
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Hidde van Steenwijk | + 4266 word(s) | 4266 | 2021-04-21 05:09:12 | | | |
| 2 | Rita Xu | Meta information modification | 4266 | 2021-06-01 11:00:01 | | |
Video Upload Options
The importance of a well-functioning and balanced immune system has become more apparent in recent decades. Various elements have however not yet been uncovered as shown, for example, in the uncertainty on immune system responses to COVID-19. Fungal beta-glucans are bioactive molecules with immunomodulating properties.
1. Introduction
Many chronic diseases can be explained by an underlying chronic inflammation; more appealing to the imagination is the recent COVID-19 pandemic, which has presented the modern world with a challenge that global health care has not faced in more than a century since the Spanish flu pandemic in 1918 [1]. A characteristic feature of an infection with COVID-19 is a pro-inflammatory status characterized by high levels of different cytokines, including interleukin (IL)-1β, IL-1Rα, IL-2, IL-10, fibroblast growth factor (FGF), granulocyte-macrophage colony stimulating factor (GM-CSF), granulocyte-colony stimulating factor (G-CSF), interferon-γ-inducible protein (IP10), monocyte chemoattractant protein (MCP1), macrophage inflammatory protein 1 alpha (MIP1A), platelet-derived growth factor (PDGF), tumor necrosis factor (TNF-α), and vascular endothelial growth factor (VEGF) [2]. These changes in cytokine levels are associated with various changes in cellular components of the immune response [3]. It becomes more evident that there is a close interaction between the virus and an individual’s immune system, resulting in different clinical manifestations of the disease [4]. Moreover, with the aid of modern immunological and biotechnological methods, the importance of a well-functioning and balanced immune system in maintaining overall health has become more apparent in recent decades [5]. In anticipation of the global immunization of the population through vaccines developed for the adaptive immune system, innate immune-based strategies for therapeutic purposes are also being investigated [2]. The innate immune system constitutes the host’s first line of defense during infection and therefore plays a critical role in the early recognition and subsequent activation of a pro-inflammatory response to invading pathogens (for review, see [6]). Nonspecific immunostimulants (NSIs) are natural, synthetic, or recombinant molecules that stimulate the innate immune system by inducing activation or increasing activity of any of its components. In contrast to specific immunostimulants such as vaccines, NSIs act irrespective of antigenic specificity to augment immune response of other antigen or stimulate components of the immune system without antigenic specificity. Despite the tremendous advances in this field of immunology over the years, many areas of uncertainty remain. For example, one question that remains is what is the precise mechanism of action of cell activation, immunomodulation, and tumor reduction for NSIs used in cancer therapy (e.g., mifamurtide, BCG vaccine). In addition to increasing our understanding of these drugs, there is an increasing interest in the development of NSIs that can be used in infectious and inflammatory diseases [6]. Recently, immunomodulators used in traditional Chinese medicine (TCM) for centuries, such as the shiitake and the pearl oyster mushroom, have gained interest for these new developments [7]. Many of the traditionally used substances, however, are substantiated by only limited scientific studies. An exception to this is the fungal beta-glucans which, with more than 20,000 published studies, are the most studied mushroom-derived molecules with potential immunomodulating properties [8][9].
Structure, Chemical Properties, and Natural Sources of Beta-Glucans
Beta-glucans are groups of polysaccharides or dietary fibers composed of D-glucose monomers, linked by (1 → 3), (1 → 4) or (1 → 6) glycosidic bonds. Beta-glucans are naturally found in the cell wall of bacteria, fungi, algae, and cereals such as oat and barley [10]. The different sources of beta-glucans, however, also differ in the linkages between the D-glucose monomers. The beta-glucans present in cereals include a mixture of (1 → 3) and (1 → 4) glycosidic bonds. Beta-glucans in mushrooms mostly contain a linear (1 → 3) backbone with (1 → 6)-linked glucose branches attached. Furthermore, beta-glucans found in yeasts, seaweeds, and bacteria display different structural forms and branching, of which curdlan, extracted from Agrobacterium, is the simplest structure; it is only composed of unbranched (1 → 3) glycosidic bonds [11]. These differences in the shape, structure, and molecular weight of beta-glucans determine their biological activity. For cereal beta-glucans (dietary fibers), mainly physicochemical properties are reported, including reactive oxygen species (ROS) scavenging activity, and their ability to lower serum cholesterol and improve gut microbiome [11]. These properties have been attributed to the mixture of only the (1 → 3) and (1 → 4) glycosidic bonds, making them resistant to absorption and digestion in the small intestine of humans [12][13]. The effects of beta-glucans containing (1 → 6) branching, such as fungal or bacterial glucans, are related to the activation/inactivation of specific receptors such as dectin-1 (mostly insoluble beta-glucans), complement receptor 3 (CR3), or toll-like receptor 2 (TLR2) (mainly water-soluble glucans) [11][14]. Individual fungi contain specific beta-glucans, which differ from each other by the amount of (1 → 6) linked side chains (Figure 1). Moreover, the content and proportions of beta-glucans in fungi is mainly determined by their genetic profile and differs between species and even cultivars. Upon ingestion, fungal glucans affect the mucosal immune system in the gastrointestinal tract. Similar to antigens, the uptake of beta-glucans occurs via microfold cells (M cells) localized within Peyer’s patches in the small intestine. M cells subsequently present the antigen or beta-glucan at their basal surfaces to immune cells, such as macrophages and dendritic cells. Here, beta-glucan particles bind with macrophages with the help of dectin-1, the primary receptor for most insoluble beta-glucans. Subsequently, dectin-1 induces the secretion of pro-inflammatory cytokines via nuclear factor kappa-B (NF-κB) and various interconnected inflammatory and immunoregulatory processes such as chemokinesis and chemotaxis (for review, see [15]). Given these immunomodulatory effects, the use of fungal glucans as pharmaceutical agents, which act as (adjuvant) immunomodulators, has been authorized in several countries, including the United States of America, Canada, Finland, Sweden, China, Japan, and Korea [16]. Lentinan, isolated from shiitake mushroom, is an example of a pharmaceutically formulated polysaccharide approved as an intravenous immunostimulant in the treatment of multiple cancers in China and Japan [17]. In addition, fungal glucans are widely used in the nutritional field as dietary supplements. Unlike pharmaceutical drugs that are supposed to suppress or stimulate the immune system in patients, dietary supplements are primarily intended as a daily oral dose to support the immune system in healthy people. Pleuran, isolated from pearl oyster mushrooms, is an example of a polysaccharide developed as a dietary supplement to support the immune system and overcome the first signs of exhaustion and fatigue in adults and children [18]. In addition to these formulations, the edible mushrooms shiitake (Lentinula edodes) and the pearl oyster mushroom (Pleurotus ostreatus) are some of the main dietary sources of beta-glucans (Figure 1) [19].
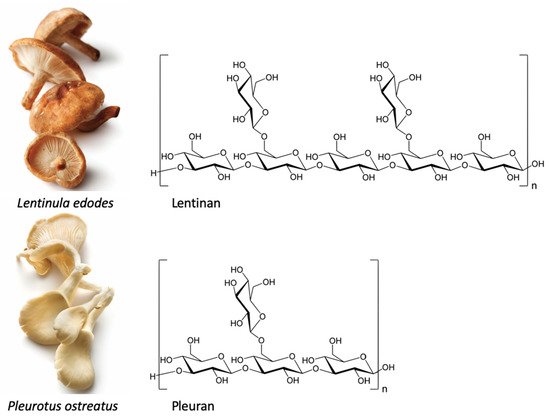
Figure 1. Shiitake (Lentinula edodes) and pearl oyster mushroom (Pleurotus ostreatus) and their specific beta-glucan structures.
2. Beta-Glucans in Immunomodulating Dietary Supplements
2.1. Health Claims in Europe
Supplement manufacturers are interested in using health claims on their product to show the health benefits of consuming these products [20][21][22]. In Europe, the use of voluntary statements related to either the nutritional content (nutrition claims) or health benefits of a food product (health claims) is regulated under the Nutrition and Health Claim Regulation (NHCR). Before such claims can be used on foods, they need to be authorized by the European Commission [23]. Scientific evidence is key in the authorization decision of the European Commission to allow new claims in the EU market [24]. A health claim is defined as any voluntary statement that refers to the relationship between food and health. Four categories of claims are known in the EU: two types of function claims that are based on generally accepted (general function claims, in Art. 13.1) or newly developed scientific evidence (new function claims, Art. 13.5), reduction of disease risk claims (Art. 14.1a) and, finally, claims referring to children’s development and health (Art. 14.1b) [23]. The food business operator needs to submit a scientific dossier along with its request for authoring a newly proposed claim. The European Commission subsequently asks the European Food Safety Authority (EFSA) to evaluate the evidence on the proposed claims [25]. This evaluation involves a critical review of three main criteria: (1) the bioactive substance is sufficiently characterized, (2) the proposed claim is well characterized and should comprise a beneficial physiological effect, and (3) the cause-and-effect relationship between the bioactive substance and the beneficial physiological effect should be established [23][24]. A beneficial effect shown in at least two independently conducted intervention trials increases the chance of receiving a positive opinion of EFSA [20][24]. Since the assessment procedure follows this specific order of evaluation of these criteria, the assessment will be discontinued if the evidence is insufficiently supporting a criterion [26].
2.2. Immune Functioning Health Claims
An effective functioning immune system is crucial for maintaining physiological integrity and, thus, for maintaining health. The immune system provides defense against infections caused by pathogenic microorganisms [27][28]. In recent years, food companies have continued to develop innovative foods in this specific field [5]. Despite its positive aim of fostering innovation, the Nutrition and Health Claims Regulation (EC) No. 1924/2006 (NHCR) may present several compliance challenges which might affect innovation in the EU food sector [29][30]. In order to provide stakeholders with greater clarity on which health effects related to immunology could be studied to support health claims, in 2011, a guidance was published by EFSA’s Panel on Dietetic Products, Nutrition and Allergies (NDA Panel) that provides more detailed guidelines for the evaluation of Articles 13.1, 13.5, and 14 health claims in this area [31]. According to the NDA Panel, maintaining a well-functioning immune function can be considered to be a beneficial physiological effect. However, given the multiple roles of the immune system, the specific aspect of immune function to which the claim relates should be noted. This means that changes in multiple biomarkers can indicate a well-functioning immune system. Markers of immune system functioning that are proposed as suitable outcomes for substantiating claims on immune function effects are listed in Table 1 [31]. In addition to a positive influence on these markers, changes should be accompanied by a favorable physiological or clinical outcome, preferably demonstrated in the same study [31].
Table 1. Changes in biomarkers that are proposed as outcomes for substantiating health claims related to immune function [31].
| Described Changes | Examples |
|---|---|
|
|
|
|
|
|
|
|
|
|
So far, ten proposed health claims have been considered to be substantiated with sufficient scientific evidence according to the NDA Panel, and have subsequently been authorized by the European Commission. The following six vitamins are reported to play a role in maintaining a well-functioning immune system: A, B6, folate (B9), B12, C, and D [32][33][34]. Meanwhile, a similar assessment was made for four essential trace elements: zinc, copper, iron, and selenium, which are considered by EFSA as necessary for the optimal functioning of the immune system [35][36][37][38][39]. Therefore, these ten micronutrients may be labeled with the health claim ‘contributes to the normal functioning of the immune system’. Moreover, foods containing 200 mg or more vitamin C may be labeled with an additional health claim: ‘Vitamin C contributes to maintain the normal function of the immune system during and after intense physical exercise’ [40]. The evidence for vitamin C’s additional health claim comes from three systematic reviews examining the role of vitamin C supplementation in the prevention, severity, and treatment of the common cold [41][42][43]. The results of the reviews showed that there is some evidence suggesting that individuals who are exposed to short periods of vigorous exercise and/or cold environments benefit from regular vitamin C intake above 200 mg/day based on the duration and severity of the common cold [40]. Next to this second substantiated claim for the effect of vitamin C, the NDA Panel considered that the role of vitamin D in the functioning of the immune system applies to all ages, including children. Therefore, vitamin D containing products may also use an additional Article 14.1(b) claim: ‘Vitamin D contributes to the normal function of the immune system in children’ [40]. This does not mean that other nutrients and foods are not also relevant; so far, however, insufficient scientific evidence has been gathered to demonstrate this [44]. In addition to the 2011 guidelines for claims related to support of the immune system, the guidelines were extended 5 years later to include claims related to stimulation of the immune system and defense against pathogenic microorganisms [31][40]. The scientific evidence to substantiate a claim related to the body’s defense against pathogens can be obtained by studying effects on clinical outcomes related to infections (e.g., incidence, severity, and/or duration of symptoms). As put forward in EFSA’s guideline, the infectious nature of the disease should be established, e.g., by clinical differential diagnosis in itself or combining this with microbiological data and/or the use of validated questionnaires, depending on the study context and type of infection [40]. Donabedian et al. (2006) concluded that the ten micronutrients which have received a positive opinion for supporting the immune system do not play a supporting role in the treatment of certain ongoing infections [45]. So far, all applications for putative health claims related to stimulation of the immune system and defense against pathogenic microorganisms have been rejected by the NDA Panel. Most of the rejected claims focused on the effects of probiotic bacteria such as Lactobacillus and Bifidobacterium strains. Claims on probiotics, however, have often been rejected because of a lack of specifying the active ingredient itself, the first step in the scientific assessment of the claim [46][47][48][49]. Other unapproved applications for products claiming immune stimulation and defense against pathogenic microorganisms mainly involved amino acids, antioxidants, oligosaccharides, and fungal compounds, including beta-glucans. The unapproved immune related applications for products containing fungal beta-glucans are listed in Table 2.
Table 2. Claims regarding fungal beta-glucan applications related to the immune system.
| Claim Type | Nutrient, Substance, Food or Food Category | Claim | Non-Authorization/Discontinuation Based on Criteria | Health Relationship | EFSA Opinion/Journal Reference | Entry ID |
|---|---|---|---|---|---|---|
| Art. 13(1) | Beta-glucan (WGP) | For immunity. Strengthens immunity. | (2) Rejected on the basis of an unclear health relationship or no clear association with health. | Immune system | 2011;9(6):2228 | 1792 |
| Art. 13(1) | Beta-glucan + olive leaf extract | Supports the body’s own defense mechanism/immunity. Maintains natural defense mechanism/immunity. Helps strengthen natural immunity. | (2) Rejected on the basis of an unclear health relationship or no clear association with health. | Immune function/immune system | 2011;9(4):2061 | 1793 |
| Art. 13(1) | Beta-glucan of Saccharomyces cerevisiae | Beta-glucan from yeast as immunomodulators. Beta-glucan from yeast support of natural defenses. | (2) Rejected on the basis of an unclear health relationship or no clear association with health. | Immune system | 2011;9(6):2228 | 847 |
| Art. 13(1) | Beta-glucan of Saccharomyces cerevisiae | Beta-glucan from yeast as immunomodulators. Beta-glucan from yeast support of natural defenses. | (2) Rejected on the basis of an unclear health relationship or no clear association with health. | Increasing nonspecific serum IgA secretion | 2011;9(6):2228 | 1944 |
| Art. 13(1) | WGP beta-glucan; (WGP® (1,3)-b-d-glucan); (from Saccharomyces cerevisiae) | WGP beta-glucan contributes to the normal function to the immune system. WGP beta-glucan naturally contributes to adequate immune responses. The daily dietary supplementation with WGP beta-glucan promotes the normal function of the immune system. WGP beta-glucan enhances the production and activity of macrophages and neutrophils. Thus, it plays an important role in the adequate function of the immune system. WGP beta-glucan contributes to maintain the normal function of the upper respiratory tract. | (3) Rejected on the basis of an unproven cause and effect relationship: no evidence (yet) for a relationship between intake and effect. | Maintenance of the upper respiratory tract defense against pathogens by maintaining immune defenses. | 2011;9(6):2248 | 1910 |
| Art. 13(5) | Yestimun® | Daily administration of Yestimun® helps to maintain the body’s defense against pathogens. | (3) Rejected on the basis of an unproven cause and effect relationship: no evidence (yet) for a relationship between intake and effect. | N/A | Q-2012-00761Commission Regulation (EU) No 1154/2014 of 29/10/2014 | N/A |
| Art. 13(5) | Yestimun®, consisting of (1,3)-(1,6)-β-d-glucans of brewer’s yeast cell wall (100% Saccharomyces cerevisiae) | Daily administration of Yestimun® strengthens the body’s defense during the cold season. | (3) Rejected on the basis of an unproven cause and effect relationship: no evidence (yet) for a relationship between intake and effect. | N/A | Q-2008-667Commission Regulation (EU) 432/2011 of 04/05/2011 | N/A |
| Art. 13(1) | Lentinula edodes (common name: Shiitake) | Contributes to natural immunological defenses. | (3) Rejected on the basis of an unproven cause and effect relationship: no evidence (yet) for a relationship between intake and effect. | Immune function/immune system | 2011;9(4):2061 | 3774 |
| Art. 13(1) | Lentinula edodes (common name: shiitake) | Contributes to natural immunological defenses. | (2) Rejected on the basis of an unclear health relationship or no clear association with health. | Stimulation of immunological responses | 2011;9(4):2061 | 2075 |
| Art. 13(1) | Pleurotus ostreatus (oyster mushroom) | Contributes to natural immunological defenses. | (2) Rejected on the basis of an unclear health relationship or no clear association with health. | Immune function/immune system | 2011;9(4):2061 | 3521 |
| Art. 13(1) | Brewer’s yeast | Strengthens immunity | (2) Rejected on the basis of an unclear health relationship or no clear association with health. | Immune function/immune system | 2010;8(10):1799 | 1384 |
| Art. 13(5) | Immune balance drink, containing vitamin C, green tea, grape skin, grape seed, and shiitake mushroom extract | The immune balance drink activates body’s defense. | (3) Rejected on the basis of an unproven cause and effect relationship: no evidence (yet) for a relationship between intake and effect. | N/A | Q-2009-517Commission Regulation (EU) No 958/2010 of 22/10/2010 | N/A |
| Art. 13(1) | Active hexose correlated compound (AHCC) | Activates immune system, exert potential effects on the immune system—stimulating immunity. | (2) Rejected on the basis of an unclear health relationship or no clear association with health. | Stimulation of immunological responses | 2011;9(4):2061 | 3139 |
| Art. 13(1) | Herbal yeast plasmolysate (Saccharomyces cerevisiae) | Strengthens the body’s defense system. Increases immunity. | (2) Rejected on the basis of an unclear health relationship or no clear association with health. | Immune function/immune system | 2011;9(4):2061 | 1817 |
These negative evaluations of putative health claims have not stopped research into the effects of specific beta-glucan-containing supplements, such as Yestimun® and pleuran. Since these two supplements are interesting cases of a growing body of evidence, the following sections discuss, in detail, the current state of the evidence in light of the requirements for substantiating health claims.
2.3. Yestimun®
Yestimun® is an insoluble, highly purified, well-characterized β-glucan from spent brewer’s yeast (Saccharomyces cerevisiae) [50]. Brewer’s yeast is grown exclusively on malt and clean spring water and is a natural byproduct of the fermentation process used for beer production. During various possessing steps, these β-glucans are further purified and soluble compounds are removed. This results in a relative β-1,6 glucan side chain binding percentage of 22% with a minimum purity of 85% [50]. Animal studies showed that orally ingested Yestimun® in rats increased the phagocytic activity of granulocytes and monocytes, the percentage of phagocytic cells and nonspecific humoral immune parameters lysozyme, ceruloplasmin and serum γ-globulin [51][52][53]. Phagocytes derived from the β-glucan fed group showed higher respiratory burst and phagocytic activity. When stimulated by LPS, the proliferation rate of lymphocytes was higher in the β-glucan group [53]. Moreover, a study in dogs with inflammatory bowel disease (IBD) showed that animals treated with β-glucan had a decreased level of IL-6 and an increased level of anti-inflammatory IL-10 as compared to untreated control animals [54]. In addition to these animal studies, clinical trials have examined the ability of Yestimun® to increase the body’s defense against invading pathogens. Auinger et al. (2013) performed a placebo-controlled, double-blind, randomized clinical trial in 162 healthy participants with recurring infections and concluded that supplementation with Yestimun® (900 mg/day) for 16 weeks reduced the number of symptomatic common cold infections by 25% compared to placebo (p = 0.041) [55]. Another trial with 100 participants confirmed these results by reporting significantly more subjects without a cold episode and its typical symptoms in the β-glucan group compared to the placebo group [56]. Although these clinical studies with Yestimun® showed positive effects on the immune system, the inclusion of these studies into the scientific dossier to support an immune claim was not considered to provide sufficient evidence for substantiating the claim [50][57][58]. The NDA Panel concluded that a cause-and-effect relationship had not been established, mainly because of study design issues: a non-validated questionnaire on common cold was used and limitations of statistical analyses were identified [50].
Recently, another intervention study was conducted that used validated questionnaires on upper respiratory tract infections (URTI) episodes as a primary endpoint [59][60]. Dharsono et al. (2019) concluded that supplementation with Yestimun® reduced the severity of physical URTI symptoms during the first week of an episode, even though the incidence and overall severity of common colds was not shown to be altered in comparison to placebo. Furthermore, accompanying health benefits in terms of lowering blood pressure and improved mood were reported [59]. However, no research has been done on the viral load and the type of viruses/bacteria causing the symptoms. This is an important limitation of the study in substantiating a health claim related to pathogen defense, as the infectious nature of the disease must be established [40]. The nature of the virus might have an impact on incidence, severity, and duration of URTI episodes [59]. In practice, however, it is uncommon to perform routine laboratory tests for the diagnosis of the common cold as it can be caused by many different agents (adenovirus, coronavirus, influenza virus, rhinovirus, etc.) [60][61]. The viral pathogens associated with the common cold may be detected by culture, antigen detection, PCR, or serologic methods. These studies are generally not indicated in patients with the common cold, because a specific etiological diagnosis is only meaningful when considering treatment with an antiviral agent [60][61].
2.4. Pleuran
Pleuran is an insoluble polysaccharide (β-(1,3/1,6)-d-glucan), isolated from the fruiting bodies of the edible mushroom Pleurotus ostreatus. Pleuran was developed as a dietary supplement to support the immune system and overcome the first signs of exhaustion and fatigue in adults and children [18]. The effect of Imunoglukan P4H®, a formulation of pleuran and vitamin C, on respiratory tract infections (RTI) and recurrent respiratory tract infections (RRTI) in children has been investigated in several clinical studies. In an open-label study, a decrease in the frequency of RRTI was observed in 153 children (71.2%). The mean annual incidence of respiratory tract infections in children with a positive response to Imunoglukan P4H® was significantly lower compared to that in unresponsive patients (3.6 vs. 8.9, p < 0.001) [62]. Another study examined the effect of Imunoglukan P4H® supplementation on the frequency of RTI in a group of 151 children with RRTI. A comparison between the number and type of RTI during the previous October period was compared to those observed during the intervention period and 6-month follow-up. Supplementation with Imunoglukan P4H® reduced the RRTI rate from 8.88 ± 3.35 episodes in the previous year to 4.27 ± 2.21 episodes in the study year (p < 0.001) [63]. Similar efficacy was observed in another prospective open-label study in 194 children in Poland, where a significant decrease in total RTI was reported during the intervention and follow-up periods (4.18 ± 2.132 vs. 8.71 ± 1.89, p < 0.001) [64].
However, the design of these studies also shows some weaknesses when considering their usage for substantiating an immune function health claim. Firstly, the prospective open-label design is a weakness. Secondly, the lack of information on viral load and the type of virus/bacteria causing symptoms are limitations. The major weakness in substantiating immune claims for beta-glucans, however, is the fact that Imunoglukan P4H® also contains vitamin C (15% of the recommended dietary allowance). The recommended daily dose contains sufficient vitamin C to make use of the health claim ‘contributes to the normal function of the immune system’. However, when looking specifically at respiratory infections, randomized, placebo-controlled studies have not clearly shown that vitamin C on its own has the potential to prevent them [45][65]. Interestingly, a double-blind, placebo-controlled, randomized trial in children with RRTI examined the effect of the Pleurotus extract in itself. Next to self-reporting RRTI symptoms, a validated health questionnaire was used to examine general health status and RRTI symptoms. Vitamin C was used as an ‘active placebo’ to investigate whether the immunomodulatory action, which is clinically manifested in the reduction of RTI, can primarily be attributed to the highly purified Pleurotus ostreatus extract [66]. In the Pleurotus extract group, 36% of the children did not suffer from any respiratory infections throughout the treatment, compared to 21% in the vitamin C group. Pleurotus extract also significantly decreased the frequency of flu and flu-like symptoms, as well as the frequency of lower respiratory tract infections compared to the vitamin C group (0.20 ± 0.55 per 12 months vs. 0.42 ± 0.78 per 12 months, p < 0.05). The results of this RCT, that uses validated questionnaires, are promising but need to be confirmed in more studies as multiple intervention studies conducted by independent institutions increase the likelihood of receiving a positive EFSA opinion [20][24]. In addition, with the target population being children with (recurring) respiratory infections instead of the general population, this evidence mainly substantiates an Article 14.1(b) claim. Should such studies yield positive results again, pleuran could apply for an additional health claim, such as the authorized claim for vitamin D discussed in Section 2.2.
Few studies have been conducted with Imunoglukan P4H® in the general population, but several clinical studies have been conducted in other populations susceptible to RTI, e.g., elite athletes [67][68]. Epidemiological evidence suggests that heavy acute or chronic exercise is related to an increased incidence of upper respiratory tract infections in athletes [69]. In a placebo-controlled study, the daily consumption of 100 mg Imunoglukan P4H® for two months prevented post-exercise immune suppression in elite athletes, and in another study, supplementation reduced RTIs (even though this was measured by a non-validated questionnaire) [67][68]. These studies demonstrate the potential for the use of Imunoglukan P4H® as an immunostimulant in elite athletes. Still, follow-up studies will have to be conducted following the guidance documents to qualify as supportive evidence for a possible health claim. When such studies would again yield positive results, Imunoglukan P4H® could apply for an additional health claim, such as the authorized claim for vitamin C: ‘Vitamin C contributes to the maintenance of the normal function of the immune system during and after intense physical exercise’.
References
- Javelle, E.; Raoult, D. COVID-19 pandemic more than a century after the Spanish flu. Lancet Infect. Dis. 2021, 21, e78.
- Rao, K.S.; Suryaprakash, V.; Senthilkumar, R.; Preethy, S.; Katoh, S.; Ikewaki, N.; Abraham, S.J.K. Role of Immune Dysregu-lation in Increased Mortality Among a Specific Subset of COVID-19 Patients and Immune-Enhancement Strategies for Com-batting Through Nutritional Supplements. Front. Immunol. 2020, 11, 154.
- Fara, A.; Mitrev, Z.; Rosalia, R.A.; Assas, B.M. Cytokine storm and COVID-19: A chronicle of pro-inflammatory cytokines. Open Biol. 2020, 10, 200160.
- Dong, X.; Cao, Y.; Lu, X.; Zhang, J.; Du, H.; Yan, Y.; Akdis, C.A.; Gao, Y. Eleven faces of coronavirus disease 2019. Allergy 2020, 75, 1699–1709.
- Wichers, H. Immunomodulation by food: Promising concept for mitigating allergic disease? Anal. Bioanal. Chem. 2009, 395, 37–45.
- Mogensen, T.H. Pathogen Recognition and Inflammatory Signaling in Innate Immune Defenses. Clin. Microbiol. Rev. 2009, 22, 240–273.
- Ma, H.-D.; Deng, Y.-R.; Tian, Z.; Lian, Z.-X. Traditional Chinese Medicine and Immune Regulation. Clin. Rev. Allergy Immunol. 2013, 44, 229–241.
- Vetvicka, V.; Vannucci, L.; Sima, P.; Richter, J. Beta Glucan: Supplement or Drug? From Laboratory to Clinical Trials. Molecules 2019, 24, 1251.
- Wasser, S. Medicinal mushrooms as a source of antitumor and immunomodulating polysaccharides. Appl. Microbiol. Biotechnol. 2002, 60, 258–274.
- Stuart, I.; Loi, L.; Fincher, G. Immunological comparison of (1→3,1→4)-β-glucan endohydrolases in germinating cereals. J. Cereal Sci. 1987, 6, 45–52.
- Nakashima, A.; Yamada, K.; Iwata, O.; Sugimoto, R.; Atsuji, K.; Ogawa, T.; Ishibashi-Ohgo, N.; Suzuki, K. β-Glucan in Foods and Its Physiological Functions. J. Nutr. Sci. Vitaminol. 2018, 64, 8–17.
- Ripsin, C.M.; Keenan, J.M.; Jacobs, D.R.; Elmer, P.J.; Welch, R.R.; Van Horn, L.; Liu, K.; Turnbull, W.H.; Thye, F.W.; Kestin, M.; et al. Oat Products and Lipid Lowering. JAMA 1992, 267, 3317–3325.
- Kim, S.Y.; Song, H.J.; Lee, Y.Y.; Cho, K.H.; Roh, Y.K. Biomedical issues of dietary fiber beta-glucan. J. Korean Med. Sci. 2006, 21, 781.
- Novak, M.; Vetvicka, V. β-Glucans, History, and the Present: Immunomodulatory Aspects and Mechanisms of Action. J. Immunotoxicol. 2008, 5, 47–57.
- Goodridge, H.S.; Wolf, A.J.; Underhill, D.M. β-glucan recognition by the innate immune system. Immunol. Rev. 2009, 230, 38–50.
- Bashir, K.M.I.; Choi, J.-S. Clinical and Physiological Perspectives of β-Glucans: The Past, Present, and Future. Int. J. Mol. Sci. 2017, 18, 1906.
- Lv, G.; Fan, L.; Zhang, Z.; Pan, H. Development of research on lentinan. Acta Agric. Zhejiangensis 2009, 21, 183–188.
- Majtan, J. Pleuran (β-Glucan fromPleurotus ostreatus): An Effective Nutritional Supplement against Upper Respiratory Tract Infections? Pediatric Fitness 2012, 59, 57–61.
- Rop, O.; Mlcek, J.; Jurikova, T. Beta-glucans in higher fungi and their health effects. Nutr. Rev. 2009, 67, 624–631.
- Lenssen, K.G.; Bast, A.; de Boer, A. Clarifying the health claim assessment procedure of EFSA will benefit functional food innovation. J. Funct. Foods 2018, 47, 386–396.
- Avermaete, T.; Viaene, J.; Morgan, E.J.; Crawford, N. Determinants of innovation in small food firms. Eur. J. Innov. Manag. 2003, 6, 8–17.
- Costa, A.; Jongen, W. New insights into consumer-led food product development. Trends Food Sci. Technol. 2006, 17, 457–465.
- The European Commission Commission Regulation (EU) No 432/2012. Off. J. Eur. Union. 2012, 13, 1–40.
- De Boer, A.; Vos, E.; Bast, A. Implementation of the nutrition and health claim regulation—The case of antioxidants. Regul. Toxicol. Pharmacol. 2014, 68, 475–487.
- Smith, A.; Terry, S.; Detken, D. 10 years of the European Food Safety Authority (EFSA) and the EU Food Safety System. Eur. Food Feed Law Rev. EFFL 2012, 7, 111–116.
- Verhagen, H.; van Loveren, H. Status of nutrition and health claims in Europe by mid 2015. Trends Food Sci. Technol. 2016, 56, 39–45.
- Mainous, M.R.; Deitch, E.A. Nutrition and Infection. Surg. Clin. N. Am. 1994, 74, 659–676.
- Van Buren, C.T. Nutrition and Immunology. J. Parenter. Enter. Nutr. 1991, 15.
- Khedkar, S.; Ciliberti, S.; Bröring, S. The EU health claims regulation: Implications for innovation in the EU food sector. Br. Food J. 2016, 118, 2647–2665.
- Khedkar, S.; Bröring, S.; Ciliberti, S. Exploring the Nutrition and Health Claims Regulation (EC) No. 1924/2006: What is the impact on innovation in the EU food sector? Int. J. Food Sci. Nutr. 2016, 68, 10–17.
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Guidance on the scientific requirements for health claims related to gut and immune function. EFSA J. 2011, 9, 2474.
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific Opinion on the substantiation of health claims related to vitamin D and normal function of the immune system and inflammatory response (ID 154, 159), maintenance of normal muscle function (ID 155) and maintenance of normal cardio-vascular function (ID 159) pursuant to Article 13(1) of Regulation (EC) No 1924/2006. EFSA J. 2010, 8, 1468.
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific Opinion on the substantiation of a health claim related to vitamin D and contribution to the normal function of the immune system pursuant to Article 14 of Regulation (EC) No 1924/2006. EFSA J. 2015, 13, 4096.
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific Opinion on the substantiation of health claims related to vitamin B6 and protein and glycogen metabolism (ID 65, 70, 71), function of the nervous system (ID 66), red blood cell formation (ID 67, 72, 186), function of the immune system (ID 68). EFSA J. 2009, 7, 1225.
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific Opinion on the substantiation of a health claim related to zinc and normal function of the immune system pursuant to Article 14 of Regulation (EC) No 1924/2006. EFSA J. 2014, 12, 3653.
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific Opinion on the substantiation of health claims related to copper and protection of DNA, proteins and lipids from oxidative damage (ID 263, 1726), function of the immune system (ID 264), maintenance of connective tissues (ID 265, 271, 1722), ene. EFSA J. 2009, 7, 1211.
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific Opinion on the substantiation of health claims related to copper and reduction of tiredness and fatigue (ID 272), maintenance of the normal function of the nervous system (ID 1723), maintenance of the normal function of the immune system (ID 17). EFSA J. 2011, 9, 2079.
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific Opinion on the substantiation of health claims related to selenium and protection of DNA, proteins and lipids from oxidative damage (ID 277, 283, 286, 1289, 1290, 1291, 1293, 1751), function of the immune system (ID 278), thyroid function (ID 2. EFSA J. 2009, 7, 1220.
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific Opinion on the substantiation of health claims related to iron and formation of red blood cells and haemoglobin (ID 249, ID 1589), oxygen transport (ID 250, ID 254, ID 256), energy-yielding metabolism (ID 251, ID 1589), function of the immune s. EFSA J. 2009, 7, 1215.
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Guidance on the scientific requirements for health claims related to the immune system, the gastrointestinal tract and defence against pathogenic microorganisms. EFSA J. 2016, 14, 4369.
- Douglas, R.; Hemilä, H.; Chalker, E.; Treacy, B. Cochrane review: Vitamin C for preventing and treating the common cold. Evid. Child Health A Cochrane Rev. J. 2008, 3, 672–720.
- Douglas, R.M.; Hemilä, H. Vitamin C for preventing and treating the common cold. PLoS Med. 2005, 2, e168.
- Hemilä, H.; Chalker, E. Vitamin C for preventing and treating the common cold. Cochrane Database Syst. Rev. 2013.
- Galmés, S.; Serra, F.; Palou, A. Current State of Evidence: Influence of Nutritional and Nutrigenetic Factors on Immunity in the COVID-19 Pandemic Framework. Nutrients 2020, 12, 2738.
- Donabedian, H. Nutritional therapy and infectious diseases: A two-edged sword. Nutr. J. 2006, 5, 21.
- Glanville, J.; King, S.; Guarner, F.; Hill, C.; Sanders, M.E. A review of the systematic review process and its applicability for use in evaluating evidence for health claims on probiotic foods in the European Union. Nutr. J. 2015, 14, 16.
- Rijkers, G.T.; De Vos, W.M.; Brummer, R.-J.; Morelli, L.; Corthier, G.; Marteau, P. Health benefits and health claims of probiotics: Bridging science and marketing. Br. J. Nutr. 2011, 106, 1291–1296.
- Miquel, S.; Beaumont, M.; Martín, R.; Langella, P.; Braesco, V.; Thomas, M. A proposed framework for an appropriate evalu-ation scheme for microorganisms as novel foods with a health claim in Europe. Microb. Cell Fact. 2015, 14, 48.
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific Opinion on the substantiation of health claims related to non characterised bacteria and yeasts pursuant to Article 13(1) of Regulation (EC) No 1924/2006. EFSA J. 2010, 8, 1470.
- Stier, H.; Ebbeskotte, V.; Gruenwald, J. Immune-modulatory effects of dietary Yeast Beta-1,3/1,6-D-glucan. Nutr. J. 2014, 13, 38.
- Wójcik, R. Effect of Biolex Beta-HP on phagocytic activity and oxidative metabolism of peripheral blood granulocytes and monocytes in rats intoxicated by cyclophosphamide. Pol. J. Vet. Sci. 2010, 13, 181–188.
- Wójcik, R.; Janowska, E.; Małaczewska, J.; Siwicki, A.K. Effect of β-1,3/1,6-D-glucan on the phagocytic activity and oxidative metabolism of peripheral blood granulocytes and monocytes in rats. Bull. Vet. Inst. Pulawy 2009, 53, 241–246.
- Małaczewska, J.; Wójcik, R.; Jung, L.; Siwicki, A.K. Effect of biolex β-HP on selected parameters of specific and non-specific humoral and cellular immunity in rats. Bull. Vet. Inst. Pulawy 2010, 54, 75–80.
- Rychlik, A.; Nieradka, R.; Kander, M.; Nowicki, M.; Wdowiak, M.; Kołodziejska-Sawerska, A. The effectiveness of natural and synthetic immunomodulators in the treatment of inflammatory bowel disease in dogs. Acta Vet. Hung. 2013, 61, 297–308.
- Auinger, A.; Riede, L.; Bothe, G.; Busch, R.; Gruenwald, J. Yeast (1,3)-(1,6)-beta-glucan helps to maintain the body’s defence against pathogens: A double-blind, randomized, placebo-controlled, multicentric study in healthy subjects. Eur. J. Nutr. 2013, 52, 1913–1918.
- Graubaum, H.-J.; Busch, R.; Stier, H.; Gruenwald, J. A Double-Blind, Randomized, Placebo-Controlled Nutritional Study Using an Insoluble Yeast Beta-Glucan to Improve the Immune Defense System. Food Nutr. Sci. 2012, 3, 738–746.
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific Opinion on the substantiation of a health claim related to Yestimun® and defence against pathogens in the upper respiratory tract pursuant to Article 13(5) of Regulation (EC) No 1924/2006. EFSA J. 2013, 11, 3159.
- Response to comments on the Scientific Opinion on the substantiation of a health claim related to Yestimun® and defence against pathogens in the upper respiratory tract pursuant to Article 13(5) of Regulation (EC) No 1924/2006. EFSA Support. Publ. 2013, 10, 500E.
- Dharsono, T.; Rudnicka, K.; Wilhelm, M.; Schoen, C. Effects of Yeast (1,3)-(1,6)-Beta-Glucan on Severity of Upper Respiratory Tract Infections: A Double-Blind, Randomized, Placebo-Controlled Study in Healthy Subjects. J. Am. Coll. Nutr. 2019, 38, 40–50.
- Barrett, B.; Locken, K.; Maberry, R.; Schwamman, J.; Brown, R.; Bobula, J.; Stauffacher, E.A. The Wisconsin Upper Respiratory Symptom Survey (WURSS): A new research instrument for assessing the common cold. J. Fam. Pr. 2002, 51, 265.
- Turner, R.B. The Common Cold. Mandell Douglas Bennett’s Princ. Pract. Infect. Dis. 2015, 748–752.e2.
- Jeseňák, M.; Sanisló, E.; Kuniaková, R.; Rennerová, Z.; Buchanec, J.; Bánovčin, P. Imunoglukan P4H® in the prevention of recurrent respiratory infections in childhood. Cesk Pediatr. 2010, 73, 639–647.
- Sapena Grau, J.; Picó Sirvent, L.; Morera Inglés, M.; Rivero Urgell, M. Beta-glucans from Pleurotus ostreatus for prevention of recurrent respiratory tract infections. Acta Pediátrica Española 2015, 73, 186–193.
- Pasnik, J.; Ślemp, A.; Cywinska-Bernas, A.; Zeman, K.; Jesenak, M. Preventive effect of pleuran (β-glucan from Pleurotus ostreatus) in children with recurrent respiratory tract infections—Open-label prospective study. Curr. Pediatr. Res. 2017, 21, 99–104.
- Moreira, A.; Kekkonen, R.A.; Delgado, L.; Fonseca, J.; Korpela, R.; Haahtela, T. Nutritional modulation of exercise-induced immunodepression in athletes: A systematic review and meta-analysis. Eur. J. Clin. Nutr. 2007, 61, 443–460.
- Jesenak, M.; Majtan, J.; Rennerova, Z.; Kyselovic, J.; Banovcin, P.; Hrubisko, M. Immunomodulatory effect of pleuran (β-glucan from Pleurotus ostreatus) in children with recurrent respiratory tract infections. Int. Immunopharmacol. 2013, 15, 395–399.
- Bergendiova, K.; Tibenska, E.; Majtan, J. Pleuran (β-glucan from Pleurotus ostreatus) supplementation, cellular immune response and respiratory tract infections in athletes. Eur. J. Appl. Physiol. 2011, 111, 2033–2040.
- Bobovčák, M.; Kuniaková, R.; Gabriž, J.; Majtán, J. Effect of Pleuran (β-glucan from Pleurotus ostreatus) supplementation on cellular immune response after intensive exercise in elite athletes. Appl. Physiol. Nutr. Metab. 2010, 35, 755–762.
- Gałązka-Franta, A.; Jura-Szołtys, E.; Smółka, W.; Gawlik, R. Upper Respiratory Tract Diseases in Athletes in Different Sports Disciplines. J. Hum. Kinet. 2016, 53, 99–106.




