1. Introduction
1.1. Stroke
Stroke is a cerebrovascular disease resulting from disturbance in normal cerebral blood flow (CBF), which causes a transient or permanent deficit in the function of one or more parts of the brain, and is becoming one of the leading causes of disability in developed countries. The disturbance of normal CBF induces metabolic and cellular changes that can lead to cell death and disruption of the nervous system
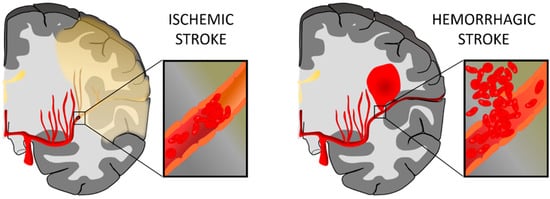
[1]. Stroke can be classified into two types: hemorrhagic and ischemic. A hemorrhagic stroke is due to a blood vessel rupture, represents up to 15% of all stroke cases, and is associated with high mortality. On the contrary, an ischemic stroke is caused by an obstruction in the vessel, often due to the presence of a clot, and constitutes approximately 85% of stroke events
[2] ().
Figure 1. Representation of ischemic stroke and hemorrhagic stroke. In an ischemic stroke, the clot blocks blood flow to an area of the brain; meanwhile, hemorrhagic stroke happens when a blood vessel breaks and bleeds into the brain. Figure created with
BioRender.com.
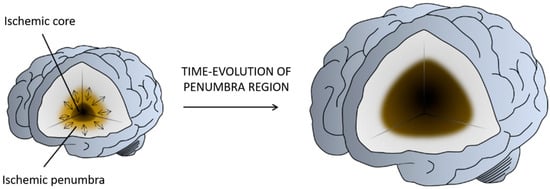
The ischemic infarct is a dynamic region in which multiple molecular and cellular processes are involved (defined as an ischemic cascade), which starts immediately after the ischemic insult and changes even weeks and months later. Based on the blood perfusion level and metabolic activity in the infarct brain, two regions have been extensively described: the ischemic core and the penumbra
[3] (). The core is the region most affected by the lack of blood flow and is irreversibly injured in a few minutes. The penumbra is a vulnerable, hypo-perfused, and metabolically compromised region that can be recovered if the blood flow supply is re-established in the first few hours after a stroke. The penumbra is also the target of multiple protective drugs aimed at reducing or blocking the progression of cell death
[4][5].
Figure 2. Illustration of the penumbra concept. Ischemic core represents the infarcted tissue and the penumbra region the salvageable tissue at risk for infarction in case of persistence vessel occlusion. While neurons in the ischemic core are considered beyond rescue, neurons in the penumbra are potential targets for therapeutic intervention. Figure created with
BioRender.com.
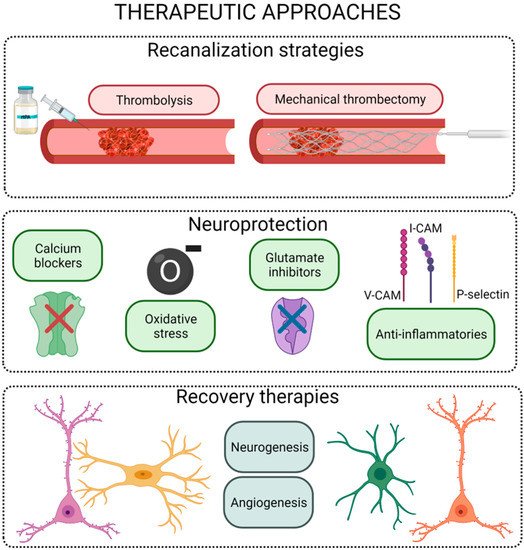
Therapeutic approaches after stroke onset may be classified into three main groups: reperfusion, protective, and recovery strategies ().
Figure 3. Scheme of the different therapeutic strategies for the ischemic stroke. Figure created with
BioRender.com.
Reperfusion therapies, based on the use of pharmacological thrombolysis and/or mechanical recanalization, are focused on restoring CBF in the first hours post stroke onset
[6]. Thrombolysis with intravenous recombinant tissue plasminogen activator (rtPA) is the only Food and Drug Administration/European Medicines Agency (FDA/EMA)-approved drug treatment for patients with acute ischemic stroke, but its use is limited by a narrow therapeutic window, selective efficacy, and hemorrhagic complications
[7]. Nowadays, advanced endovascular recanalization technology based on the use of mechanical catheters has significantly improved the recanalization rates, showing a better overall outcome in treated patients. However, this mechanical therapy is effective when stroke events are caused by a large vessel occlusion, which represents less than 30% of the patients. Moreover, in occlusions located in small vessels, where the rtPA has a successful recanalization rate, the efficacy of mechanical thrombectomy is also limited. In addition, some clinical trials have shown that pretreatment with intravenous rtPA provides additional benefits to patients undergoing mechanical thrombectomy. These evidences reflect that rtPA thrombolysis remains the gold standard treatment for acute stroke
[7].
Neuronal protection is a term that conglomerates a variety of strategies focused on reducing cell death after an ischemic event without affecting tissue reperfusion. To date, several compounds have been proposed to block the pathway leading to ischemia-induced cell death at different steps of the ischemic cascade. These interventions range from physical approaches such as hypothermia to the use of pharmacological drugs
[8]. Despite the positive results obtained in preclinical studies, there is no protective compound for stroke patients yet, due to the narrow therapeutic window, ability of the drug to reach brain targets, side effects, or short half-life after drug administration
[8].
Neurorepair strategies, usually based on cell therapies or growth factor administration, involve the restoration of brain function, either by regeneration of the damaged cerebral tissue or by the establishment of alternative neural pathways or synapses (referred to as brain plasticity). The therapeutic window for these therapies is wider than the one in thrombolytic or neuroprotective approaches. The aim of the treatments for neurological function recovery after stroke is not restricted to the neurons; it is more focused on the neurovascular unit, including procedures that enhance synaptogenesis and angiogenesis. Thus, neurorepair treatments may use stem cells and pro-neurogenic, pro-angiogenic, and/or pro-synaptogenic drug delivery, among others
[9].
1.2. Nanomedicine
Nanotechnology emerged in 1959 as a new area of study that involved the creation of materials or systems at the nanometer scale, defined as nanoparticles (NPs). Later, this technology was applied to the medical field, opening a new era in the diagnosis and treatment of many diseases, currently known as nanomedicine
[10]. The development of NPs for clinical use has allowed, for instance, the encapsulation of hydrophilic drugs, a decrease in the therapeutic doses, a reduction in side effects, an improvement in the biocompatibility and therapeutic effect, or targeting and delivery of the drug to the desired region
[11].
Over the last few years, the complexity of nanosystems has increased exponentially. Initially, the first formulations were mainly focused on the encapsulation of drugs for increasing their safety and efficacy; however, the new advances in the field of nanomedicine have allowed the development of “smart” delivery nanoplatforms that, after administration, can specifically recognize the pathological region and induce a controlled drug delivery that can be induced by internal or external stimuli. Internal stimuli involve chemical and biochemical changes in the targeted region, such as pH, temperature, redox, and ionic changes, or even enzymatic activities, whereas external stimuli comprise, for instance, light, magnetic field, or ultrasound
[11].
Owing to the versatility of nanomedicine, as observed in other pathologies, this technology has been widely applied in the field of stroke, not only for diagnosis but also to improve the efficacy of thrombolytic therapy and protective drugs, as well as to help in the development of recovery therapies.
2. Nanomedicine for Stroke Diagnosis
Computed tomography (CT) and magnetic resonance imaging (MRI) have revolutionized ischemic stroke diagnosis and management. Initially restricted to structural imaging to exclude bleeding, these imaging modalities can now detect intracranial vessel occlusion, evaluate the ischemic penumbra to select candidates for thrombectomy, and play a key role in identifying stroke etiology. Molecular imaging has the potential to further expand the information provided by CT and MRI by revealing the biological processes that constitute potential diagnostic or therapeutic targets
[12]. The use of nanotechnology to design contrast agents has led to significant advances in the field
[13].
The recent development of a new family of contrast agents for MRI based on micro-sized particles of iron oxide (MPIOs) has allowed a large increase in the sensitivity and specificity of this imaging modality, paving the way for clinical application
[14]. The use of larger particles with a diameter of at least 1 µm has several advantages compared to the more classically used ultrasmall superparamagnetic iron oxide particles (20–50 nm). Indeed, it prevents the passive leakage of particles in the brain parenchyma and increases the payload of the contrast material per particle
[15]. Using MPIOs coupled with monoclonal antibodies, non-invasive detection of specific proteins expressed by the cerebrovasculature has been demonstrated in neurovascular disorders in the last few years
[16].
The combination of in vivo MRI with NP contrast agents has allowed the clarification of many aspects of post-stroke inflammation, which is one of the main pathogenic processes occurring in the acute and subacute phases of stroke
[17]. Monitoring the temporo-spatial regulation of the inflammatory reaction in the affected brain parenchyma could have significant clinical applications, such as identifying patient subsets that could benefit from immunomodulatory treatments. In this regard, the endothelial cells of the blood–brain barrier (BBB) are key players in post-stroke inflammation by mediating the diapedesis of leukocytes from the blood to the brain through the expression of adhesion molecules. Since these adhesion molecules are easily accessible by large contrast-carrying particles, they constitute interesting targets for molecular imaging. Using MPIOs coupled with monoclonal antibodies targeting vascular cell adhesion molecule-1 (VCAM-1), molecular MRI has provided evidence for the existence of a new concept defined as “inflammatory penumbra” in ischemic stroke
[18]. In mice, 24 h after ischemic stroke, this technique unveiled an inflammatory area at risk, surrounding the initial ischemic lesion, which was secondarily infiltrated by lymphocytes and ultimately recruited by the ischemic core
[18]. By analogy with the ischemic penumbra, the mismatch between the VCAM-1-overexpressing region (revealed by molecular MRI) and the ischemic core (revealed by diffusion-weighted imaging 24 h post onset) was called the “inflammatory penumbra”. Interestingly, the size of this “inflammatory penumbra” varied according to the duration and subtypes of ischemic stroke in experimental models, suggesting that there could be a wide variability in the intensity and spatial extent of the inflammatory reaction between patients.
Besides VCAM-1, other adhesion molecules are involved in the diapedesis of leukocytes from the blood to the brain. For instance, Deddens and co-workers performed molecular MRI of intercellular adhesion molecule-1 (ICAM-1) in an experimental model of ischemic stroke in mice. They used MPIOs targeting ICAM-1 to reveal the overexpression of ICAM-1 by activated endothelial cells at different time points after ischemic onset
[19]. They found that ICAM-1 expression was maximal at 48 h and extended in the peri-infarct area, in line with the “inflammatory penumbra” concept. Whether VCAM-1 and ICAM-1 imaging methods provide differential information on stroke, pathophysiology remains to be explored. Moreover, Quenault et al. demonstrated that molecular MRI of P-selectin using MPIOs could be employed to diagnose a transient ischemic attack (TIA). In a TIA, the duration of ischemia is too short to induce changes on an unenhanced MRI scan. Interestingly, experimental studies have revealed that P-selectin is overexpressed for at least 24 h in the affected vascular territory. Using molecular MRI of P-selectin, it is therefore possible to detect the endothelial activation triggered by the TIA and distinguish TIA from stroke mimics, such as epilepsy or migraine
[20].
Molecular MRI can also aid in the etiologic assessment of ischemic stroke. Indeed, it has been previously demonstrated that ruptured atherosclerotic plaques overexpress endothelial activation markers. Using MPIOs targeting P-selectin and VCAM-1, McAteer et al. demonstrated that it is possible to detect the activated endothelium inside atherosclerotic plaques using MRI
[21]. Molecular MRI of endothelial activation in stroke patients could thus be utilized to identify the culprit vascular lesion and therefore better characterize stroke etiology.
Even if its sensitivity to detect contrast material is lower than that of MRI, CT can also be used for molecular imaging using NPs. This has been demonstrated in experimental models of carotid artery thrombosis and embolic ischemic stroke
[22]. Kim et al. showed that fibrin-targeted gold NPs can reveal cerebral thrombus as a high-density endovascular material on CT images. In acute stroke settings, this would allow the assessment of thrombus burden and monitoring of thrombolytic therapy in a non-invasive manner.
Given the demonstration of the high sensitivity and specificity of molecular imaging of inflammation offered by MPIO-enhanced molecular MRI in experimental models, efforts are ongoing to translate this method to clinical imaging. Indeed, the MPIOs used in preclinical studies are non-biodegradable because of their coating and inner structure. In this context, the development of biocompatible MPIOs is mandatory. To overcome this limitation, Perez-Balderas et al. recently reported the production of multimeric magnetite particles that form large MPIO-like particles that are biodegradable
[23]. In an experimental model of neuroinflammation, they demonstrated that this method allowed non-invasive imaging of activated endothelial cells.