
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Silvio Ionta | + 2149 word(s) | 2149 | 2021-06-01 08:53:38 | | | |
| 2 | Vicky Zhou | Meta information modification | 2149 | 2021-06-01 09:49:44 | | |
Video Upload Options
The so-called cortical silent period (CSP) refers to the temporary interruption of electromyographic signal from a muscle following a motor-evoked potential (MEP) triggered by transcranial magnetic stimulation (TMS) over the primary motor cortex (M1). The neurophysiological origins of the CSP are debated. Previous evidence suggests that both spinal and cortical mechanisms may account for the duration of the CSP. However, contextual factors such as cortical fatigue, experimental procedures, attentional load, as well as neuropathology can also influence the CSP duration. The present paper summarizes the most relevant evidence on the mechanisms underlying the duration of the CSP, with a particular focus on the central role of the basal ganglia in the “direct” (excitatory), “indirect” (inhibitory), and “hyperdirect” cortico-subcortical pathways to manage cortical motor inhibition. We propose new methods of interpretation of the CSP related, at least partially, to the inhibitory hyperdirect and indirect pathways in the basal ganglia. This view may help to explain the respective shortening and lengthening of the CSP in various neurological disorders.
1. Introduction
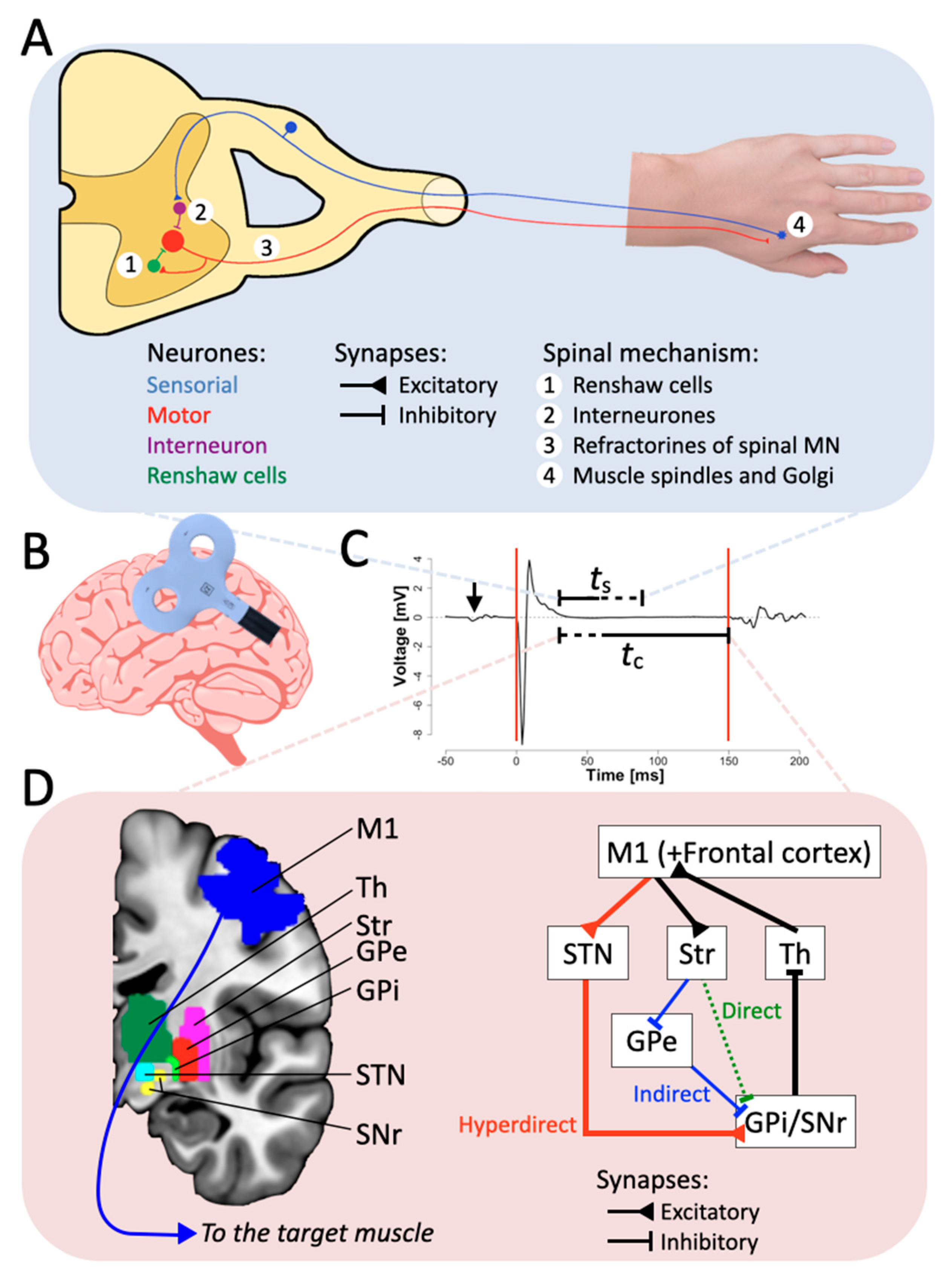 Figure 1. The inhibitory hyperdirect pathway contributes to the CSP duration. (A) Schematic representation of the spinal components of the CSP. Excitation of M1 through single TMS pulse reaches spinal interneuronal circuits, which in turn excite hand muscles. The four spinal mechanisms presented in the scheme contribute only to the first 50 ms of the CSP. (B) Schematic representation of the TMS coil stimulation in M1. (C) Example of an MEP. The red lines represent the usual limit defined for the CSP. “ts” and “tc” stand for the duration elicited by the spinal and the cortical part, respectively, and the arrow represents the TMS pulse artifact. (D) Simplified schematic diagram of the CBGTC loop, at least partially accounting for the later part of the CSP. Abbreviations: Str: striatum; STN: subthalamic nucleus; GPe: external segment of the globus pallidus; GPi: internal segment of the globus pallidus; SNr: substantia nigra pars reticulata; Th: thalamus.
Figure 1. The inhibitory hyperdirect pathway contributes to the CSP duration. (A) Schematic representation of the spinal components of the CSP. Excitation of M1 through single TMS pulse reaches spinal interneuronal circuits, which in turn excite hand muscles. The four spinal mechanisms presented in the scheme contribute only to the first 50 ms of the CSP. (B) Schematic representation of the TMS coil stimulation in M1. (C) Example of an MEP. The red lines represent the usual limit defined for the CSP. “ts” and “tc” stand for the duration elicited by the spinal and the cortical part, respectively, and the arrow represents the TMS pulse artifact. (D) Simplified schematic diagram of the CBGTC loop, at least partially accounting for the later part of the CSP. Abbreviations: Str: striatum; STN: subthalamic nucleus; GPe: external segment of the globus pallidus; GPi: internal segment of the globus pallidus; SNr: substantia nigra pars reticulata; Th: thalamus.2. Neural Mechanisms of CSP
2.1. Basal Ganglia Involvement in Motor Control and CSP
2.2. Hyperdirect Pathway and Motor Neurological Disorders Influencing CSP
3. Conclusions
References
- Hupfeld, K.E.; Swanson, C.W.; Fling, B.W.; Seidler, R.D. TMS-Induced Silent Periods: A Review of Methods and Call for Consistency. J. Neurosci. Methods 2020, 346, 108950.
- Cacchio, A.; Cimini, N.; Alosi, P.; Santilli, V.; Marrelli, A. Reliability of Transcranial Magnetic Stimulation-Related Measurements of Tibialis Anterior Muscle in Healthy Subjects. Clin. Neurophysiol. 2009, 120, 414–419.
- Adrian, E.D.; Moruzzi, G. Impulses in the Pyramidal Tract. J. Physiol. 1939, 97, 153–199.
- Krnjević, K.; Randić, M.; Straughan, D.W. Cortical Inhibition. Nature 1964, 201, 1294–1296.
- Krnjević, K.; Randić, M.; Straughan, D.W. An Inhibitory Process in the Cerebral Cortex. J. Physiol. 1966, 184, 16–48.
- Cantello, R.; Gianelli, M.; Civardi, C.; Mutani, R. Magnetic Brain Stimulation: The Silent Period after the Motor Evoked Potential. Neurology 1992, 42, 1951.
- Marsden, C.D.; Merton, P.A.; Morton, H.B. Direct Electrical Stimulation of Corticospinal Pathways through the Intact Scalp in Human Subjects. Adv. Neurol. 1983, 39, 387–391.
- De Benedictis, A.; Sarubbo, S.; Duffau, H. Subcortical Surgical Anatomy of the Lateral Frontal Region: Human White Matter Dissection and Correlations with Functional Insights Provided by Intraoperative Direct Brain Stimulation. J. Neurosurg. 2012, 117, 1053–1069.
- Montemurro, N.; Herbet, G.; Duffau, H. Right Cortical and Axonal Structures Eliciting Ocular Deviation during Electrical Stimulation Mapping in Awake Patients. Brain Topogr. 2016, 29, 561–571.
- Calancie, B.; Nordin, M.; Wallin, U.; Hagbarth, K.E. Motor-Unit Responses in Human Wrist Flexor and Extensor Muscles to Transcranial Cortical Stimuli. J. Neurophysiol. 1987, 58, 1168–1185.
- DeLong, M.R.; Wichmann, T. Circuits and Circuit Disorders of the Basal Ganglia. Arch. Neurol. 2007, 64, 20.
- Farzan, F.; Barr, M.S.; Hoppenbrouwers, S.S.; Fitzgerald, P.B.; Chen, R.; Pascual-Leone, A.; Daskalakis, Z.J. The EEG Correlates of the TMS-Induced EMG Silent Period in Humans. NeuroImage 2013, 83, 120–134.
- Alexander, G.E.; Crutcher, M.D. Functional Architecture of Basal Ganglia Circuits: Neural Substrates of Parallel Processing. Trends Neurosci. 1990, 13, 266–271.
- DeLong, M.R.; Georgopoulos, A.P. Motor Functions of the Basal Ganglia. Compr. Physiol. 2011, 1017–1061.
- Smith, Y.; Bevan, M.; Shink, E.; Bolam, J.P. Microcircuitry of the Direct and Indirect Pathways of the Basal Ganglia. Neuroscience 1998, 86, 353–387.
- Parent, A.; Hazrati, L.-N. Functional Anatomy of the Basal Ganglia. I. The Cortico-Basal Ganglia-Thalamo-Cortical Loop. Brain Res. Rev. 1995, 20, 91–127.
- Waldvogel, H.J.; Billinton, A.; White, J.H.; Emson, P.C.; Faull, R.L.M. Comparative Cellular Distribution of GABAA and GABAB Receptors in the Human Basal Ganglia: Immunohistochemical Colocalization of the ?1 Subunit of the GABAA Receptor, and the GABABR1 and GABABR2 Receptor Subunits. J. Comp. Neurol. 2004, 470, 339–356.
- Kita, H.; Kita, S.T. The Morphology of Globus Pallidus Projection Neurons in the Rat: An Intracellular Staining Study. Brain Res. 1994, 636, 308–319.
- Mink, J.W.; Thach, W.T. Basal Ganglia Motor Control. III. Pallidal Ablation: Normal Reaction Time, Muscle Cocontraction, and Slow Movement. J. Neurophysiol. 1991, 65, 330–351.
- Nambu, A.; Tokuno, H.; Takada, M. Functional Significance of the CorticoÁ/SubthalamoÁ/Pallidal ‘Hyperdirect’ Pathway. Neurosci. Res. 2002, 7.
- Braak, H.; Del Tredici, K. Cortico-Basal Ganglia-Cortical Circuitry in Parkinson’s Disease Reconsidered. Exp. Neurol. 2008, 212, 226–229.
- Cai, W.; Duberg, K.; Padmanabhan, A.; Rehert, R.; Bradley, T.; Carrion, V.; Menon, V. Hyperdirect Insula-Basal-Ganglia Pathway and Adult-like Maturity of Global Brain Responses Predict Inhibitory Control in Children. Nat. Commun. 2019, 10, 4798.
- Wang, H.; Fan, L.; Song, M.; Liu, B.; Wu, D.; Jiang, R.; Li, J.; Li, A.; Banaschewski, T.; Bokde, A.L.W.; et al. Functional Connectivity Predicts Individual Development of Inhibitory Control during Adolescence. Cereb. Cortex 2020, bhaa383.
- Greenhouse, I.; Oldenkamp, C.L.; Aron, A.R. Stopping a Response Has Global or Nonglobal Effects on the Motor System Depending on Preparation. J. Neurophysiol. 2012, 107, 384–392.
- Levin, O.; Netz, Y.; Ziv, G. Behavioral and Neurophysiological Aspects of Inhibition—The Effects of Acute Cardiovascular Exercise. J. Clin. Med. 2021, 10, 282.
- Aron, A.R. Cortical and Subcortical Contributions to Stop Signal Response Inhibition: Role of the Subthalamic Nucleus. J. Neurosci. 2006, 26, 2424–2433.
- Aron, A.R.; Durston, S.; Eagle, D.M.; Logan, G.D.; Stinear, C.M.; Stuphorn, V. Converging Evidence for a Fronto-Basal-Ganglia Network for Inhibitory Control of Action and Cognition. J. Neurosci. 2007, 27, 11860–11864.
- Badry, R.; Mima, T.; Aso, T.; Nakatsuka, M.; Abe, M.; Fathi, D.; Foly, N.; Nagiub, H.; Nagamine, T.; Fukuyama, H. Suppression of Human Cortico-Motoneuronal Excitability during the Stop-Signal Task. Clin. Neurophysiol. 2009, 120, 1717–1723.
- Jahfari, S.; Waldorp, L.; van den Wildenberg, W.P.M.; Scholte, H.S.; Ridderinkhof, K.R.; Forstmann, B.U. Effective Connectivity Reveals Important Roles for Both the Hyperdirect (Fronto-Subthalamic) and the Indirect (Fronto-Striatal-Pallidal) Fronto-Basal Ganglia Pathways during Response Inhibition. J. Neurosci. 2011, 31, 6891–6899.
- Mathis, J.; de Quervain, D.; Hess, C.W. Dependence of the Transcranially Induced Silent Period on the ‘instruction Set’ and the Individual Reaction Time. Electroencephalogr. Clin. Neurophysiol. Mot. Control 1998, 109, 426–435.
- Fecteau, S.; Lassonde, M.; Théoret, H. Intrahemispheric Dysfunction in Primary Motor Cortex without Corpus Callosum: A Transcranial Magnetic Stimulation Study. BMC Neurol. 2006, 6, 21.
- Kühn, A.A.; Brandt, S.A.; Kupsch, A.; Trottenberg, T.; Brocke, J.; Irlbacher, K.; Schneider, G.H.; Meyer, B.-U. Comparison of Motor Effects Following Subcortical Electrical Stimulation through Electrodes in the Globus Pallidus Internus and Cortical Transcranial Magnetic Stimulation. Exp. Brain Res. 2004, 155, 48–55.
- Chu, H.-Y.; McIver, E.L.; Kovaleski, R.F.; Atherton, J.F.; Bevan, M.D. Loss of Hyperdirect Pathway Cortico-Subthalamic Inputs Following Degeneration of Midbrain Dopamine Neurons. Neuron 2017, 95, 1306–1318.e5.
- Young, M.S.; Triggs, W.J.; Bowers, D.; Greer, M.; Friedman, W.A. Stereotactic Pallidotomy Lengthens the Transcranial Magnetic Cortical Stimulation Silent Period in Parkinson’s Disease. Neurology 1997, 49, 1278–1283.
- Greenhouse, I.; Gould, S.; Houser, M.; Aron, A.R. Stimulation of Contacts in Ventral but Not Dorsal Subthalamic Nucleus Normalizes Response Switching in Parkinson’s Disease. Neuropsychologia 2013, 51, 1302–1309.
- Nambu, A.; Takada, M.; Inase, M.; Tokuno, H. Dual Somatotopical Representations in the Primate Subthalamic Nucleus: Evidence for Ordered but Reversed Body-Map Transformations from the Primary Motor Cortex and the Supplementary Motor Area. J. Neurosci. 1996, 16, 2671–2683.
- Deuschl, G.; Schade-Brittinger, C.; Krack, P.; Volkmann, J.; Schäfer, H.; Bötzel, K.; Daniels, C.; Deutschländer, A.; Dillmann, U.; Eisner, W. A Randomized Trial of Deep-Brain Stimulation for Parkinson’s Disease. N. Engl. J. Med. 2006, 355, 896–908.
- Limousin, P.; Krack, P.; Pollak, P.; Benazzouz, A.; Ardouin, C.; Hoffmann, D.; Benabid, A.-L. Electrical Stimulation of the Subthalamic Nucleus in Advanced Parkinson’s Disease. N. Engl. J. Med. 1998, 339, 1105–1111.
- Bäumer, T.; Hidding, U.; Hamel, W.; Buhmann, C.; Moll, C.K.E.; Gerloff, C.; Orth, M.; Siebner, H.R.; Münchau, A. Effects of DBS, Premotor RTMS, and Levodopa on Motor Function and Silent Period in Advanced Parkinson’s Disease. Mov. Disord. 2009, 24, 672–676.
- Schroll, H.; Beste, C.; Hamker, F.H. Combined Lesions of Direct and Indirect Basal Ganglia Pathways but Not Changes in Dopamine Levels Explain Learning Deficits in Patients with Huntington’s Disease. Eur. J. Neurosci. 2015, 41, 1227–1244.
- Beaumont, V.; Zhong, S.; Lin, H.; Xu, W.; Bradaia, A.; Steidl, E.; Gleyzes, M.; Wadel, K.; Buisson, B.; Padovan-Neto, F.E.; et al. Phosphodiesterase 10A Inhibition Improves Cortico-Basal Ganglia Function in Huntington’s Disease Models. Neuron 2016, 92, 1220–1237.
- Vitek, J.L. Pathophysiology of Dystonia: A Neuronal Model. Mov. Disord. Off. J. Mov. Disord. Soc. 2002, 17, S49–S62.
- Granert, O.; Peller, M.; Jabusch, H.-C.; Altenmüller, E.; Siebner, H.R. Sensorimotor Skills and Focal Dystonia Are Linked to Putaminal Grey-Matter Volume in Pianists. J. Neurol. Neurosurg. Psychiatry 2011, 82, 1225–1231.
- Silberstein, P.; KuÈhn, A.A.; Kupsch, A.; Trottenberg, T.; Krauss, J.K.; WoÈhrle, J.C.; Mazzone, P.; Insola, A.; Di Lazzaro, V.; Oliviero, A. Patterning of Globus Pallidus Local Field Potentials Differs between Parkinson’s Disease and Dystonia. Brain 2003, 126, 2597–2608.
- Zeuner, K.E.; Knutzen, A.; Granert, O.; Götz, J.; Wolff, S.; Jansen, O.; Dressler, D.; Hefter, H.; Hallett, M.; Deuschl, G. Increased Volume and Impaired Function: The Role of the Basal Ganglia in Writer’s Cramp. Brain Behav. 2015, 5, e00301.
- Simonyan, K.; Cho, H.; Hamzehei Sichani, A.; Rubien-Thomas, E.; Hallett, M. The Direct Basal Ganglia Pathway Is Hyperfunctional in Focal Dystonia. Brain 2017, 140, 3179–3190.
- Cotroneo, M.; Ciacciarelli, A.; Cosenza, D.; Casella, C.; Dell’Aera, C.; Grillo, F.; Fazio, M.C.; La Spina, P.; Musolino, R.F. Hemiballism: Unusual Clinical Manifestation in Three Patients with Frontoparietal Infarct. Clin. Neurol. Neurosurg. 2020, 188, 105612.




