
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Claudio Tana | + 1610 word(s) | 1610 | 2021-05-08 09:36:53 | | | |
| 2 | Vicky Zhou | Meta information modification | 1610 | 2021-05-31 15:14:02 | | |
Video Upload Options
Cardiac sarcoidosis (CS) is an unusual, but potentially harmful, manifestation of systemic sarcoidosis (SA), a chronic disease characterized by organ involvement from noncaseating and nonnecrotizing granulomas. Lungs and intrathoracic lymph nodes are usually the sites that are most frequently affected, but no organ is spared and CS can affect a variable portion of SA patients, up to 25% from post-mortem studies. The cardiovascular involvement is usually associated with a bad prognosis and is responsible for the major cause of death and complications, particularly in African American patients. Furthermore, the diagnosis is often complicated by the occurrence of non-specific clinical manifestations, which can mimic the effect of more common heart disorders, and imaging and biopsies are the most valid approach to avoid misdiagnosis.
1. Introduction
Sarcoidosis is a chronic disease characterized by organ involvement from noncaseating and non-necrotizing granulomas. Genetic predisposition and environmental risk factors were hypothesized as the main actors of the disease pathogenesis [1]. Lungs and intrathoracic lymph nodes are usually the sites that are most frequently affected [2][3] but no organ is spared as the involvement can be also cardiovascular, gastrointestinal, neurological and of the genitourinary system and skin [4][5][6][7].
The cardiovascular involvement is usually associated with a bad prognosis and is responsible for the major cause of death and complications, particularly in African American patients. The diagnosis is often complicated by the occurrence of non-specific clinical manifestations [8], which can mimic the effect of more common heart disorders, and imaging and biopsies are the most valid approach to avoid misdiagnosis.
2. Diagnosis of Cardiac Sarcoidosis
The diagnostic criteria of cardiac sarcoidosis were presented by many international scientific societies and an optimal diagnostic algorithm is still under discussion.
The JMHW guidelines of 2006 [9] have suggested that a diagnosis of cardiac sarcoidosis can be reached by (1) histological demonstration of the presence of noncaseating granulomas in the myocardium in a patient with a histological or clinical diagnosis of sarcoidosis in other organs or tissues, and (2) a clinical or histological diagnosis of extra-cardiac sarcoidosis in association with at least three major cardiac criteria: advanced atrioventricular block (AVB), basal interventricular septal thickening and cardiac uptake of gallium-67, left ventricular ejection fraction is less than 50% for four minor criteria: (1) ECG abnormalities (ventricular extrasystoles, ventricular tachycardia, right bundle branch block, Q wave abnormalities and axial deviation on the standard electrocardiogram); (2) echocardiographic abnormalities (segmental-type morphological or wall mobility abnormalities, ventricular aneurysms, or wall thickening); (3) perfusion defects revealed by thallium or technetium scintigraphy; (4) late gadolinium reinforcement on cardiovascular magnetic resonance imaging; (5) diffuse infiltration or interstitial fibrosis in the myocardium on the biopsy.
The JMHW guidelines emphasize the importance of an endomyocardial biopsy to demonstrate cardiac involvement by sarcoid tissue. However, this approach has the great limitation of being extremely invasive in the clinical practice. Furthermore, myocardial involvement in cardiac sarcoidosis is patchy and multifocal and, when combined with the limitations of current sampling techniques, many patients can have nondiagnostic biopsies [10]. Despite its high specificity, therefore, an endomyocardial biopsy can have a low sensitivity for the diagnosis of cardiac sarcoidosis. Another limit of the JMH guidelines is that the uptake of Gallium-67 (67 Ga), still considered among the diagnostic criteria, is not currently used in most centers due to its limited diagnostic accuracy, as demonstrated by several studies [10][11][12][13].
In the latest update, fatal ventricular arrhythmias (e.g., sustained ventricular tachycardia and ventricular fibrillation) and anatomic abnormalities of the ventricular wall (e.g., aneurysm of the ventricular wall, midbasal septum thickening) were included in the major criteria, and diagnostic methods such as the 18F-FDG PET and CMR were included in the major criteria as diagnostic tools [14].
The consensus statement from the Heart Rhythm Society (HRS), in association with the American College of Chest Physicians (ACCP), the American Heart Association (AHA), the Asia Pacific Heart Rhythm Society (APHRS), the European Heart Rhythm Association (EHRA), and the World Association of Sarcoidosis and other Granulomatous Disorders (WASOG) in 2014 recognized both histological (defined) and clinical (probable) criteria for the diagnosis of cardiac sarcoidosis. These guidelines have included both the use of LGE-CMR and 18F-FDG PET in the diagnostic criteria [15].
2.1. Patient Screening for Cardiac Involvement
The first evaluation of patients with suspected CS should include a baseline ECG, which is useful to reveal the most common alterations. A normal ECG, however, does not exclude the presence of even minimal cardiac involvement, but is most often altered in overt CS. Echocardiography is important to reveal the most frequent, although nonspecific, abnormalities such as segmental-type morphological or wall-thickening wall hypomobility abnormalities. Such findings, however, can be found in other cardiac disorders, and are not specific to CS, but can be useful for the first screening of the severity of the disease [9].
Some abnormalities such as the cardiac right ventricular involvement can lead to the misdiagnosis of arrhythmogenic right ventricular cardiomyopathy (ARVC). The 2010 ARVC task force criteria failed to differentiate between CS and hereditary ARVC. Some authors have recently demonstrated how prolonged PR interval, advanced AVB, long duration of QRS associated with reduced LVEF, right involvement of the ventricular apex and positive findings on the 18F-FDG PET should be considered as suspected for CS [16].
2.2. Cardiac MRI
CMR allows a rapid, accurate and non-invasive evaluation of clinical or sub-clinical cardiac sarcoidosis, thanks to the high spatial and soft-tissue resolution [17]. At present, it is one of the preferred techniques for evaluating cardiac sarcoidosis [18][19], having a high sensitivity and specificity of 75–100% and 76–78%, respectively [11], and thanks to the lack of ionizing radiation. Bright blood cine sequences allow an accurate evaluation of biventricular volume and function, mass and myocardial segment thickness (Figure 1c,d).
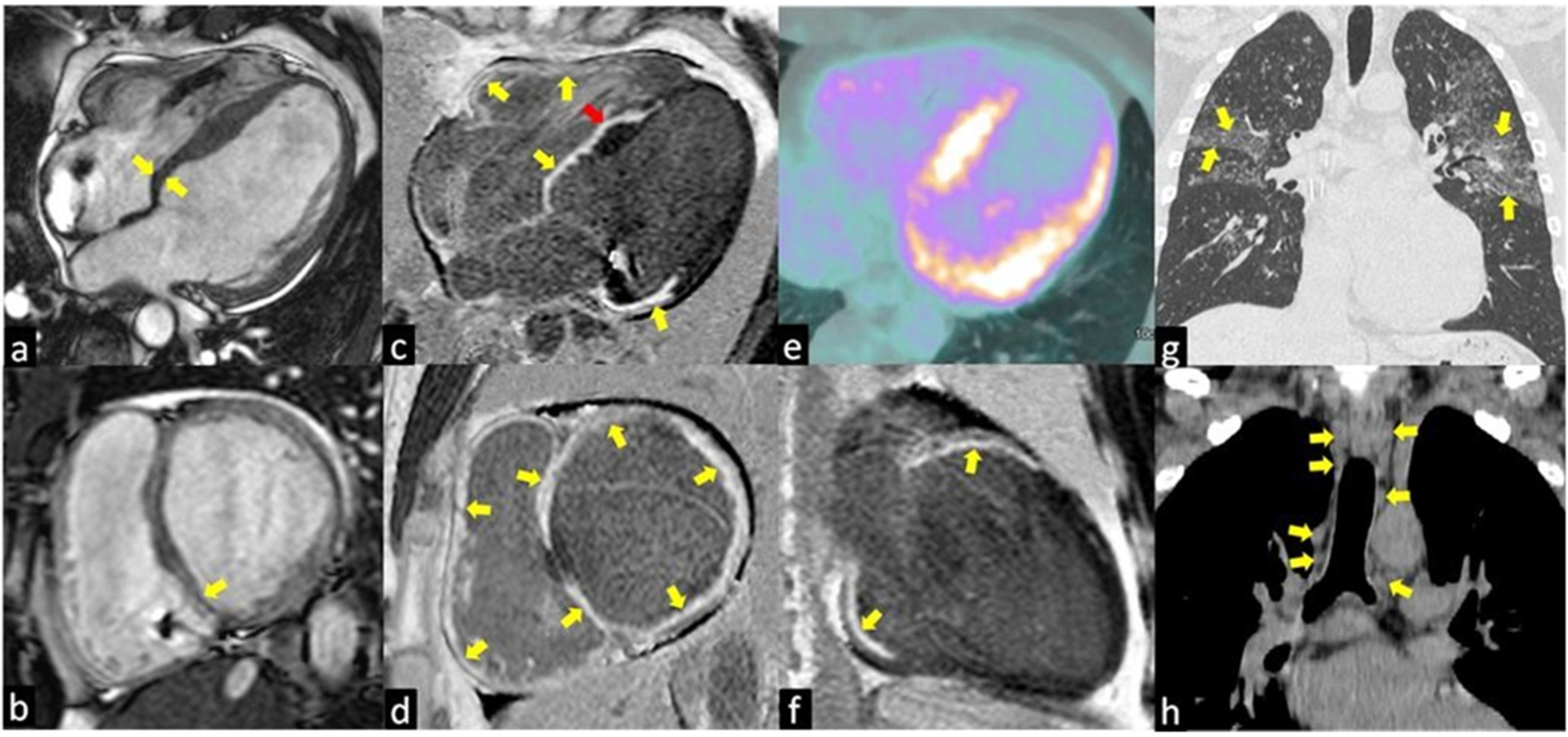
Figure 1. The images show a case of typical Cardiac Sarcoidosis characterized by the presence of LGE of all basal segments of both ventricles (yellow arrows in c,d,f) with the predominantly transmural distribution involving more than one coronary territory and the right ventricular side of the interventricular septum (red arrow in c). Bright blood cine sequences show the thinning of the basal septum (yellow arrows in a,b). Coronal computed tomography (CT) scans show the typical perilymphatic distribution of micronodules with upper lobe predilection (yellow arrows in g) and hilar and mediastinal bilateral lymphadenopathy (yellow arrows in h). The 18F-fluorodeoxyglucose positron emission tomography (e) revealed an increased uptake in the septal and lateral left ventricle myocardial segments in a patient with systemic sarcoidosis.
With the T2-weighted images and the evaluation of early gadolinium uptake, it is possible to reveal respectively the focal presence of acute inflammation (edema) (Figure 2a) and myocardial hyperemia in the thickened myocardium where the granulomas infiltration is located (Figure 2a,b) [20]. Instead, the chronic phase is characterized by ventricular systolic dysfunctions and dilatation often associated with basal septum wall thinning (Figure 1c,d) [21].
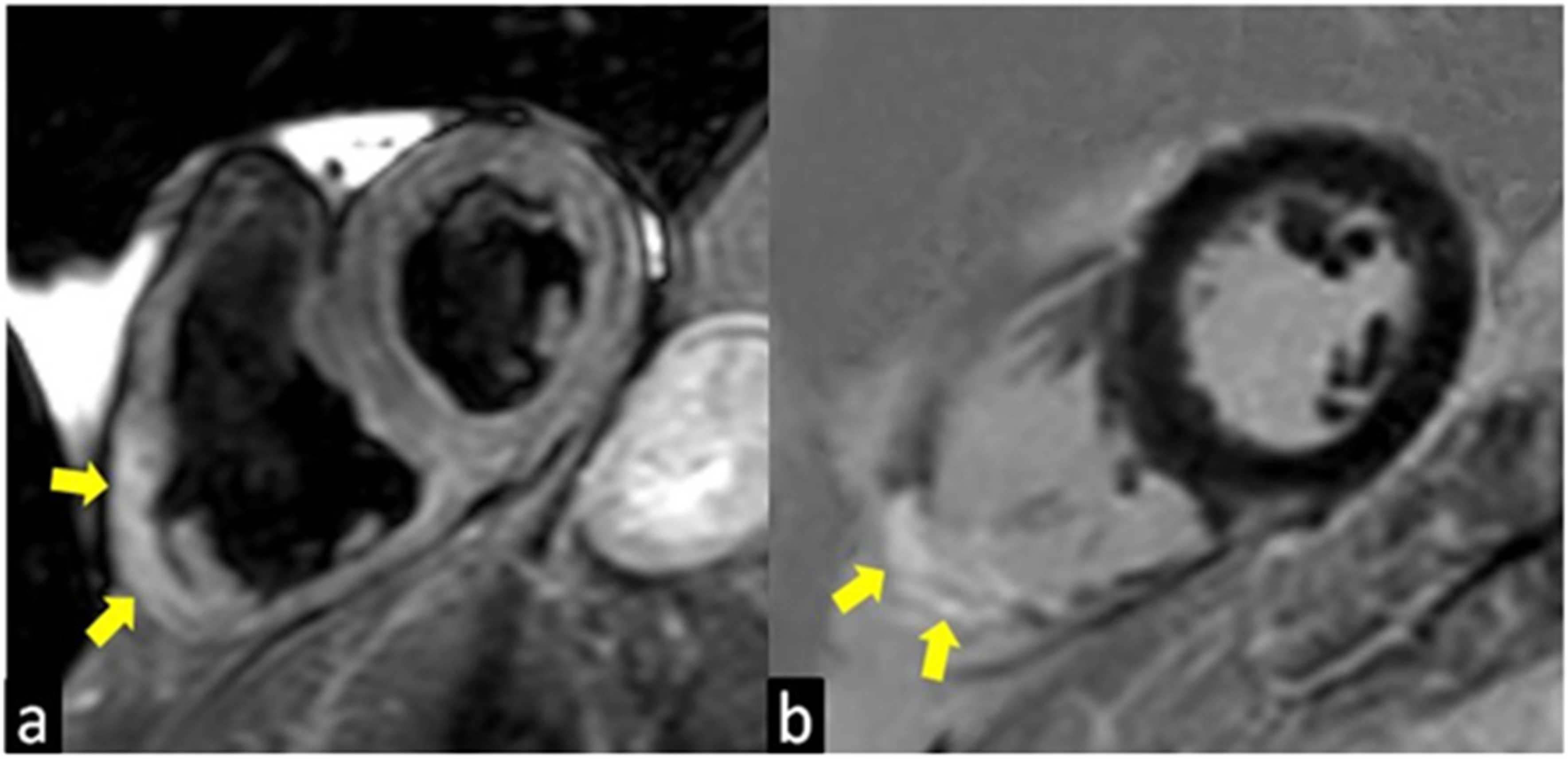
Figure 2. T2-weighted (a) and Late Gadolinium Enhancement (LGE; b) images show a case of atypical presentation of the acute phase of Cardiac Sarcoidosis, characterized by the presence of edema (yellow arrow in a) and LGE (yellow arrow in b) of the inferolateral wall of the right ventricle with the transmural distribution.
Gadolinium is a biologically inert tracer that diffuses freely into the extracellular space, but is unable to cross the intact cell membrane and exhibits a slow washout from damaged cardiomyocytes, identifying areas of myocyte necrosis during the acute phase of CS and areas of macroscopic interstitial fibrosis (scar) during the chronic phase of CS. It is important to underline that late enhancement with gadolinium is not specific for cardiac sarcoidosis but can be observed also in other infiltrative pathologies such as amyloidosis, cardiomyopathies (e.g., hypertrophic cardiomyopathy), myocarditis and ischemic lesions [22].
Typical aspects of cardiac sarcoidosis are: (1) presence of edema (acute phase) and LGE in the basal and mid interventricular septum (Figure 1c,f); (2) presence of both non-ischemic (intramyocardial or subepicardial) (Figure 1e) and ischemic (subendocardial or transmural) (Figure 1f,h) LGE patterns, the latter characterized by the involvement of more than one coronary territories [23]; (3) LGE of the right ventricular side of the interventricular septum (Figure 1e); (4) transmural LGE of thin and akinetic segments in the chronic phase of CS (Figure 1e,f) [22]. The presence of extensive LGE was associated with a poor prognosis by several studies, index of strong sarcoid activity. A meta-analysis including 7 studies and 694 subjects suggested that the presence of LGE among CS patients was associated with an increased risk of cardiovascular death or ventricular arrhythmia [24].
LGE, as well as T1 and T2 mapping techniques, can be used for monitoring the response to anti-inflammatory therapies.
CMR has some advantages over PET imaging, as there is no exposure to ionizing radiation, there is no need for patient preparation such as a specific diet prior to the image acquisition, and it also allows the assessment of cardiovascular morphology, ventricular function, valve and flow quantification [24][25] and the identification of several extracardiac collateral findings [26][27]. However, CMR imaging is limited in patients with pacemakers, or other recently implanted metal devices, and gadolinium is contraindicated in patients with advanced renal disease (estimated glomerular filtration rate [eGFR] < 30 mL/min) [28].
3. Conclusions
As systemic sarcoidosis, CS remains a challenging issue in the matter of diagnostics [29][30]. The evolution of diagnostic techniques in recent years has led to a significant improvement in the detection and classification of the severity of the disease.
Despite the fact that the present gold standard is represented by endomyocardial biopsies, the high invasiveness and risk of false negatives suggests the need for searching and validating new diagnostic algorithms including non invasive methods. As increasing information is available, there is less need for an invasive diagnostic approach to reach a definitive diagnosis of cardiac sarcoidosis. We think that the use of Hybrid PET/CMR imaging could change the global approach to this harmful and complex disease in the future.
References
- Birnie, D.; Ha, A.C.; Gula, L.J.; Chakrabarti, S.; Beanlands, R.S.; Nery, P. Cardiac Sarcoidosis. Clin. Chest Med. 2015, 36, 657–668.
- Tana, C.; Schiavone, C.; Cipollone, F.; Giamberardino, M.A. Management issues of sarcoidosis in the time of COVID. Chest 2021, 159, 1306–1307.
- Tchernev, G.; Cardoso, J.C.; Chokoeva, A.A.; Verma, S.B.; Tana, C.; Ananiev, J.; Gulubova, M.; Philipov, S.; Kanazawa, N.; Nenoff, P.; et al. The "mystery" of cutaneous sarcoidosis: Facts and controversies. Int. J. Immunopathol. Pharmacol. 2014, 27, 321–330.
- Silverman, K.J.; Hutchins, G.M.; Bilkley, B.H. Cardiac sarcoidosis: A clinicopathologic study of 84 unselected patients with systemic sarcoidosis. Circulation 1978, 58, 1204–1211.
- Tana, C.; Tchernev, G.; Chokoeva, A.A.; Wollina, U.; Lotti, T.; Fioranelli, M.; Roccia, M.G.; Maximov, G.K.; Silingardi, M. Pulmonary and abdominal sarcoidosis, the great imitators on imaging? J. Biol. Regul. Homeost. Agents 2016, 30, 45–48.
- Iannuzzi, M.C.; Rybicki, B.A.; Teirstein, A.S. Sarcoidosis. N. Engl. J. Med. 2007, 357, 2153–2165.
- Tchernev, G.; Chokoeva, A.A.; Tana, M.; Tana, C. Transcriptional blood signatures of sarcoidosis, sarcoid-like reactions and tubercolosis and their diagnostic implications. Sarcoidosis Vasc. Diffus. Lung Dis. Off. J. WASOG 2016, 33, 5030.
- Tana, C.; Donatiello, I.; Coppola, M.G.; Ricci, F.; Maccarone, M.T.; Ciarambino, T.; Cipollone, F.; Giamberardino, M.A. CT Findings in Pulmonary and Abdominal Sarcoidosis. Implications for Diagnosis and Classification. J. Clin. Med. 2020, 9, 3028.
- Guideline for Diagnosis of Cardiac Sarcoidosis. Study Report on Diffuse Pulmonary Diseases; Ministry of Health, Labour and Welfare: Tokyo, Japan, 1993; pp. 23–24.
- Hulten, E.; Agarwal, V.; Cahill, M.; Cole, G.; Vita, T.; Parrish, S.; Bittencourt, M.S.; Murthy, V.L.; Kwong, R.; di Carli, M.F.; et al. Presence of late gadolinium enhancement by cardiac magnetic resonance among patients with suspected cardiac sarcoidosis is associated with adverse cardiovascular prognosis: A systematic review and meta-analysis. Circ. Cardiovasc. Imaging 2016, 9, 1–9.
- Smedema, J.-P.; Snoep, G.; van Kroonenburgh, M.P.; van Geuns, R.-J.; Dassen, W.R.; Gorgels, A.P.; Crijns, H.J. Evaluation of the Accuracy of Gadolinium-Enhanced Cardiovascular Magnetic Resonance in the Diagnosis of Cardiac Sarcoidosis. J. Am. Coll. Cardiol. 2005, 45, 1683–1690.
- Hamzeh, N.Y.; Wamboldt, F.S.; Weinberger, H.D. Management of Cardiac Sarcoidosis in the United States: A Delphi Study. Chest 2012, 141, 154–162.
- Tchernev, G.; Chokoeva, A.A.; Schiavone, C.D.; Erme, A.M.; Tana, C.; Darling, M.; Kaley, J.; Gianfaldoni, S.; Wollina, U.; Lotti, T.; et al. Sarcoidosis exclusion criteria: The “simple truth” for a complicated diagnosis. J. Biol. Regul. Homeost. Agents 2015, 29 (Suppl. 1), 5–9.
- Larson, S.R.; Pieper, J.A.; Hulten, E.A.; Ficaro, E.P.; Corbett, J.R.; Murthy, V.L.; Weinberg, R.L. Characterization of a highly effective preparation for suppression of myocardial glucose utilization. J. Nucl. Cardiol. 2020, 27, 849–861.
- Birnie, D.H.; Sauer, W.H.; Bogun, F.; Cooper, J.M.; Culver, D.A.; Duvernoy, C.S.; Judson, M.A.; Kron, J.; Mehta, D.; Nielsen, J.C.; et al. HRS Expert Consensus Statement on the Diagnosis and Management of Arrhythmias Associated With Cardiac Sarcoidosis. Hear. Rhythm. 2014, 11, 1304–1323.
- Gasperetti, A.; Rossi, V.A.; Chiodini, A.; Casella, M.; Costa, S.; Akdis, D.; Büchel, R.; Deliniere, A.; Pruvot, E.; Gruner, C.; et al. Differentiating hereditary arrhythmogenic right ventricular cardiomyopathy from cardiac sarcoidosis fulfilling 2010 ARVC Task Force Criteria. Heart Rhythm. 2021, 18, 231–238.
- Pontone, G.; Di Cesare, E.; Castelletti, S.; De Cobelli, F.; De Lazzari, M.; Esposito, A.; Focardi, M.; Di Renzi, P.; Indolfi, C.; Lanzillo, C.; et al. Appropriate use criteria for cardiovascular magnetic resonance imaging (CMR): SIC-SIRM position paper part 1 (ischemic and congenital heart diseases, cardio-oncology, cardiac masses and heart transplant). Radiol. Med. 2021, 126, 365–379.
- Dubrey, S.W.; Grocott-Mason, R.; Mittal, T.K. Images in cardiology: Cardiac sarcoidosis with delayed enhanced MRI. Heart 2005, 91, 1185.
- Dubrey, S.W.; Sharma, R.; Underwood, R.; Mittal, T. Cardiac sarcoidosis: Diagnosis and management. Postgrad. Med. J. 2015, 91, 384–394.
- Tadamura, E.; Yamamuro, M.; Kubo, S.; Kanao, S.; Saga, T.; Harada, M.; Ohba, M.; Hosokawa, R.; Kimura, T.; Kita, T.; et al. Effectiveness of Delayed Enhanced MRI for Identification of Cardiac Sarcoidosis: Comparison with Radionuclide Imaging. Am. J. Roentgenol. 2005, 185, 110–115.
- Greulich, S.; Deluigi, C.C.; Gloekler, S.; Wahl, A.; Zürn, C.; Kramer, U.; Nothnagel, D.; Bültel, H.; Schumm, J.; Grün, S.; et al. CMR Imaging Predicts Death and Other Adverse Events in Suspected Cardiac Sarcoidosis. JACC Cardiovasc. Imaging 2013, 6, 501–511.
- Nagai, T.; Kohsaka, S.; Okuda, S.; Anzai, T.; Asano, K.; Fukuda, K. Incidence and Prognostic Significance of Myocardial Late Gadolinium Enhancement in Patients With Sarcoidosis Without Cardiac Manifestation. Chest 2014, 146, 1064–1072.
- Patel, M.R.; Cawley, P.J.; Heitner, J.F.; Klem, I.; Parker, M.A.; Jaroudi, W.A.; Meine, T.J.; White, J.B.; Elliott, M.D.; Kim, H.W.; et al. Detection of Myocardial Damage in Patients With Sarcoidosis. Circulation 2009, 120, 1969–1977.
- Sharma, S. Cardiac imaging in myocardial sarcoidosis and other cardiomyopathies. Curr. Opin. Pulm. Med. 2009, 15, 507–512.
- Mantini, C.; Di Giammarco, G.; Pizzicannella, J.; Gallina, S.; Ricci, F.; D’Ugo, E.; Marchetti, M.; Cotroneo, A.R.; Ahmed, N.; Bucciarelli-Ducci, C.; et al. Grading of aortic stenosis severity: A head-to-head comparison between cardiac magnetic resonance imaging and echocardiography. Radiol. Med. 2018, 123, 643–654.
- Ricci, F.; Aung, N.; Gallina, S.; Zemrak, F.; Fung, K.; Bisaccia, G.; Paiva, J.M.; Khanji, M.Y.; Mantini, C.; Palermi, S.; et al. Cardiovascular magnetic resonance reference values of mitral and tricuspid annular dimensions: The UK Biobank cohort. J. Cardiovasc. Magn. Reson. 2021, 23, 1–13.
- Mantini, C.; Mastrodicasa, D.; Bianco, F.; Bucciarelli, V.; Scarano, M.; Mannetta, G.; Gabrielli, D.; Gallina, S.; Petersen, S.E.; Ricci, F.; et al. Prevalence and Clinical Relevance of Extracardiac Findings in Cardiovascular Magnetic Resonance Imaging. J. Thorac. Imaging 2019, 34, 48–55.
- Slart, R.H.; Glaudemans, A.W.; Lancellotti, P.; Hyafil, F.; Blankstein, R.; Schwartz, R.G.; Document Reading Group. A joint procedural position statement on imaging in cardiac sarcoidosis: From the Cardiovascular and Inflammation & Infection Committees of the European Association of Nuclear Medicine, the European Association of Cardiovascular Imaging, and the American Society of Nuclear Cardiology. J. Nucl. Cardiol. 2018, 25, 298–319.
- Tana, C.; Mantini, C.; Cipollone, F.; Giamberardino, M.A. Chest Imaging of Patients with Sarcoidosis and SARS-CoV-2 Infection. Current Evidence and Clinical. Diagnostics 2021, 11, 183.
- Tana, C.; Schiavone, C.; Ticinesi, A.; Ricci, F.; Giamberardino, M.A.; Cipollone, F.; Silingardi, M.; Meschi, T.; Dietrich, C.F. Ultrasound imaging of abdominal sarcoidosis: State of the art. World J. Clin. Cases 2019, 7, 809–818.




