1595 word(s) | 1595 | 2021-05-28 04:52:53 | | | |
| 2 | Enzi Gong | Meta information modification | 1595 | 2021-06-09 05:33:22 | | | | |
| 3 | Enzi Gong | Meta information modification | 1595 | 2021-06-09 05:33:50 | | | | |
| 4 | Enzi Gong | -71 word(s) | 1524 | 2021-06-18 04:27:39 | | | | |
| 5 | Conner Chen | Meta information modification | 1524 | 2021-10-12 05:58:36 | | |
Video Upload Options
Hypothermic Oxygenated Perfusion (HOPE) is a new liver preservation technique used in clinical trials. It may mitigate ischemia/reperfusion injury and improve organ function and patient outcomes.
1. Overview
Although machine perfusion is a hot topic today, we are just at the beginning of understanding the underlying mechanisms of protection. Recently, the first randomized controlled trial reported a significant reduction of ischemic cholangiopathies after transplantation of livers donated after circulatory death, provided the grafts were treated with an endischemic hypothermic oxygenated perfusion (HOPE). This approach has been known for more than fifty years, and was initially mainly used to preserve kidneys before implantation. Today there is an increasing interest in this and other dynamic preservation technologies and various centers have tested different approaches in clinical trials and cohort studies. Based on this, there is a need for uniform perfusion settings (perfusion route and duration), and the development of general guidelines regarding the duration of cold storage in context of the overall donor risk is also required to better compare various trial results. This article will highlight how cold perfusion protects organs mechanistically, and target such technical challenges with the perfusion setting. Finally, the options for viability testing during hypothermic perfusion will be discussed.
2. Background
The field of liver transplantation has recently seen a boost in the development of machine perfusion technology with the first randomized controlled trials published and the availability of several devices on the market. To further drive this technology successfully into routine clinical practice, the community needs to first understand underlying mechanisms of protection by different perfusion approaches. Secondly, transparent reports of study results and the identification of gaps in the literature are needed. With this review article we summarize the clinical studies on hypothermic machine perfusion (HMP) in liver transplantation. Next, the protective mechanism of this cold oxygenation is described. Third, a few methodological differences of HMP performed by different groups are identified and discussed followed by the impact of HMP on organ selection through viability assessment. Finally, this review will project future trials needed to answer the here described ambiguities.
3.What Is the Real HOPE-Effect in Solid Organ Transplantation?
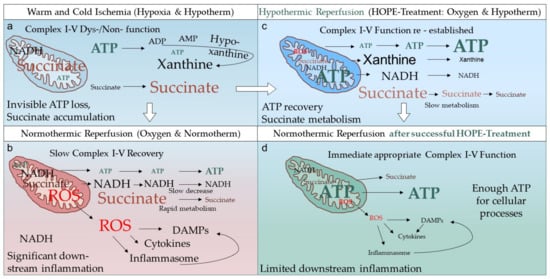
4. Conclusions
References
- Jameel, N.M.; Thirunavukkarasu, C.; Murase, N.; Cascio, M.; Prelich, J.; Yang, S.; Harvey, S.A.K.; Gandhi, C.R. Constitutive release of powerful antioxidant-scavenging activity by hepatic stellate cells: Protection of hepatocytes from ischemia/reperfusion injury. Liver Transpl. 2010, 16, 1400–1409.
- Jaeschke, H. Reactive oxygen and mechanisms of inflammatory liver injury: Present concepts. J. Gastroenterol. Hepatol. 2011, 26, 173–179.
- Lindell, S.L.; Klahn, S.L.; Piazza, T.M.; Mangino, M.J.; Torrealba, J.R.; Southard, J.H.; Carey, H.V. Natural resistance to liver cold ischemia-reperfusion injury associated with the hibernation phenotype. Am. J. Physiol. Gastrointest. Liver Physiol. 2005, 288, G473–G480.
- Dutkowski, P.; Clavien, P. Uploading cellular batteries: Caring for mitochondria is key. Liver Transpl. 2018, 24, 462–464.
- Schlegel, A.; Muller, X.; Mueller, M.; Stepanova, A.; Kron, P.; de Rougemont, O.; Muiesan, P.; Clavien, P.A.; Galkin, A.; Meierhofer, D.; et al. Hypothermic oxygenated perfusion protects from mitochondrial injury before liver transplantation. EBioMedicine 2020.
- Speijer, D. Being right on Q: Shaping eukaryotic evolution. Biochem. J. 2016.
- Dar, W.A.; Sullivan, E.; Bynon, J.S.; Eltzschig, H.; Ju, C. Ischaemia reperfusion injury in liver transplantation: Cellular and molecular mechanisms. Liver Int. 2019.
- Kim, M.; Stepanova, A.; Niatsetskaya, Z.; Sosunov, S.; Arndt, S.; Murphy, M.P.; Galkin, A.; Ten, V.S. Attenuation of oxidative damage by targeting mitochondrial complex I in neonatal hypoxic-ischemic brain injury. Free Radic. Biol. Med. 2018.
- Murphy, M.P. How mitochondria produce reactive oxygen species. Biochem. J. 2009, 417, 1–13.
- Burlage, L.C.; Karimian, N.; Westerkamp, A.C.; Visser, N.; Matton, A.P.M.; van Rijn, R.; Adelmeijer, J.; Wiersema-Buist, J.; Gouw, A.S.H.; Lisman, T.; et al. Oxygenated hypothermic machine perfusion after static cold storage improves endothelial function of extended criteria donor livers. HPB 2017, 19, 538–546.
- Schlegel, A.; Rougemont ODe Graf, R.; Clavien, P.A.; Dutkowski, P. Protective mechanisms of end-ischemic cold machine perfusion in DCD liver grafts. J. Hepatol. 2013, 58, 278–286.
- Schlegel, A.; Dutkowski, P. Impact of Machine Perfusion on Biliary Complications after Liver Transplantation. Int. J. Mol. Sci. 2018, 19, 3567.
- Kron, P.; Schlegel, A.; Mancina, L.; Clavien, P.-A.; Dutkowski, P. Hypothermic oxygenated perfusion (HOPE) for fatty liver grafts in rats and humans. J. Hepatol. 2018, 68, 82–91.
- Zhai, Y.; Shen XDa Gao, F.; Zhao, A.; Freitas, M.C.; Lassman, C.; Luster, A.D.; Busuttil, R.W.; Kupiec-Weglinski, J.W. CXCL10 regulates liver innate immune response against ischemia and reperfusion injury. Hepatology 2008.
- Mills, E.L.; Kelly, B.; Logan, A.; Costa, A.S.H.; Varma, M.; Bryant, C.E.; Tourlomousis, P.; Däbritz, J.H.M.; Gottlieb, E.; Latorre, I.; et al. Succinate Dehydrogenase Supports Metabolic Repurposing of Mitochondria to Drive Inflammatory Macrophages. Cell 2016, 167, 457–470.
- Chouchani, E.T.; Pell, V.R.; Gaude, E.; Aksentijević, D.; Sundier, S.Y.; Robb, E.L.; Logan, A.; Nadtochiy, S.M.; Ord, E.N.J.; Smith, A.C.; et al. Ischaemic accumulation of succinate controls reperfusion injury through mitochondrial ROS. Nature 2014, 515, 431–435.
- Martin, J.; Costa, A.; Gruszczyk, A.; Beach, T.; Allen, F.; Prag, H.A.; Hinchy, E.C.; Mahbubani, K.; Hamed, M.; Tronci, L.; et al. Succinate accumulation drives ischaemia-reperfusion injury during organ transplantation. Nat. Metab. 2019, 1, 966–974.
- Schlegel, A.; Kron, P.; Graf, R.; Dutkowski, P.; Clavien, P.A. Warm vs. cold perfusion techniques to rescue rodent liver grafts. J. Hepatol. 2014, 61, 1267–1275.
- Saeb-Parsy, K.; Martin, J.L.; Summers, D.M.; Watson, C.J.E.; Krieg, T.; Murphy, M.P. Mitochondria as Therapeutic Targets in Transplantation. Trends. Mol. Med. 2021, 27, 185–198.
- Berman, D.I.; Bulakhova, N.A.; Meshcheryakova, E.N. The Siberian wood frog survives for months underwater without oxygen. Sci. Rep. 2019, 9.
- Boteon, Y.; Laing, R.; Schlegel, A.; Wallace, L.; Smith, A.; Attard, J.; Bhogal, R.H.; Neil, D.A.H.; Hübscher, S.; Perera, M.T.P.R.; et al. Combined Hypothermic and Normothermic Machine Perfusion Improves Functional Recovery of Extended Criteria Donor Livers. Liver Transpl. 2018, 24, 1699–1715.
- Van Golen, R.F.; Van Gulik, T.M.; Heger, M. Mechanistic overview of reactive species-induced degradation of the endothelial glycocalyx during hepatic ischemia/reperfusion injury. Free Radic. Biol. Med. 2012, 52, 1382–1402.
- Longatto Boteon, Y.; Schlegel, A.; Laing, R.; Attard, J.; Bhogal, R.; Wallace, L.; Reynolds, G.; Mirza, D.; Mergental, H.; Afford, S.; et al. Combination of hypothermic oxygenated machine perfusion followed by normothermic machine perfusion optimises the reconditioning of marginal human donor livers. HPB 2018.
- Darius, T.; Vergauwen, M.; Smith, T.; Gerin, I.; Joris, V.; Mueller, M.; Aydin, S.; Muller, X.; Schlegel, A.; Nath, J.; et al. Brief O2 uploading during continuous hypothermic machine perfusion is simple yet effective oxygenation method to improve initial kidney function in a porcine autotransplant model. Am. J. Transpl. 2020.
- Wyss, R.; Méndez Carmona, N.; Arnold, M.; Segiser, A.; Mueller, M.; Dutkowski, P.; Carrel, T.P.; Longnus, S.L. Hypothermic, oxygenated perfusion (HOPE) provides cardioprotection via succinate oxidation prior to normothermic perfusion in a rat model of donation after circulatory death (DCD). Am. J. Transpl. 2020, 12.
- Mergental, H.; Laing, R.W.; Kirkham, A.J.; Perera, M.T.P.R.; Boteon, Y.L.; Attard, J.; Barton, D.; Curbishley, S.; Wilkhu, M.; Neil, D.A.H.; et al. Transplantation of discarded livers following viability testing with normothermic machine perfusion. Nat. Commun. 2020.
- Boteon, Y.; Afford, S.; Mergental, H. Pushing the Limits: Machine Preservation of the Liver as a Tool to Recondition High-Risk Grafts. Curr. Transpl. Rep. 2018, 5, 113–120.
- Laing, R.; Mergental, H.; Yap, C.; Kirkham, A.; Whilku, M.; Barton, D.; Curbishley, S.; Boteon, Y.L.; Neil, D.A.; Hübscher, S.G.; et al. Viability testing and transplantation of marginal livers (VITTAL) using normothermic machine perfusion: Study protocol for an open-label, non-randomised, prospective, single-arm trial. BMJ Open 2017.
- Panconesi, R.; Flores Carvalho, M.; Mueller, M.; Meierhofer, D.; Dutkowski, P.; Muiesan, P.; Schlegel, A. Viability Assessment in Liver Transplantation—What Is the Impact of Dynamic Organ Preservation? Biomedicines 2021, 9, 161.
- Watson, C.; Kosmoliaptsis, V.; Pley, C.; Randle, L.; Fear, C.; Crick, K.; Gimson, A.E.; Allison, M.; Upponi, S.; Brais, R.; et al. Observations on the ex situ perfusion of livers for transplantation. Am. J. Transpl. 2018.
- Van Leeuwen, O.B.; De Vries, Y.; Fujiyoshi, M.; Nijsten, M.W.N.; Ubbink, R.; Pelgrim, G.J.; Werner, M.J.M.; Reyntjens, K.M.E.; van den Berg, A.P.; de Boer, M.T.; et al. Transplantation of high-risk donor livers after ex situ resuscitation and assessment using combined hypo- A nd normothermic machine perfusion: A prospective clinical trial. Ann. Surg. 2019.
- Matton, A.P.M.; de Vries, Y.; Burlage, L.C.; van Rijn, R.; Fujiyoshi, M.; de Meijer, V.E.; de Boer, M.T.; de Kleine, R.H.J.; Verkade, H.J.; Gouw, A.S.H.; et al. Biliary Bicarbonate, pH, and Glucose Are Suitable Biomarkers of Biliary Viability during Ex Situ Normothermic Machine Perfusion of Human Donor Livers. Transplantation 2019.
- Watson, C.J.E.; Jochmans, I. From “Gut Feeling” to Objectivity: Machine Preservation of the Liver as a Tool to Assess Organ Viability. Curr. Transpl. Rep. 2018.
- Stepanova, A.; Sosunov, S.; Niatsetskaya, Z.; Konrad, C.; Starkov, A.; Manfredi, G.; Wittig, I.; Ten, V.; Galkin, A. Redox-Dependent Loss of Flavin by Mitochondrial Complex I in Brain Ischemia/Reperfusion Injury. Antioxid Redox Signal 2019, 20, 608–622.
- Muller, X.; Schlegel, A.; Kron, P.; Eshmuminov, D.; Würdinger, M.; Meierhofer, D.; Clavien, P.-A.; Dutkowski, P. Novel real time prediction of liver graft function during hypothermic oxygenated machine perfusion prior to liver transplantation. Ann. Surg. 2019, 270, 783–790.
- Wang, L.; Thompson, E.; Bates, L.; Pither, T.L.; Hosgood, S.A.; Nicholson, M.L.; Watson, C.J.E.; Wilson, C.; Fisher, A.J.; Ali, S.; et al. Flavin mononucleotide as a biomarker of organ quality—A pilot study. Transpl. Direct. 2020.
- Oi, K.; Davies, W.; Tazelaar, H.; Bailey, K.; Federspiel, M.; Russell, S.; McGregor, C.G.A. Ex vivo hypothermic recirculatory adenoviral gene transfer to the transplanted pig heart. J. Gene Med. 2006, 8, 795–803.




