Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Dong Sui | + 2304 word(s) | 2304 | 2021-05-27 06:24:11 | | | |
| 2 | Peter Tang | Meta information modification | 2304 | 2021-06-01 11:40:54 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Sui, D. Catalytic Cathodes for Na-CO2 Batteries. Encyclopedia. Available online: https://encyclopedia.pub/entry/10356 (accessed on 07 February 2026).
Sui D. Catalytic Cathodes for Na-CO2 Batteries. Encyclopedia. Available at: https://encyclopedia.pub/entry/10356. Accessed February 07, 2026.
Sui, Dong. "Catalytic Cathodes for Na-CO2 Batteries" Encyclopedia, https://encyclopedia.pub/entry/10356 (accessed February 07, 2026).
Sui, D. (2021, June 01). Catalytic Cathodes for Na-CO2 Batteries. In Encyclopedia. https://encyclopedia.pub/entry/10356
Sui, Dong. "Catalytic Cathodes for Na-CO2 Batteries." Encyclopedia. Web. 01 June, 2021.
Copy Citation
Na-CO2 batteries with high energy density are drawing tremendous attention because of their advantages of combining cost-effective energy conversion and storage with CO2 clean recycle and utilization. Nevertheless, their commercial applications are impeded by unsatisfactory electrochemical performance including large overpotentials, poor rate capability, fast capacity deterioration, and inferior durability, which mainly results from the inefficient electrocatalysts of cathode materials.
Na-CO2 batteries
CO2 capture and recycle
energy storage
electrocatalyst
electrocatalysis
1. Introduction
With the rapid development and wide spread of electric vehicle and consuming electronics, energy storage devices featuring in high energy and power density, low cost, and long cycle life are highly desired [1][2][3][4][5][6][7][8]. Rechargeable batteries, supercapacitors and metal–ion hybrid devices are commonly used, but all of these systems have to deal with some drawbacks in terms of energy density, lifespan, and/or cost [9][10][11][12][13][14]. In addition, renewable and clean power sources such as wind and solar energy also need high-performance energy storage devices because of their intermittent and unstable characteristics [15][16]. On the other hand, the over consumption of non-renewable fossil fuels have not only caused energy crisis and environmental pollution but also produced large amount of greenhouse gas [17][18][19]. It is well accepted that CO2 is the leading greenhouse gas, and its ever-growing emissions will lead to the catastrophic consequences of climate change, sea level rise, and glacier melting [20][21]. Therefore, researchers across the world devote extensive efforts to developing various chemical and physical routes to capture and store CO2 or convert CO2 into value-added materials [17][22][23][24][25][26][27][28][29][30]. However, most of these technologies deliver unsatisfactory performance due to the low efficiency conversion rate and high energy consumption, raising the overall cost and inhibiting the extensive applications.
Recently, metal-CO2 batteries, a type of metal–air batteries, have become one of the most appealing choices due to the unique feature of simultaneously consuming greenhouse gas CO2 and generating electrical energy [16][18][20][31]. Arther et al. proposed the first protype of the Li-CO2 battery, which served as a primary battery and was supposed to capture and utilize CO2 [32]. Later, Zhang et al. designed the first rechargeable Li-CO2 battery, and a high capacity was harvested at room temperature by applying a catalytic cathode to promote the decomposition of insulating Li2CO3 [33]. Afterwards, a prosperous research field began rising, and various cathode materials, electrocatalysts, and electrolytes have been developed to improve the electrochemical performances of rechargeable Li-CO2 batteries [34][35][36]. However, the limited and uneven distribution of lithium resources in the earth boost the price and impede the large-scale utilization of Li-based energy storage systems, motivating researchers to investigate alternatives to Li-CO2 batteries.
Among various metal–CO2 batteries, more research attention is being engendered to the Na-CO2 batteries recently, which show the similar characteristics as their Li-based analogous, including high energy density (1125 W h kg–1) and effective utilization of CO2 but with low cost (vs. Li) and relatively high working voltage of 2.0 V (vs. Mg and Al, <1.0 V) [37][38][39][40][41][42]. Moreover, the low free energy (ΔrG0m = −905.6 kJ mol−1) for the reaction between Na and CO2 leads to a smaller charge potential than that of Li (ΔrG0m = −1081 kJ mol−1), which is beneficial to suppressing electrolyte decomposition and thus helps to enhance round-trip efficiency and increase the lifespan [37]. Na+ as a charge carrier also possesses other advantages compared with Li+, such as less polarizing because of larger ion radius and higher coordination number, smaller charge transfer resistance, and faster electrode kinetics due to the lower solvation energy, demonstrating promising potential in Na-CO2 batteries [43]. Das et al. reported a pioneering work of using a mixture of CO2 and O2 as the air cathode in a non-aqueous Na-O2 battery with the main discharge products of Na2O2, Na2C2O4, and Na2CO3, proving that CO2 could be applied as an active material in Na-air batteries [44]. After that, Hu et al. reported a Na-CO2 battery using multi-wall carbon tubes on Ni mesh as cathode in pure CO2, and a lifespan of 200 cycles was successfully obtained [37]. Inspired by these exciting and promising investigations, researchers devoted more efforts into the development of novel structured cathodes and efficient catalysts, the anode surface modification as well as the electrolyte regulation for high-performance Na-CO2 batteries [31][45][46].
However, the practical application of Na-CO2 batteries is still inhibited by the unsatisfactory electrochemical performance involving limited cycle lifespan, low rate capability, high polarization, and low energy efficiency, which is mainly attributed to the inferior electrocatalyst cathodes [36][47].
2. Na-CO2 Batteries
Typically, Na-CO2 batteries are composed of metallic Na anodes, CO2-involved porous cathodes with sufficient gas-diffusion pathways and effective electrocatalyst, and ion-conducting separators immersing in sodium salts-containing electrolytes, as shown in Figure 1a. Na-CO2 batteries have a working potential of 2.35 V (vs. Na/Na+) and follow a charge/discharge mechanism, as demonstrated in Equation (1): [37]
4Na + 3CO2 ↔ 2Na2CO3 + C.
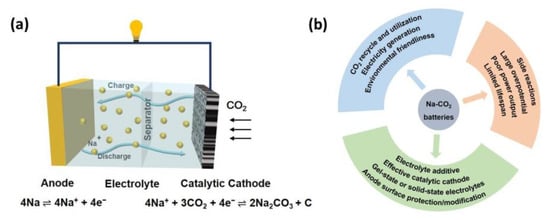
Figure 1. (a) The schematic configuration of rechargeable Na-CO2 batteries; (b) The importance, existing challenges, and potential solutions for Na-CO2 batteries.
Overall, the reactions of Na-CO2 batteries are the reversible reduction and evolution of CO2 accompanied by the formation and decomposition of Na2CO3. Specifically, during the discharge process, the stripping of the Na anode occurs with Na+ and electrons moving to the porous cathode where CO2 is reduced via a carbon dioxide reduction reaction and the products of Na2CO3 and amorphous carbon generate, corresponding to the reaction: 4 Na+ + 4e− + 2CO2 → Na2CO3 + C. For the charge process, promoting by the catalytic cathode, the discharge products decompose into CO2 through a carbon dioxide evolution reaction with Na+ and electron moving back and depositing on the anode, corresponding to the reaction 2Na2CO3 + C → 4Na + 3CO2 [46]. The specific reactions taking place in the cathode and anode are also demonstrated in Figure 1a.
Obviously, as an emerging energy storage system with the merits of low cost and high energy density, Na-CO2 batteries are of great significance for alleviating the global climate change and energy shortage by CO2 recycle and utilization as well as electricity generation. Nevertheless, the inherent disadvantages pertaining to large overpotential (resulting in low energy efficiency), limited cycling life, poor rate capability, and serious side reactions hamper their practical applications. All these challenges urgently call for high-performance cathodes with effective catalysts, anode with stable solid–electrolyte interface as well as advanced electrolyte, as demonstrated in Figure 1b and discussed in the following paragraphs.
As one of the key components, cathode materials are in the spotlight in the realm of metal-CO2 batteries. It is in the cathode where CO2 is captured and utilized as well as the formation and decomposition of discharge products. Therefore, idea cathode materials should have merits of high electrical conductivity, effective catalytic activity, excellent mechanical and electrochemical stability, and low cost. Moreover, the structure and porosity of the cathode materials (i.e., morphology, crystalline form, specific surface area (SSA), pore volume, and pore size distribution) also have a great impact on the catalytic effect and thus should be rationally designed. In Li-CO2 batteries, carbon-based materials [33][48][49] have been widely used as cathodes and sometimes porous gold [50], NiO [51], Ag nanowires [37][52], and platinum net [33][53] have been applied to investigate the reaction products thus far. Similarly, these cathodes/catalysts are quite suitable for Na-CO2 batteries, too. More importantly, to promote the CO2 reduction and evolution reactions, highly efficient catalysts are urgently desired. The design strategies of catalytic cathodes and the understanding of their catalytic mechanism are the hottest research topics for Na-CO2 batteries. In order to achieve high-performance cathode, various preparation techniques including solution-based processing, physical roll pressing and thin film-based technology have been used. Among these, solution-based cathode fabrication featuring mass production and ease of operation is the most commonly utilized. The typical process involves slurry preparing, coating, drying and pressing, which consume large amounts of organic solvents and energy and cause pollution and safety issues. On the other hand, mechanical roll pressing such as dry electrode coating has been developed by physically mixing the raw materials without solvent and then directly pressed into electrode with desired thickness. Obviously, this emerging technology is pollute-free, energy saving, and much safer [54]. Nevertheless, the uniformity of the electrode prepared by this method needs to further be improved. In addition, other technologies such as vacuum filtration and chemical vapor deposition serve as facile methods to prepare thin film-based electrodes with adjusting thickness, which can be applied to fabricate binder-free and self-standing electrodes for wearable electronics.
Sodium metal is generally used as anode for Na-CO2 batteries. However, as other sodium metal-based energy storage devices, serious side reactions and uncontrollable dendrite growth during the repeated charge/discharge process are two main obstacles for their practical applications. Therefore, one of the top priorities is to develop an effective way to inhibit the side reactions and form a compatible interface between the electrolytes and Na anode. Normally, both the liquid and solid-state electrolytes are unstable and have serious side reactions with the highly active Na metal, resulting in low Coulomb efficiency, high interfacial resistance, and sluggish Na+ ion transfer [55]. In order to form stable interface between Na anode and electrolytes, two strategies are commonly applied, namely, employing Na alloys instead of Na metal as anodes and adding buffer layers on the Na surface [56][57]. Na dendrite formation is another notorious problem for Na metal-based batteries and always leads to short circuit and safety issues. Recently, tremendous efforts have been devoted to solve the annoying problem, and several strategies have been proposed to construct stable dendrite-free sodium metal anodes, including designing effective Na hosts, electrolyte modification, sodium surface protection, or artificial solid electrolyte interface regulation [55][58][59][60][61]. Among these methods, special attention should be given to nanostructured framework for the Na anode, which can efficiently homogenize the near surface electric field and regulate the electron transport and ion flux [62][63]. Therefore, the local current density could be sufficiently reduced, and a smooth deposition without dendrite could be obtained.
Organic solvent-based liquid electrolytes are commonly used in Na-CO2 batteries, but they face the challenges of decomposition because of high overpotential and safety issues such as flammability, volatilization, electrochemical instability and potential leakage risk [55]. In order to enhance the electrolyte stability and suppress the decomposition at high potential, Xu et al. reported an organic–inorganic hybrid liquid electrolyte by adding 10% ionic-liquid-tethered silica nanoparticles [64]. By using this modified electrolyte, the prepared devices could even be operated at a high voltage of 5 V. Furthermore, the quasi-solid- or all-solid-state electrolytes are employed to replace the conventionally used liquid ones due to two main reasons: (i) the safety issues of flammability and leakage of liquid electrolyte because of the open cell configuration of Na-CO2 batteries can be effectively avoided; (ii) the long-term stability of Na metal anode can be realized by suppressing dendrite growth and inhibiting CO2 corrosion [55][63][65][66].
However, the development of Na-CO2 batteries is in a nascent stage, and several big challenges still exist, for instance, the irreversible capacity loss due to the formation of metal carbonates and the unwanted side reactions such as the decomposition of electrolyte and carbon cathode, which impedes the practical applications. Similar to the Li-CO2 batteries, the sluggish kinetics of CO2 reduction reaction (corresponding to the formation of Na2CO3 during the discharge process) and CO2 evolution reaction (corresponding to the decomposition of the thermodynamically stable Na2CO3) in the cathode are the most critical steps affecting the rate and cycling performance of Na-CO2 batteries, which is influenced by several factors [67]. For example, the CO2 reduction and evolution is seriously restrained by the insufficient catalytic activity, resulting in large overpotential and inferior round-trip efficiency. Poor electronic conductivity and slow mass diffusion rate lead to inferior rate performance. Moreover, the surface or porous channels of the cathode materials are easily coated or blocked by the insulating and insoluble discharge products, which can passivate the catalytic centers and thus reduce the electronic conductivity, causing low capacity and unfavorable durability. Hence, advanced cathode materials with high efficiency and robust catalysts are highly needed to reduce the overpotential and enhance the rate properties and cycling stability. In the following section of this minireview, we will focus on the recent progresses of catalytic cathode materials for Na-CO2 batteries.
3. Catalytic Cathode Materials for Na-CO2 Batteries
To realize the commercial implementations of Na-CO2 batteries, it is of extensive significance to develop efficient cathode materials. During the past decades, fruitful achievements have been made for Na-CO2 batteries, especially in the design of novel cathodes with highly efficient electrocatalyst materials. Electrocatalysts usually play an important role in promoting the reduction and evolution of CO2 and the formation and decomposition of carbonates, which has a critical influence on the electrochemical performance of Na-CO2 batteries.
Several figures of merit should be emphasized to obtain effective catalytic cathodes, which are exhibited in Figure 2. Firstly, materials with high electrical conductivity, rationally designed porous structure and large pore volume are preferable to enable fast electron transport, facilitate the mass (Na+ and CO2) diffusion, and accommodate the insulating discharge products, aiming to reduce the impendence and thus to improve rate capability. Secondly, it is of equal importance for the cathode materials to possess strong binding affinity with CO2, helping to decrease the reaction barriers at the gas/electrolyte/solid interfaces [65]. Last but not the least, ideal cathodes should have highly efficient catalytic activity to promote the CO2 reduction and evolution and the decomposition of discharge products, which is beneficial to reduce the overpotential during charge and discharge and lead to high electrochemical performance. Therefore, high SSA with abundant and accessible catalytic sites is extremely desired [67]. Other factors about the catalytic cathodes, including resources abundance, environmental friendliness, and facile preparation process, are also vital for the practical applications of Na-CO2 batteries.
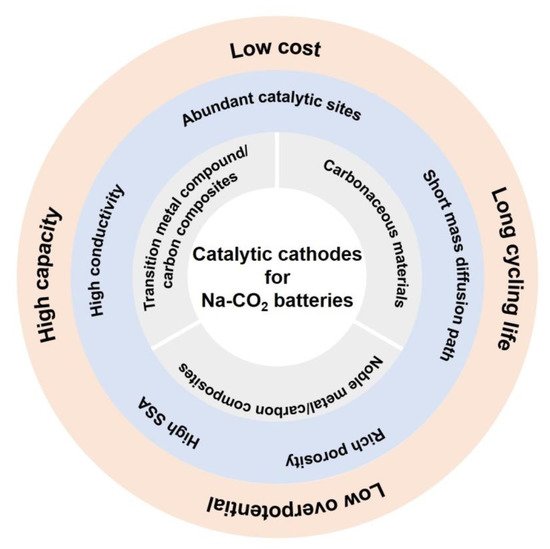
Figure 2. Effective catalytic cathode materials for high-performance Na-CO2 batteries.
Typical catalytic cathode materials for high-performance Na-CO2 batteries include carbon-based materials, noble metal/carbon-based materials, and transition metal oxide/carbon-based composites, as demonstrated in Figure 2. In this section, the latest achievements of catalytic cathode materials for Na-CO2 batteries are reviewed with a special focus on the synthetic methods, structures, and catalytic mechanisms.
References
- Liu, Y.; Zhu, Y.; Cui, Y. Challenges and opportunities towards fast-charging battery materials. Nat. Energy 2019, 4, 540–550.
- Wu, N.; Qiao, X.; Shen, J.; Liu, G.; Sun, T.; Wu, H.; Hou, H.; Liu, X.; Zhang, Y.; Ji, X. Anatase inverse opal TiO2 C induced the dominant pseudocapacitive effect for durable and fast lithium/sodium storage. Electrochim. Acta 2019, 299, 540–548.
- Simon, P.; Gogotsi, Y. Perspectives for electrochemical capacitors and related devices. Nat. Mater. 2020, 19, 1151–1163.
- Sui, D.; Xie, Y.; Zhao, W.; Zhang, H.; Zhou, Y.; Qin, X.; Ma, Y.; Yang, Y.; Chen, Y. A high-performance ternary Si composite anode material with crystal graphite core and amorphous carbon shell. J. Power Sources 2018, 384, 328–333.
- Guo, D.; Yang, M.; Zhang, L.; Li, Y.; Wang, J.; Liu, G.; Wu, N.; Kim, J.-K.; Liu, X. Cr2O3 nanosheet/carbon cloth anode with strong interaction and fast charge transfer for pseudocapacitive energy storage in lithium-ion batteries. RSC Adv. 2019, 9, 33446–33453.
- Zhao, Z.; Yi, Z.; Li, H.; Pathak, R.; Yang, Z.; Wang, X.; Qiao, Q. Synergetic effect of spatially separated dual co-catalyst for accelerating multiple conversion reaction in advanced lithium sulfur batteries. Nano Energy 2021, 81, 105621.
- Pathak, R.; Zhou, Y.; Qiao, Q. Recent Advances in Lithiophilic Porous Framework toward Dendrite-Free Lithium Metal Anode. Appl. Sci. 2020, 10, 4185.
- Zhou, F.; Li, Z.; Lu, Y.-Y.; Shen, B.; Guan, Y.; Wang, X.-X.; Yin, Y.-C.; Zhu, B.-S.; Lu, L.-L.; Ni, Y.; et al. Diatomite derived hierarchical hybrid anode for high performance all-solid-state lithium metal batteries. Nat. Commun. 2019, 10, 1–11.
- Sui, D.; Wu, M.; Liu, Y.; Yang, Y.; Zhang, H.; Ma, Y.; Zhang, L.; Chen, Y. High performance Li-ion capacitor fabricated with dual graphene-based materials. Nanotechnology 2020, 32, 015403.
- Wang, F.; Liu, Y.; Wei, H.-J.; Li, T.-F.; Xiong, X.-H.; Wei, S.-Z.; Ren, F.-Z.; Volinsky, A.A. Recent advances and perspective in metal coordination materials-based electrode materials for potassium-ion batteries. Rare Met. 2021, 40, 448–470.
- Liu, G.; Li, M.; Wu, N.; Cui, L.; Huang, X.; Liu, X.; Zhao, Y.; Chen, H.; Yuan, W.; Bai, Y. Single-Crystalline Particles: An Effective Way to Ameliorate the Intragranular Cracking, Thermal Stability, and Capacity Fading of the LiNi0.6Co0.2Mn0.2O2 Electrodes. J. Electrochem. Soc. 2018, 165, A3040–A3047.
- Wu, N.; Wu, H.; Kim, J.-K.; Liu, X. Zhang, Y. Restoration of Degraded Nickel-Rich Cathode Materials for Long-Life Lith-ium-Ion Batteries. ChemElectroChem 2018, 5, 78–83.
- Zhou, Z.; Ma, L.; Tan, C. Preparation of Layered (NH4)2V6O16·H2O Nanosheets as an Anode for Li-ion Batteries. Chem. J. Chin. Univ. 2021, 42, 662–670.
- Sheberla, D.; Bachman, J.C.; Elias, J.S.; Sun, C.-J.; Shao-Horn, Y.; Dincă, M. Conductive MOF electrodes for stable superca-pacitors with high areal capacitance. Nat. Mater. 2017, 16, 220–224.
- Sui, D.; Xu, L.; Zhang, H.; Sun, Z.; Kan, B.; Ma, Y.; Chen, Y. A 3D cross-linked graphene-based honeycomb carbon composite with excellent confinement effect of organic cathode material for lithium-ion batteries. Carbon 2020, 157, 656–662.
- Xu, C.; Fang, X.; Zhan, J.; Chen, J.; Liang, F. Progress for Metal-CO2 Batteries: Mechanism and Advanced Materials. Prog. Chem. 2020, 32, 836–850.
- Licht, S.; Douglas, A.; Ren, J.; Carter, R.; Lefler, M.; Pint, C.L. Carbon Nanotubes Produced from Ambient Carbon Dioxide for Environmentally Sustainable Lithium-Ion and Sodium-Ion Battery Anodes. ACS Central Sci. 2016, 2, 162–168.
- Xie, Z.; Zhang, X.; Zhang, Z.; Zhou, Z. Metal-CO2 Batteries on the Road: CO2 from Contamination Gas to Energy Source. Adv. Mater. 2017, 29, 1605891.
- Chen, H.; Liu, P.; Liu, J.; Feng, X.; Zhou, S. Mechanochemical in-situ incorporation of Ni on MgO/MgH2 surface for the selective O-/C-terminal catalytic hydrogenation of CO2 to CH4. J. Catal. 2021, 394, 397–405.
- Xie, J.; Zhou, Z.; Wang, Y. Metal–CO2 Batteries at the Crossroad to Practical Energy Storage and CO2 Recycle. Adv. Funct. Mater. 2020, 30, 1908285.
- Chang, S.; Liang, F.; Yao, Y.; Ma, W.; Yang, B.; Dai, Y. Research Progress of Metallic Carbon Dioxide Batteries. Acta Chim. Sin. 2018, 76, 515–525.
- Appaturi, J.N.; Ramalingam, R.J.; Gnanamani, M.K.; Periyasami, G.; Arunachalam, P.; Adnan, R.; Adam, F.; Wasmiah, M.D.; Al-Lohedan, H.A. Review on Carbon Dioxide Utilization for Cycloaddition of Epoxides by Ionic Liquid-Modified Hybrid Catalysts: Effect of Influential Parameters and Mechanisms Insight. Catalysts 2020, 11, 4.
- Maina, J.W.; Pringle, J.M.; Razal, J.M.; Nunes, S.; Vega, L.; Gallucci, F.; Dumée, L.F. Strategies for Integrated Capture and Conversion of CO2 from Dilute Flue Gases and the Atmosphere. ChemSusChem 2021, 14, 1805–1820.
- Zurrer, T.; Wong, K.; Horlyck, J.; Lovell, E.C.; Wright, J.; Bedford, N.M.; Han, Z.; Liang, K.; Scott, J.; Amal, R. Mixed-Metal MOF-74 Templated Catalysts for Efficient Carbon Dioxide Capture and Methanation. Adv. Funct. Mater. 2021, 31, 2007624.
- Bai, S.-T.; De Smet, G.; Liao, Y.; Sun, R.; Zhou, C.; Beller, M.; Maes, B.U.W.; Sels, B.F. Homogeneous and heterogeneous catalysts for hydrogenation of CO2 to methanol under mild conditions. Chem. Soc. Rev. 2021, 50.
- Klinkenberg, N.; Kraft, S.; Polarz, S. Great Location: About Effects of Surface Bound Neighboring Groups for Passive and Active Fine-Tuning of CO2 Adsorption Properties in Model Carbon Capture Materials. Adv. Mater. 2021, 33, 2007734.
- Mgolombane, M.; Majodina, S.; Bankole, O.; Ferg, E.; Ogunlaja, A. Influence of surface modification of zinc oxide–based nanomaterials on the photocatalytic reduction of carbon dioxide. Mater. Today Chem. 2021, 20, 100446.
- Keith, D.W.; Holmes, G.; Angelo, D.S.; Heidel, K. A Process for Capturing CO2 from the Atmosphere. Joule 2018, 2, 1573–1594.
- Chen, H.; Liu, J.; Liu, P.; Wang, Y.; Xiao, H.; Yang, Q.; Feng, X.; Zhou, S. Carbon-confined magnesium hydride nano-lamellae for catalytic hydrogenation of carbon dioxide to lower olefins. J. Catal. 2019, 379, 121–128.
- Chen, H.; Yang, M.; Liu, J.; Lu, G.; Feng, X. Insight into the effects of electronegativity on the H2 catalytic activation for CO2 hydrogenation: Four transition metal cases from a DFT study. Catal. Sci. Technol. 2020, 10, 5641–5647.
- Cai, F.; Hu, Z.; Chou, S.-L. Progress and Future Perspectives on Li(Na)-CO2 Batteries. Adv. Sustain. Syst. 2018, 2, 1800060.
- Xu, S.; Das, S.K.; Archer, L.A. The Li-CO2 battery: A novel method for CO2 capture and utilization. RSC Adv. 2013, 3, 6656–6660.
- Zhang, Z.; Zhang, Q.; Chen, Y.; Bao, J.; Zhou, X.; Xie, Z.; Wei, J.; Zhou, Z. The First Introduction of Graphene to Rechargeable Li-CO2Batteries. Angew. Chem. Int. Ed. 2015, 54, 6550–6553.
- Liu, B.; Sun, Y.; Liu, L.; Chen, J.; Yang, B.; Xu, S.; Yan, X. Recent advances in understanding Li-CO2 electrochemistry. Energy Environ. Sci. 2019, 12, 887–922.
- Xie, J.; Wang, Y. Recent Development of CO2 Electrochemistry from Li-CO2 Batteries to Zn-CO2 Batteries. Acc. Chem. Res. 2019, 52, 1721–1729.
- Zhang, Z.; Bai, W.-L.; Wang, K.-X.; Chen, J.-S. Electrocatalyst design for aprotic Li-CO2 batteries. Energy Environ. Sci. 2020, 13, 4717–4737.
- Hu, X.; Sun, J.; Li, Z.; Zhao, Q.; Chen, C.; Chen, J. Rechargeable Room-Temperature Na-CO2 Batteries. Angew. Chem. Int. Ed. 2016, 55, 6482–6486.
- Hu, X.; Li, Z.; Chen, J. Flexible Li-CO2Batteries with Liquid-Free Electrolyte. Angew. Chem. Int. Ed. 2017, 56, 5785–5789.
- Ma, W.; Liu, X.; Li, C.; Yin, H.; Xi, W.; Liu, R.; He, G.; Zhao, X.; Luo, J.; Ding, Y. Rechargeable Al-CO2 Batteries for Reversible Utilization of CO2. Adv. Mater. 2018, 30, e1801152.
- Kim, J.; Seong, A.; Yang, Y.; Joo, S.; Kim, C.; Jeon, D.H.; Dai, L.; Kim, G. Indirect surpassing CO2 utilization in membrane-free CO2 battery. Nano Energy 2021, 82, 105741.
- Wang, Y.; Wang, Y.; Feng, X.; Chen, W.; Ai, X.; Yang, H.; Cao, Y. Developments and Perspectives on Emerging High-Energy-Density Sodium-Metal Batteries. Chem 2019, 5, 2547–2570.
- Zheng, Z.; Wu, C.; Gu, Q.; Konstantinov, K.; Wang, J. Research Progress and Future Perspectives on Rechargeable Na-O2 and Na-CO2 Batteries. Energy Environ. Mater. 2020, 4.
- Okoshi, M.; Yamada, Y.; Yamada, A.; Nakai, H. Theoretical Analysis on De-Solvation of Lithium, Sodium, and Magnesium Cations to Organic Electrolyte Solvents. J. Electrochem. Soc. 2013, 160, A2160–A2165.
- Das, S.K.; Xu, S.; Archer, L.A. Carbon dioxide assist for non-aqueous sodium–oxygen batteries. Electrochem. Commun. 2013, 27, 59–62.
- Mu, X.; Pan, H.; He, P.; Zhou, H. Li-CO2 and Na-CO2 Batteries: Toward Greener and Sustainable Electrical Energy Storage. Adv. Mater. 2020, 32, 1903790.
- Jena, A.; Tong, Z.; Chang, H.; Hu, S.; Liu, R. Capturing carbon dioxide in Na-CO2 batteries: A route for green energy. J. Chin. Chem. Soc. 2021, 68, 421–428.
- Hu, A.; Shu, C.; Xu, C.; Liang, R.; Li, J.; Zheng, R.; Li, M.; Long, J. Design strategies toward catalytic materials and cathode structures for emerging Li–CO2 batteries. J. Mater. Chem. A 2019, 7, 21605–21633.
- Zhang, Z.; Wang, X.-G.; Zhang, X.; Xie, Z.; Chen, Y.-N.; Ma, L.; Peng, Z.; Zhou, Z. Verifying the Rechargeability of Li-CO2 Batteries on Working Cathodes of Ni Nanoparticles Highly Dispersed on N-Doped Graphene. Adv. Sci. 2018, 5, 1700567.
- Zhang, X.; Zhang, Q.; Zhang, Z.; Chen, Y.; Xie, Z.; Wei, J.; Zhou, Z. Rechargeable Li–CO2 batteries with carbon nanotubes as air cathodes. Chem. Commun. 2015, 51, 14636–14639.
- Liu, Y.; Wang, R.; Lyu, Y.; Li, H.; Chen, L. Rechargeable Li/CO2–O2 (2:1) battery and Li/CO2 battery. Energy Environ. Sci. 2014, 7, 677–681.
- Wang, R.; Yu, X.; Bai, J.; Li, H.; Huang, X.; Chen, L.; Yang, X. Electrochemical decomposition of Li2CO3 in NiO–Li2CO3 nanocomposite thin film and powder electrodes. J. Power Sources 2012, 218, 113–118.
- Liu, Q.; Tang, Y.; Sun, H.; Yang, T.; Sun, Y.; Du, C.; Jia, P.; Ye, H.; Chen, J.; Peng, Q.; et al. In Situ Electrochemical Study of Na-O2/CO2 Batteries in an Environmental Transmission Electron Microscope. ACS Nano 2020, 14, 13232–13245.
- Meini, S.; Tsiouvaras, N.; Schwenke, K.U.; Piana, M.; Beyer, H.; Lange, L.; Gasteiger, H.A. Rechargeability of Li–air cathodes pre-filled with discharge products using an ether-based electrolyte solution: Implications for cycle-life of Li–air cells. Phys. Chem. Chem. Phys. 2013, 15, 11478–11493.
- Zhou, H.; Liu, M.; Gao, H.; Hou, D.; Yu, C.; Liu, C.; Zhang, D.; Wu, J.-C.; Yang, J.; Chen, D. Dense integration of solvent-free electrodes for Li-ion supercabattery with boosted low temperature performance. J. Power Sources 2020, 473, 228553.
- Lu, Y.; Cai, Y.; Zhang, Q.; Liu, L.; Niu, Z.; Chen, J. A compatible anode/succinonitrile-based electrolyte interface in all-solid-state Na–CO2 batteries. Chem. Sci. 2019, 10, 4306–4312.
- Chi, X.; Liang, Y.; Hao, F.; Zhang, Y.; Whiteley, J.; Dong, H.; Hu, P.; Lee, S.; Yao, Y. Tailored Organic Electrode Material Compatible with Sulfide Electrolyte for Stable All-Solid-State Sodium Batteries. Angew. Chem. Int. Ed. 2018, 57, 2630–2634.
- Tang, H.; Deng, Z.; Lin, Z.; Wang, Z.; Chu, I.-H.; Chen, C.; Zhu, Z.; Zheng, C.; Ong, S.P. Probing Solid–Solid Interfacial Re-actions in All-Solid-State Sodium-Ion Batteries with First-Principles Calculations. Chem. Mater. 2018, 30, 163–173.
- Zhao, S.; Wang, C.; Du, D.; Li, L.; Chou, S.; Li, F.; Chen, J. Bifunctional Effects of Cation Additive on Na-O2 Batteries. Angew. Chem. Int. Ed. 2021, 60, 3205–3211.
- Lin, X.; Sun, Y.; Sun, Q.; Luo, J.; Zhao, Y.; Zhao, C.; Yang, X.; Wang, C.; Huo, H.; Li, R.; et al. Reviving Anode Protection Layer in Na-O2 Batteries: Failure Mechanism and Resolving Strategy. Adv. Energy Mater. 2021, 11, 2003789.
- Fan, L.; Li, X. Recent advances in effective protection of sodium metal anode. Nano Energy 2018, 53, 630–642.
- Luo, W.; Lin, C.-F.; Zhao, O.; Noked, M.; Zhang, Y.; Rubloff, G.W.; Hu, L. Ultrathin Surface Coating Enables the Stable Sodium Metal Anode. Adv. Energy Mater. 2017, 7, 1601526.
- Xu, Y.; Wang, C.; Matios, E.; Luo, J.; Hu, X.; Yue, Q.; Kang, Y.; Li, W. Sodium Deposition with a Controlled Location and Orientation for Dendrite-Free Sodium Metal Batteries. Adv. Energy Mater. 2020, 10, 2002308.
- Hu, X.; Li, Z.; Zhao, Y.; Sun, J.; Zhao, Q.; Wang, J.; Tao, Z.; Chen, J. Quasi–solid state rechargeable Na-CO2 batteries with reduced graphene oxide Na anodes. Sci. Adv. 2017, 3, e1602396.
- Xu, S.; Lu, Y.; Wang, H.; Abruña, H.D.; Archer, L.A. A rechargeable Na-CO2/O2 battery enabled by stable nanoparticle hybrid electrolytes. J. Mater. Chem. A 2014, 2, 17723–17729.
- Hu, X.; Joo, P.H.; Matios, E.; Wang, C.; Luo, J.; Yang, K.; Li, W. Designing an All-Solid-State Sodium-Carbon Dioxide Battery Enabled by Nitrogen-Doped Nanocarbon. Nano Lett. 2020, 20, 3620–3626.
- Wang, X.; Zhang, X.; Lu, Y.; Yan, Z.; Tao, Z.; Jia, D.; Chen, J. Flexible and Tailorable Na-CO2 Batteries Based on an All-Solid-State Polymer Electrolyte. ChemElectroChem 2018, 5, 3628–3632.
- Xu, C.; Zhan, J.; Wang, Z.; Fang, X.; Chen, J.; Liang, F.; Zhao, H.; Lei, Y. Biomass-derived highly dispersed Co/Co9S8 nanoparticles encapsulated in S, N-co-doped hierarchically porous carbon as an efficient catalyst for hybrid Na-CO2 batteries. Mater. Today Energy 2021, 19, 100594.
More
Information
Subjects:
Electrochemistry
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.5K
Revisions:
2 times
(View History)
Update Date:
23 Jun 2021
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




