Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Hiroki Kuniyasu | + 2157 word(s) | 2157 | 2021-05-28 04:42:49 | | | |
| 2 | Bruce Ren | -21 word(s) | 2136 | 2021-06-04 10:26:00 | | | | |
| 3 | Conner Chen | Meta information modification | 2136 | 2021-10-12 05:57:26 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Kuniyasu, H. CML-HMGB1 in Gastric Cancer. Encyclopedia. Available online: https://encyclopedia.pub/entry/10507 (accessed on 07 February 2026).
Kuniyasu H. CML-HMGB1 in Gastric Cancer. Encyclopedia. Available at: https://encyclopedia.pub/entry/10507. Accessed February 07, 2026.
Kuniyasu, Hiroki. "CML-HMGB1 in Gastric Cancer" Encyclopedia, https://encyclopedia.pub/entry/10507 (accessed February 07, 2026).
Kuniyasu, H. (2021, June 04). CML-HMGB1 in Gastric Cancer. In Encyclopedia. https://encyclopedia.pub/entry/10507
Kuniyasu, Hiroki. "CML-HMGB1 in Gastric Cancer." Encyclopedia. Web. 04 June, 2021.
Copy Citation
Advanced glycation end products (AGEs) are produced in response to a high-glucose environment and oxidative stress and exacerbate various diseases. Nε-(Carboxymethyl)lysine (CML) is an AGE that is produced by the glycation of lysine residues of proteins.
HMGB1
glycation
Nε-(Carboxymethyl)lysine
gastric cancer
1. Introduction
Gastric cancer is the third leading cause of cancer deaths in Japan [1]. Early diagnosis and early endoscopic treatment have improved the 5-year survival rate of gastric cancer patients (73.1%) [2]. However, the 5-year survival rate for advanced gastric cancer still remains poor at 47.2 and 7.3% for stages III and IV, respectively [2]. Elucidation of the mechanism of acquisition of malignant phenotypes is expected to lead to the development of novel treatments.
Taguchi et al. reported that high-mobility group box-1 (HMGB1) promotes the progression and metastasis of various cancer types [3]. We have previously reported the involvement of HMGB1 in malignancy in gastric, colorectal, prostate, and oral cancers [4][5][6][7][8][9]. Notably, the high expression of HMGB1 along with that of its receptor, i.e., receptor for advanced glycation end products (RAGE), correlates well with the progression of gastric cancer [4]. HMGB1 provides cancer cells’ proliferation, invasion, anti-apoptotic survival, and metastatic ability [10].
RAGE is a member of the immunoglobulin superfamily. It is a cell membrane receptor that has the ability to bind to multiple ligands, such as AGE, HMGB1, and S100B [11]. HMGB1 enhances oxidative stress by binding to RAGE via multiple pathways, such as mitogen-activated protein kinase, p21ras, nuclear factor (NF)-κB, AKT, phosphoinositide 3-kinase, and Wnt [12]. Importantly, RAGE also binds to AGE [13][14], which is generated at high levels in diabetes mellitus. AGE is involved in the development of complications, such as diabetic retinopathy, through RAGE activation [14][15]. We have previously reported that AGE promotes the development and progression of colorectal cancer [16][17]; it is also a risk factor for breast cancer [18].
Nε-(Carboxymethyl)lysine (CML) is an AGE produced by the glycation of lysine residues under physiological conditions [19]. Non-enzymatic glycation of proteins occurs through a wide range of processes, including condensation, rearrangement, fragmentation, and oxidation reactions, with Schiff bases and Amadori products as intermediates [20]. CML is one of the most abundant AGEs and correlates well with the total level of AGEs [21][22]. It has been reported that CML promotes cancer progression through RAGE in prostate, pancreatic, lung, and breast cancers [23][24][25][26][27]. HMGB1 is a 215-amino-acid-long protein that has a molecular weight of 30 kDa [28]. The HMGB1 protein possesses 44 lysine residues, which might serve as the target sites for the CML modification. We have previously reported that a high-glucose and high-fat diet increases HMGB1 levels in the colonic mucosa in a rat colon carcinogenesis model [29]. In the rats, the levels of mRNA and protein of HMGB1 were increased before forming aberrant crypt foci. In contrast, downregulation of HMGB1 inhibits cancer formation and reduces progression of the colorectal cancer [7][30][31]. Although HMGB1 has been suggested to be associated with hyperglycemic condition, glycation of HMGB1 remains poorly understood.
2. CML Modification of Cancer-Related Proteins in Human Gastric Cancer Cell Lines
We investigated the formation of CML in cancer-related proteins after treating the human gastric cancer cell line TMK1 with glyoxal (GLX) or high-concentration glucose (GLC) (Figure 1A). The nuclear proteins HMGB1, mutant p53, and histone H4 were CML-modified by GLX and GLC treatment. Oxidative stress (read-out; 4-hyroxynonenal, 4-HNE) was increased in all the cell lines, especially TMK1 upon treatment with GLX and GLC (Figure 1B). HMGB1 concentrations in the culture medium increased in response to GLX and GLC treatment in all the cell lines, and was the highest in TMK1 (Figure 1C). GLX and GLC treatment increased the levels of CML-modified HMGB1 in the culture medium, which were higher in the culture media of TMK1 cells than in those of other cells (Figure 1D). These results suggest that CML-modified HMGB1 is extracellularly secreted. U937 is a macrophage cell line, which produces oxidative stresses as inflammatory responses. In U937 cells, GLC or GLX induced 4-HNE and total HMGB1, but CML-HMGB1 levels were lower than in gastric cancer cell lines (Figure 1B–D).
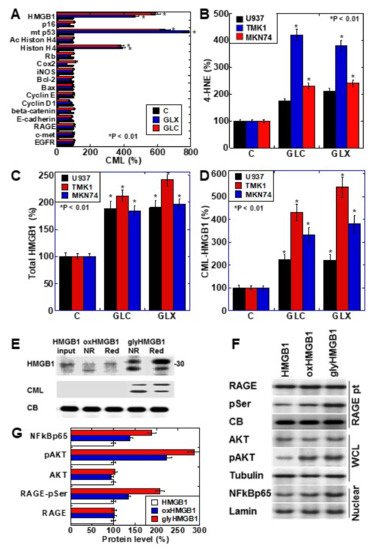
Figure 1. CML-HMGB1 formation in gastric cancer cells. (A) CML formation in various cancer-associated proteins was analyzed by antibody array using immunoprecipitant by anti-CML antibody of TMK-1 cell lysate, which was treated with GLX or GLC. The Scheme 100. (B) Oxidative stress levels in cells treated with GLX or GLC as measured by ELISA. (C) Total HMGB1 levels in cells treated with GLX or GLC as measured by ELISA. (D) CML-HMGB1 levels in cells treated with GLX or GLC measured by immunoprecipitation; immunoprecipitant by anti-CML antibody was detected by anti-HMGB1 antibody. (E) H2O2-treated HMGB1 (oxidized HMGB1) and GLX-treated HMGB1 (glycated HMGB1) were examined by Western blot analysis under non-reduced (NR) or reduced (Red) conditions. The membrane was re-probed with an anti-CML antibody (CML). (F) Alterations in HMGB1-associated intracellular signals in TMK-1 cells treated with naïve HMGB1 (10 μg/mL), oxidized HMGB1 (10 μg/mL), or glycated HMGB1 (10 μg/mL). (G) Semi-quantification of protein levels with standardization by CB, tubulin, or lamin. Phosphorylated RAGE (pSer) was examined by immunoprecipitation; RAGE precipitant was detected using an anti-pSer antibody. CB, tubulin, and lamin were used as the loading controls. Error bar and standard deviation calculated by ordinary analysis of variance from three independent experiments. CML, Nε-(Carboxymethyl)lysine; HMGB1, high-mobility group box-1; C, untreated control; GLX, glyoxal; GLC, glucose; RBP, retinol-binding protein; 4-HNE, 4-hydroxynonenal; NR, non-reduced condition; Red, reduced condition; CB, Coomassie blue; RAGE, receptor for advanced glycation end products; pSer, phosphorylated serine; pAKT, phosphorylated AKT; .NF-κB, nuclear factor-κB; RAGE pt, anti-RAGE antibody precipitant; WCL, whole cell lysate; Nuclear, nuclear fraction. * P < 0.01.
To further examine the properties of CML-HMGB1, recombinant human HMGB1 was treated with H2O2 and GLX to generate oxidized HMGB1 and glycated HMGB1, respectively (Figure 1E). Oxidized HMGB1 had higher mobility than naïve HMGB1, and under reducing conditions, the mobility of oxidized HMGB1 was similar to that of naïve HMGB1. The molecular weight of glycated HMGB1 was higher than that of naïve HMGB1; however, the mobility was lower under reducing conditions. This suggests that the SH group of HMGB1 is oxidized in glycated HMGB1. Furthermore, when the immunoblot membrane was re-probed with an anti-CML antibody after being probed with anti-HMGB1 antibody, CML was observed only in glycated HMGB1. These results indicate that HMGB1 undergoes CML modification by glycation.
Next, the effects of naïve HMGB1, oxidized HMGB1, and glycated HMGB1 on intracellular signals were examined (Figure 1F). The phosphorylation of RAGE and AKT, and the nuclear translocation of NF-κB, were more pronounced upon treatment with oxidized HMGB1 compared to those observed on treatment with naïve HMGB1; glycated HMGB1 resulted in more potent effects in this respect. Therefore, it was confirmed that glycated HMGB1 (CML-HMGB1) has a significant ability to activate intracellular signals.
3. CML Formation and HMGB1 Secretion in Human Gastric Cancer Cell Lines
CML formation occurred in a GLX concentration-dependent manner in TMK1 cells (Figure 2A). The DNA-binding ability of CML-HMGB1—produced by treating recombinant HMGB1 with GLX—was markedly decreased in comparison with that of HMGB1 (Figure 2B). Similarly, when the ability of HMGB1 to bind to histone H3 was examined (Figure 2C), a marked decrease was observed in the case of CML-HMGB1 compared to HMGB1. When TMK1 cells were treated with GLX, nuclear HMGB1 levels were reduced temporarily (Figure 2D). At this time, no significant changes were observed in HMGB1 in the cytoplasm (Figure 2E), but HMGB1 levels in the culture medium also increased (Figure 2F). These findings indicate that GLX deposited CML on nuclear HMGB1, following which the binding affinity of HMGB1 to DNA and histones in the nucleus was decreased, and HMGB1 was translocated outside the nucleus and secreted extracellularly.
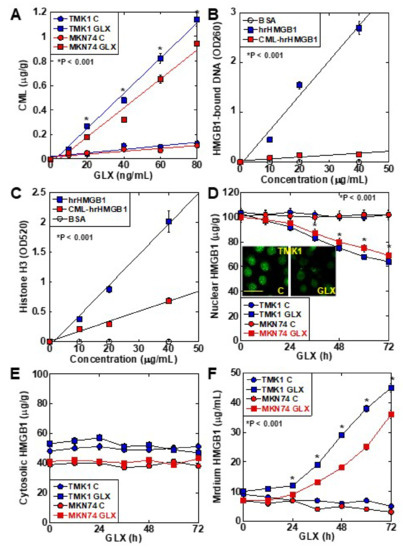
Figure 2. Properties of CML formation in HMGB1 in TMK-1 gastric cancer cells. (A) CML formation in GLX-treated TMK-1 cells measured using ELISA. (B) Effect of glycation on DNA binding of HMGB1 in TMK-1 cells treated with HMGB1 or GLX (CML-HMGB1). DNA extracted from the nuclear protein immunoprecipitated by HMGB1 antibody was measured using a spectrophotometer (A260). (C) Effect of glycation on histone H3 binding to rhHMGB1 or CML-rhHMGB1. rhHMGB1 or GLX-treated rhHMGB1 (CML-HMGB1) were mixed with FITC-labeled histone H3. Histone H3 was measured using a fluorescence microplate reader (A520). (D–F) HMGB1 in the nuclear fraction (D), cytosol (E), and cultured medium (F) measured by ELISA in GLX-treated TMK-1 cells. The inset of panel D shows the fluorescent immunocytochemistry of HMGB1. Scale bar, 50 μm. Error bar and standard deviation calculated by ordinary analysis of variance from three independent experiments. CML, Nε-(Carboxymethyl)lysine; HMGB1, high-mobility group box-1; hr, human recombinant; GLX, glyoxal; OD, optical density; FITC, fluorescent isothiocyanate. * P < 0.001.
4. Role of CML-HMGB1 in Gastric Cancer Cells
The role of CML-HMGB1 was compared to that of HMGB1 in the context of gastric cancer (Figure 3). First, both HMGB1 and CML-HMGB1 promoted the proliferation of TMK1 and MKN74 cell lines, but the effect of CML-HMGB1 was significantly more pronounced than that of HMGB1 (Figure 3A,B). In vitro invasion, sphere formation, and protection against thapsigargin-induced apoptosis were also promoted by HMGB1 and CML-HMGB1, but the effects were more pronounced with CML-HMGB1 (Figure 3C–E). In addition, 5-FU sensitivity (IC50) increased by 1.05-fold for HMGB1 and 1.3-fold for CML-HMGB1 in comparison with untreated TMK1 cells (Figure 3F). Thus, CML-HMGB1 exhibited a more significant tumor-promoting effect than HMGB1.
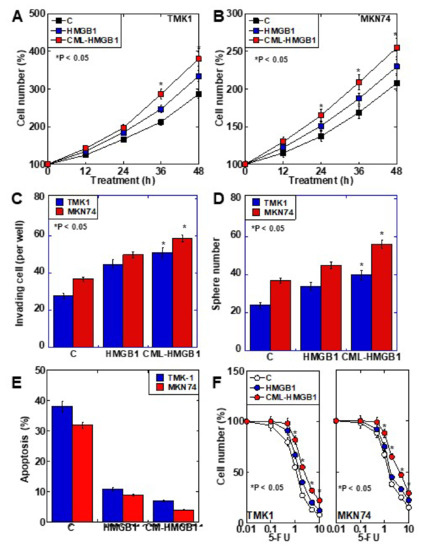
Figure 3. Effect of CML-HMGB1 on malignant properties of TMK-1 and MKN74 gastric cancer cells. (A,B) Effect of CML-HMGB1 (10 μg/mL) and HMGB1 (10 μg/mL) on cell proliferation. (C,D) Effect of CML-HMGB1 and HMGB1 on cell invasion by in vitro invasion assay and stemness by sphere formation. (E) Effect of CML-HMGB1 and HMGB1 on protection from apoptosis. Cells were untreated or treated with thapsigargin (5 μM) for 48 h with CML-HMGB1 or HMGB1. (F) Effect of CML-HMGB1 and HMGB1 on sensitivity to 5-FU. Cells were untreated or treated with 5-FU for 48 h with CML-HMGB1 or HMGB1 (C). Error bar and standard deviation calculated by ordinary analysis of variance from three independent experiments. CML, Nε-(Carboxymethyl)lysine; HMGB1, high-mobility group box-1; C, untreated control; 5-FU, 5-fluorouracil. * P < 0.05.
5. Clinical Significance of CML-HMGB1
To examine the clinical significance of CML-HMGB1, the protein level of CML-HMGB1 was examined by Western blotting in 10 gastric cancer samples (Figure 4A). CML-HMGB1 was detected at various levels in all the samples. The protein levels of CML-HMGB1 and HMGB1 were semi-quantified and a correlation with clinicopathological parameters was investigated (Table 1). HMGB1 levels were correlated with T factor (primary tumor) and M factor (distant metastasis), whereas CML-HMGB1 levels were correlated with all T, N (nodal metastasis), and M factors, and stage. No correlation was found with grade for either CML-HMGB1 or HMGB1.
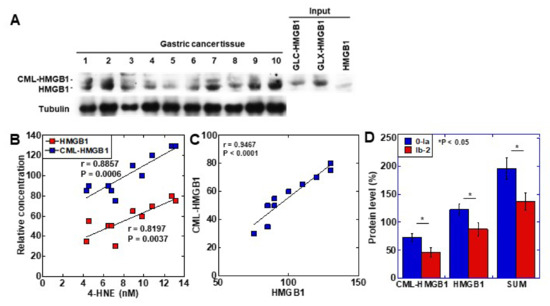
Figure 4. Formation of CML-HMGB1 in human gastric cancer. (A) CML-HMGB1 formation was examined by Western blot analysis in 10 human gastric cancer tumor tissues. (B) Comparison of HMGB1 and CML-HMGB1 levels with oxidative stress (4-HNE levels). The correlation was calculated using the Pearson’s correlation test. (C) Association between the relative levels of HMGB1 and CML-HMGB1. The correlation was calculated using the Pearson’s correlation test. (D) Effect of CML-HMGB1 on drug resistance in gastric cancer patients. Histological grade of therapeutic effect was determined according to Japanese Gastric Cancer Classification [32]. Grade 0, no effect; Grade 1a, very slight effect; Grade 1b, slight effect; Grade 2, considerable effect. Error bar, standard deviation calculated by ordinary analysis of variance from three independent experiments. CML, Nε-(Carboxymethyl)lysine; HMGB1, high-mobility group box-1;.4-HNE, 4-hydroxynonenal; SUM, summation of HMGB1 and CML-HMGB1 protein levels. * P < 0.05.
Table 1. Relationship between pathological parameters and protein levels of HMGB1 or CML-HMGB1 in gastric cancer samples.
| Parameter 1 | Level | Number | Protein Level | |||
|---|---|---|---|---|---|---|
| HMGB1 2 | p3 | CML-HMGB1 2 | p3 | |||
| Grade | 1 | 6 | 104 ± 21 | NS | 59 ± 17 | NS |
| 2 | 4 | 98 ± 21 | 54 ± 18 | |||
| pT | 2 | 3 | 8 ± 3 | 0.0015 | 43 ± 12 | 0.0245 |
| 3 | 4 | 96 ± 11 | 54 ± 13 | |||
| 4 | 3 | 127 ± 8 | 75 ± 2 | |||
| pN | 0 | 3 | 83 ± 8 | NS | 38 ± 10 | 0.0069 |
| 1 and 2 | 7 | 109 ± 10 | 65 ± 10 | |||
| pM | 0 | 8 | 94 ± 4 | 0.0123 | 52 ± 14 | 0.0377 |
| 1 | 2 | 130 ± 7 | 78 ± 4 | |||
| pStage | 1 and 2 | 3 | 83 ± 8 | NS | 38 ± 10 | 0.0069 |
| 3 and 4 | 7 | 109 ± 18 | 65 ± 11 | |||
The levels of the oxidative stress marker 4-HNE were measured in the same tissue specimens and compared to those of CML-HMGB1 and HMGB1 (Figure 4B). Both CML-HMGB1 and HMGB1 levels correlated with 4-HNE, but CML-HMGB1 exhibited a more significant correlation with oxidative stress (p = 0.0006 vs. p = 0.0037). A correlation was identified between the levels of CML-HMGB1 and HMGB1 (p < 0.0001) (Figure 4C).
As HMGB1 was found to be correlated with drug resistance [33], we examined the relationship between drug sensitivity and protein levels of CML-HMGB1 or HMGB1 in the above 10 samples (Figure 4D). CML-HMGB1 and HMGB1—and the sum of both—were found to be expressed at higher levels in the insensitive group (grade 0–1a) than in the sensitive group (grade 1b–2). By multivariate analysis (Table 2), CML-HMGB1 showed a less significant correlation with drug resistance than HMGB1; however, summation of HMGB1 and CML-HMGB1 showed more significant correlation with drug resistance than HMGB1 alone. This suggests that CML-HMGB1 promotes drug resistance accompanying HMGB1.
Table 2. Multivariate analysis of HMGB1, CML-HMGB1, and the sum for drug resistance.
| Variable | Coefficient | SE | 95% Confidence Interval | p |
|---|---|---|---|---|
| HMGB1 | −0.01398 | 0.01444 | −0.04812 to 0.02016 | 0.0014 |
| CML-HMGB1 | 0.03455 | 0.01189 | 0.006428 to 0.06267 | |
| SUM | −1.612 | 0.3951 | −2.523 to −0.7007 | 0.0008 |
References
- Statistics Bureau Ministry of Internal Affairs and Communications Japan. Statistical Handbook of Japan 2017; Statistics Bureau Ministry of Internal Affairs and Communications Japan: Tokyo, Japan, 2017.
- Mikami, H.; Nagase, H. Cancer Survival Rates at Japanese Association of Clinical Cancer Centers. Available online: (accessed on 12 May 2021).
- Taguchi, A.; Blood, D.C.; del Toro, G.; Canet, A.; Lee, D.C.; Qu, W.; Tanji, N.; Lu, Y.; Lalla, E.; Fu, C.; et al. Blockade of RAGE-amphoterin signalling suppresses tumour growth and metastases. Nature 2000, 405, 354–360.
- Kuniyasu, H.; Oue, N.; Wakikawa, A.; Shigeishi, H.; Matsutani, N.; Kuraoka, K.; Ito, R.; Yokozaki, H.; Yasui, W. Expression of receptors for advanced glycation end-products (RAGE) is closely associated with the invasive and metastatic activity of gastric cancer. J. Pathol. 2002, 196, 163–170.
- Kuniyasu, H.; Chihara, Y.; Kondo, H. Differential effects between amphoterin and advanced glycation end products on colon cancer cells. Int. J. Cancer 2003, 104, 722–727.
- Kuniyasu, H.; Chihara, Y.; Kondo, H.; Ohmori, H.; Ukai, R. Amphoterin induction in prostatic stromal cells by androgen deprivation is associated with metastatic prostate cancer. Oncol. Rep. 2003, 10, 1863–1868.
- Luo, Y.; Yoneda, J.; Ohmori, H.; Sasaki, T.; Shimbo, K.; Eto, S.; Kato, Y.; Miyano, H.; Kobayashi, T.; Sasahira, T.; et al. Cancer usurps skeletal muscle as an energy repository. Cancer Res. 2014, 74, 330–340.
- Kuniyasu, H.; Chihara, Y.; Takahashi, T. Co-expression of receptor for advanced glycation end products and the ligand amphoterin associates closely with metastasis of colorectal cancer. Oncol. Rep. 2003, 10, 445–448.
- Sasahira, T.; Kirita, T.; Oue, N.; Bhawal, U.K.; Yamamoto, K.; Fujii, K.; Ohmori, H.; Luo, Y.; Yasui, W.; Bosserhoff, A.K.; et al. High mobility group box-1-inducible melanoma inhibitory activity is associated with nodal metastasis and lymphangiogenesis in oral squamous cell carcinoma. Cancer Sci. 2008, 99, 1806–1812.
- Ohmori, H.; Luo, Y.; Kuniyasu, H. Non-histone nuclear factor HMGB1 as a therapeutic target in colorectal cancer. Expert Opin. Ther. Targets 2011, 15, 183–193.
- Schmidt, A.M.; Hori, O.; Cao, R.; Yan, S.D.; Brett, J.; Wautier, J.L.; Ogawa, S.; Kuwabara, K.; Matsumoto, M.; Stern, D. RAGE: A novel cellular receptor for advanced glycation end products. Diabetes 1996, 45, S77–S80.
- Azizian-Farsani, F.; Abedpoor, N.; Hasan Sheikhha, M.; Gure, A.O.; Nasr-Esfahani, M.H.; Ghaedi, K. Receptor for Advanced Glycation End Products Acts as a Fuel to Colorectal Cancer Development. Front. Oncol. 2020, 10, 552283.
- Schmidt, A.M.; Stern, D.M. RAGE: A new target for the prevention and treatment of the vascular and inflammatory complications of diabetes. Trends Endocrinol. Metab. 2000, 11, 368–375.
- Yamagishi, S.; Takeuchi, M.; Inagaki, Y.; Nakamura, K.; Imaizumi, T. Role of advanced glycation end products (AGEs) and their receptor (RAGE) in the pathogenesis of diabetic microangiopathy. Int. J. Clin. Pharmacol. Res. 2003, 23, 129–134.
- Wautier, J.L.; Guillausseau, P.J. Diabetes, advanced glycation endproducts and vascular disease. Vasc. Med. 1998, 3, 131–137.
- Shimomoto, T.; Luo, Y.; Ohmori, H.; Chihara, Y.; Fujii, K.; Sasahira, T.; Denda, A.; Kuniyasu, H. Advanced glycation end products (AGE) induce the receptor for AGE in the colonic mucosa of azoxymethane-injected Fischer 344 rats fed with a high-linoleic acid and high-glucose diet. J. Gastroenterol. 2012, 47, 1073–1083.
- Shimomoto, T.; Ohmori, H.; Luo, Y.; Chihara, Y.; Denda, A.; Sasahira, T.; Tatsumoto, N.; Fujii, K.; Kuniyasu, H. Diabetes-associated angiotensin activation enhances liver metastasis of colon cancer. Clin. Exp. Metastasis 2012, 29, 915–925.
- Omofuma, O.O.; Turner, D.P.; Peterson, L.L.; Merchant, A.T.; Zhang, J.; Steck, S.E. Dietary Advanced Glycation End-products (AGE) and Risk of Breast Cancer in the Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial (PLCO). Cancer Prev. Res. 2020, 13, 601–610.
- Delgado-Andrade, C. Carboxymethyl-lysine: Thirty years of investigation in the field of AGE formation. Food Funct. 2016, 7, 46–57.
- Cho, S.J.; Roman, G.; Yeboah, F.; Konishi, Y. The road to advanced glycation end products: A mechanistic perspective. Curr. Med. Chem. 2007, 14, 1653–1671.
- Ikeda, K.; Higashi, T.; Sano, H.; Jinnouchi, Y.; Yoshida, M.; Araki, T.; Ueda, S.; Horiuchi, S. Nε-(carboxymethyl)lysine protein adduct is a major immunological epitope in proteins modified with advanced glycation end products of the Maillard reaction. Biochemistry 1996, 35, 8075–8083.
- Ferreira, A.E.; Ponces Freire, A.M.; Voit, E.O. A quantitative model of the generation of Nε-(carboxymethyl)lysine in the Maillard reaction between collagen and glucose. Biochem. J. 2003, 376, 109–121.
- Bartling, B.; Desole, M.; Rohrbach, S.; Silber, R.E.; Simm, A. Age-associated changes of extracellular matrix collagen impair lung cancer cell migration. FASEB J. 2009, 23, 1510–1520.
- Hsia, T.C.; Yin, M.C.; Mong, M.C. Advanced Glycation End-Products Enhance Lung Cancer Cell Invasion and Migration. Int. J. Mol. Sci. 2017, 18, 1642.
- Menini, S.; Iacobini, C.; de Latouliere, L.; Manni, I.; Ionta, V.; Blasetti Fantauzzi, C.; Pesce, C.; Cappello, P.; Novelli, F.; Piaggio, G.; et al. The advanced glycation end-product Nε -carboxymethyllysine promotes progression of pancreatic cancer: Implications for diabetes-associated risk and its prevention. J. Pathol. 2018, 245, 197–208.
- Nass, N.; Ignatov, A.; Andreas, L.; Weißenborn, C.; Kalinski, T.; Sel, S. Accumulation of the advanced glycation end product carboxymethyl lysine in breast cancer is positively associated with estrogen receptor expression and unfavorable prognosis in estrogen receptor-negative cases. Histochem. Cell Biol. 2017, 147, 625–634.
- Yang, S.; Pinney, S.M.; Mallick, P.; Ho, S.M.; Bracken, B.; Wu, T. Impact of Oxidative Stress Biomarkers and Carboxymethyllysine (an Advanced Glycation End Product) on Prostate Cancer: A Prospective Study. Clin. Genitourin. Cancer 2015, 13, e347–e351.
- Wen, L.; Huang, J.K.; Johnson, B.H.; Reeck, G.R. A human placental cDNA clone that encodes nonhistone chromosomal protein HMG-1. Nucleic Acids Res. 1989, 17, 1197–1214.
- Ohmori, H.; Luo, Y.; Fujii, K.; Sasahira, T.; Shimomoto, T.; Denda, A.; Kuniyasu, H. Dietary linoleic acid and glucose enhances azoxymethane-induced colon cancer and the metastasis through the expression of high mobility group box 1. Pathobiology 2010, 77, 210–217.
- Wang, S.Y.; Li, J.Y.; Xu, J.H.; Xia, Z.S.; Cheng, D.; Zhong, W.; Lai, Y.; Yu, T.; Chen, Q.K. Butyrate suppresses abnormal proliferation in colonic epithelial cells under diabetic state by targeting HMGB1. J. Pharmacol. Sci. 2019, 139, 266–274.
- Huang, W.S.; Lin, C.T.; Chen, C.N.; Chang, S.F.; Chang, H.I.; Lee, K.C. Metformin increases the cytotoxicity of oxaliplatin in human DLD-1 colorectal cancer cells through down-regulating HMGB1 expression. J. Cell Biochem. 2018, 119, 6943–6952.
- Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer 2011, 14, 101–112.
- Nishiguchi, Y.; Oue, N.; Fujiwara-Tani, R.; Sasaki, T.; Ohmori, H.; Kishi, S.; Mori, S.; Mori, T.; Ikeda, N.; Matsumoto, S.; et al. Role of Metastasis-Related Genes in Cisplatin Chemoresistance in Gastric Cancer. Int. J. Mol. Sci. 2020, 21, 254.
More
Information
Subjects:
Oncology
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
946
Revisions:
3 times
(View History)
Update Date:
12 Oct 2021
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




