
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Oleg Kolodiazhnyi | + 2917 word(s) | 2917 | 2021-05-26 06:15:46 |
Video Upload Options
Natural phosphorus compounds are essential for modern biological systems, and their diverse biological properties testify to their importance in the world of living organisms. They provide stable ligation necessary for fixing information in RNA and DNA, contribute to cellular structure in phospholipids, serve as the main source of biochemical energy (eg, ATP, phosphoenolpyruvate, creatinephosphate), and are present in a large number of metabolites. Сentral place that phosphates retain in biological systems allows us to conclude that they played an important role in the emergence of life on Earth. In recent years, a large number of natural phosphorus compounds have been isolated from living organisms and significant advances have been made in understanding the effect of phosphates on prebiotic chemistry.
1. Introduction
Phosphorus compounds are essential for modern biological systems, and their diverse biological properties testify to their importance in the world of living organisms. They provide stable ligation necessary for fixing information in RNA and DNA, contribute to cellular structure in phospholipids, serve as the main source of biochemical energy (eg, ATP, phosphoenolpyruvate, creatinephosphate, and others), and are present in a large number of metabolites. Natural phosphorus compounds are invaluable sources for modeling and creating new biologically active substances on their basis. A number of organophosphorus compounds that are C-P analogs of natural compounds are used in the clinic as drugs for the treatment of various diseases. Among the natural phosphorus compounds, effective pharmaceuticals, antibiotics, herbicides, insecticides, and various bioregulators were found. Сentral place that phosphates retain in biological systems allows us to conclude that they played an important role in the emergence of life on Earth (Figure 1) [1].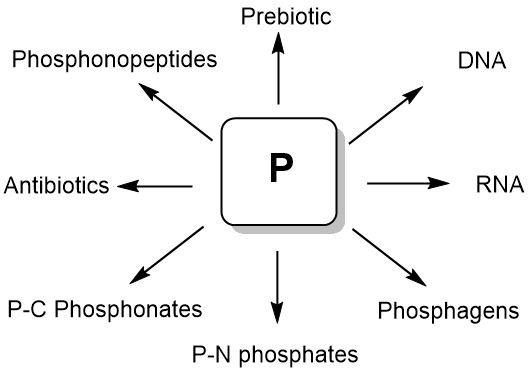
Figure 1. Natural organophosphotus compounds
The study of polyphosphates in the structure of DNA and RNA allows a better understanding of the functioning of these polymeric molecules, vital for life (Figure 2).
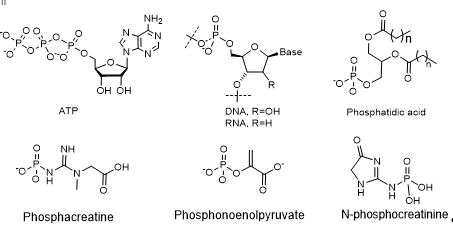
Figure 2. Biologically important phosphates
2. Phosphorus as a key element of living organisms starting from origin of life
Phosphorus is one of the main elements of living organisms. Despite its low content in organisms, phosphorus plays an important role in biochemical reactions. In the human body, phosphorus is not only the main component of bones and teeth, but phosphate groups are also connect deoxyriboses in sugar-phosphate "backbone" of DNA and riboses in the RNA scaffold. Besides the ATP molecules acts as a form of energy conversion in living cells, whereas protein phosphorylation/dephosphorylation is a way of transmitting the life signal. There are reasons to assert that under the conditions of the ancient Earth, phosphorus could contribute to the formation of amino acids, nucleosides/nucleotides, basic groups and peptides. It is believed that phosphorus was the catalyst for the evolution of the ancient Earth[2]. The negative charges of phosphoric acid diesters in DNA help retain molecules within the cell membrane and give nucleic acid the stability necessary for genetic reproducibility. The ionic nature makes phosphates hydrophilic and promotes their retention in the cell membrane. It is important to note that in the case of RNA and DNA ligation, the ionic structure of phosphates allows the ligation of two nucleosides whilst retaining a negative charge at the phosphodiester. The charge carried by the bonds of phosphodiesters between nucleotides provides an important solubilizing element and, importantly, protects the phosphodiesters from hydrolysis. Unlike carboxylic acid anhydrides, phosphoric anhydrides are protected by their negative charges from the rapid impact of water and other nucleophiles, so they can persist in an aqueous environment[3]. Considering the influence of phosphates on the most important life processes, it is necessary to take into account the role of phosphate in the formation of primary organisms, lipids and genetics, considering phosphates as the main element in the energy system of nascent organisms. Life can be seen as a way of existence and transmission of information recorded on an organic matrix. An elementary representative of such an information state is a living cell, which is essentially a biocomputer filled with a huge amount of information concentrated in DNA or RNA[4]. Geological studies have established that life on Earth appeared about 4 billion years ago, in time period between the Hadean and Archean eons, almost immediately as soon as the Earth cooled down to an acceptable temperature. At that time, there was no oxygen atmosphere on Earth yet, but the first anaerobic bacteria appeared and formed many of the currently existing deposits of minerals: sulfur, graphite, iron and nickel. Anaerobic bacteria obtained energy in the absence of oxygen through substrate-level phosphorylation, i.e., reaction that results in the production of ATP by the transfer of a phosphate group from a substrate directly to ADP (Figure 3)[5].
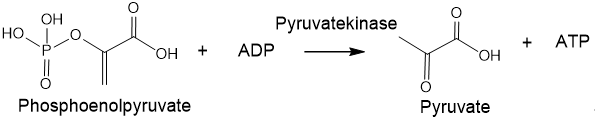
Figure 3. An example of the substrate-level phosphorylation.
3. Stereochemistry of DNA and RNA
NA macromolecule consists of two chains oriented by nitroge-nous bases to each other. This double-stranded molecule is twisted along a helical line, which can be right (A- and B-forms of DNA) or left (Z-form of DNA)[6]. Helical junctions are important architectural elements in RNA, and three- and four-way DNA junctions is a central intermediate in homologous genetic recombinations. The ideal (4H) junctions is created by four double helixes that are held together by interchange of strands. Experimental confirmation of the existence of quadrilateral bonds of cruciform DNA molecules was obtained using electron microscopy. The cruciform shape is another form of quadrilateral bonds found in DNA derived from natural sources. The folded X-structure is formed by paired coaxial superposition of spirals forming a right-sided antiparallel cross. Last years, the DNA successfully used as chiral ligands in transition metal complexes combining the catalytic capabilities of the transition metal with the chiral architecture of the biopolymer[7]. The hybrid catalyst comprises a synthetic catalyst, usually a transition metal complex, which is attached to a chiral biomolecular backbone. For example, salmon testes DNA (stDNA) and calf thymus DNA (ctDNA) are used as ligands. As a result, the catalyzed reaction takes place inside the DNA helix or very close to it, that is determined by DNA chirality, leading to products having an excess one of enantiomers. The chirality of the DNA double helix can be transferred directly to the reaction sites in reactions catalyzed by metal complexes.
4. Natural compounds with P-N bonds
A large number of various organophosphorus compounds with P-N bonds have been isolated from living organisms, which indicates the importance of these compounds in nature. For example, the N-phosphocreatine is one of natural P-N-amidophosphates, which have a significant impact on the formation and development of living organisms, starting from the prebiotic period[8]. Many amidophosphates have cytostatic, antitumor, chemotherapeutic properties to be alkylating-type drugs such as cyclophosphamide, diphosphamide, mafosfamide, trophosphamide, perphosphamide and others Guanidine compounds containing PN-bonds play a central role in the physiology and biochemistry. For example, up to 20-25% of N-phosphocreatine is converted in vivo, through the intermediate formation of N-phosphocreatinine to creatinine (Figure 4).
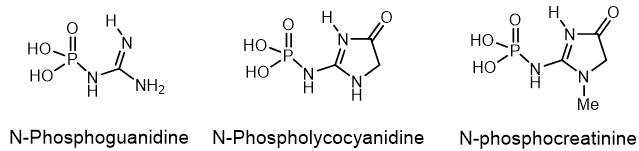
Figure 4. Examples of Phosphaguanidines
The stereochemistry of P-N phosphates, isolated from natural sources, is relatively monotonous. Most natural P-N phosphates whose stereochemistry has been established by reliable methods are derivatives of L-amino acids and D-carbohydrates. Therefore, they contain asymmetric centers of the corresponding absolute configurations. For example. Sutherland et al.[9] investigated the potentially prebiotic synthesis of nucleotides via arabinose-3-phosphate and its cyanamide derivative in an attempt to effect constitutional self-assembly of RNA via a simple pathway to RNA, including nucleotide polymerization. All organisms have proteins that can be involved in NH2-pA catabolism. They are members of the HIT family of proteins and catalyze the hydrolytic cleavage of NH2-pA into 5'-AMP and ammonia. Hint1 (Histidine triad nucleotide binding protein 1) is a protein encoded by the gene of the same name, located in humans on the short arm of chromosome 5. It is involved in such biological processes as apoptosis, transcription, regulation of transcription. Hint proteins are present in almost all types of living organisms[9].
Among the nucleotides containing the P-N bond, nucleotide antibiotics, which are adenosine-containing phospholipids, attract attention. For example, stearic and palmitic acid dinogunellins and their structurally related dinogunellins A-D. A distinctive feature in dynogunellins is the presence of a phosphoramide P-N bond common to many nucleotide antibiotics, for example, fosmidosines, antifungal nucleotide antibiotics from Streptomyces durhameusiz. The fosmidosine has a specific inhibitory activity against spores of Botrytis cinerea, a worldwide pathogenic fungus that causes gray rot disease in various fruits and vegetables. Fosmidosine has a powerful antitumor effect and is able to stop cell growth in the G1 phase of the cell cycle (Figure 5).
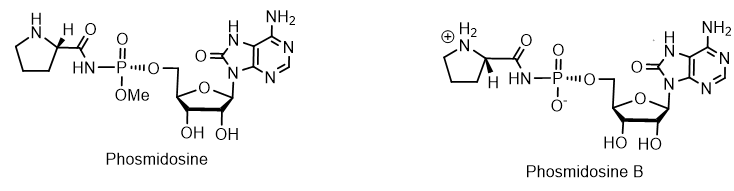
Figure 5. Phosmidosines
The selective antibiotic Agrocin 84, which is a member of the adenine nucleotide family, the first member of which is 6-N-AMP (R = H)[10], has attracted close attention. Agrocin 84, which is a 6-N-phosphoramide, was isolated from Agrobacterium radiobacter K84 found in Australia[11]. Agrocin 84 is selectively active against several strains of phytopathogenic agrobacteria such as Agrobacterium tumefaciens and Agrobacterium rhizogenes. Agrocin 84 is an active factor in the biological control of coronal gall, a plant cancer caused by certain strains of Agrobacterium. Agrocin 84 neutralizes the pathogenic plant bacterium Agrobacterium tumefaciens. Agrocin 84 acts as a molecular Trojan horse, mimicking a substrate derived from a plant tumor, and thus gaining access to the interior of the cell, where it is converted into a toxic moiety (TM84). TM84 is a potent inhibitor of leucyl-tRNA synthetase (LeuRS), an important enzyme that catalyzes the condensation of leucine with its related tRNA and is responsible for the correct translation of the genetic code. It was found that TM84 uses a unique tRNA-dependent mechanism to inhibit leucyl-tRNA synthetase (LeuRS), while the TM84 producer prevents self-poisoning by expressing resistant LeuRS and AgnB2 (Figure 6).
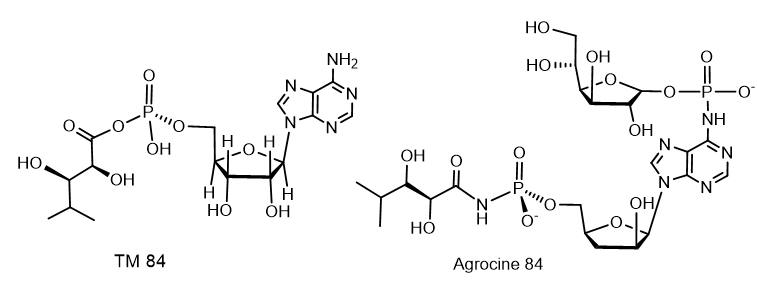
Figure 6. Agrocine 84 and TM 84
Microcin C (McC) is a member of the microcin family containing a heptapeptide covalently linked to 3-aminopropyl AMP via an acylphosphoramide bond. McC is a «Trojan horse»-like inhibitor of aspartyl-tRNA synthetase, endowed with strong antibacterial properties, in which the heptapeptide fragment is responsible for the transport of the inhibitory metabolite into the bacterial cell[12]. The analogue of aspartyl AMP formed inside the cell carries a chemically more stable phosphoramide bond in comparison with labile aspartyl adenylate and, in addition, is esterified with a 3-aminopropyl group. Some species of bacteria produce antibiotics containing peptide and nucleotide moieties for protection. Escherichia coli, for example, produces the antibiotic Microcin C7. The peptide moiety of this phosphoramide mediates the transport of the antibiotic to the target bacterial cell, where processing leads to the breaking of the peptide bond between alanine and aspartic acid, releasing adenylphosphoramide aspartic acid, which acts as an inhibitor of protein synthesis in the target bacterium. Microcins C (McC) - consists of a peptide with formylmethionine at the lateral nitrogen and C-terminal asparagine linked to nebularin-5'-monophosphate through a trimethylene bridge. MccC51 is produced by E. coli extract and inhibits the growth of a number of bacteria (Figure 7).
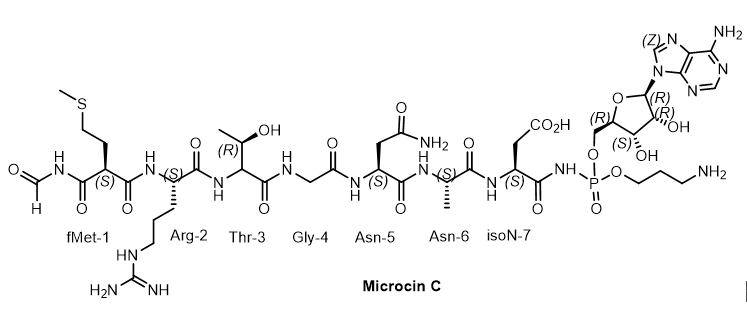
Figure 7. Microcine C
The Nobel laureate Philip Cohen said that that “Reversible phosphorylation of protein side chains regulates almost all aspects of cell life”[13]. Phosphorylation and dephosphorylation, catalyzed by protein kinases and protein phosphatases, can alter protein function in almost every possible way; for example, by increasing or decreasing its biological activity. 30% of the proteins encoded by the human genome contain covalently bound phosphate, and abnormal phosphorylation is currently recognized as a cause or consequence of many human diseases. Phosphorylation of amino acids is a widespread way for regulating the activity of key cell proteins, including enzymes and proteins of signaling pathways. Phosphorylation of amino acid residues (Asp, Glu, forming an anhydride P-O bond) and basic (His, Arg, Lys, forming a P–N bond, namely N-phosphorylation) amino acid residues usually generates high-energy molecules that are intermediates for enzymatic catalysis. Arginine is one of the main amino acid residues involving into the formation of protein-DNA interfaces (Figure 8)
Figure 8. Protein-DNA intermediates
5. Natural organopho sphorus compounds with P-C bond
Natural phosphonates are represented by low molecular weight metabolites and phosphonylated macromolecules such as lipids, polysaccharides, and glycoproteins. The widespread use of phosphonates is illustrated by the fact that in some organisms, PC compounds form the overwhelming majority of cellular phosphorus-containing molecules. Aminophosphonic acids[14] and hydroxyphosphonic acids[15] are widely known, many of which have been studied in detail and found practical application[16]. These compounds are analogs of natural amino and hydroxycarboxylic acids, in which the planar carboxyl group is replaced by a tetrahedral phosphonic acid fragment. aminophosphonic acids and their conjugates with peptides have antibacterial, antitumor, antiviral and antifungal effects. Fosfomycin is a structural analogue of phosphoenol pyruvate; it competes with the enzyme N-acetyl-D-glucosamino-3-o-enolpyruvyl transferase. The result is a specific, selective and irreversible inhibition of this enzyme, which ensures the absence of cross-resistance with other classes of antibiotics and the possibility of synergy with other antibiotics. Fosfomycin is used for the treatment of diseases of urinary tract and prostatitis; it is active as an antibiotic against gram-positive and gram-negative bacteria MDR and XDR. Fosmidomycin and FR-900098 have been suggested as drugs against Plasmodium, the causative agent of malaria[17]. The Streptomyces bacteria produces a tripeptide which was called bialaphos. It consists of two alanine residues and a unique amino acid "phosphinothricin". The phosphinothricin (Gliphosate) was first synthesized as a racemate which is used as an active ingredient in several commercial herbicidal formulations (Figure 9)
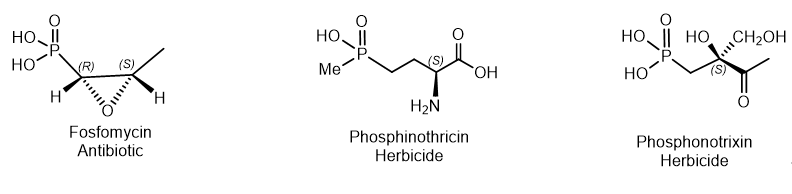
Figure 9. Examples of natural phosphonates
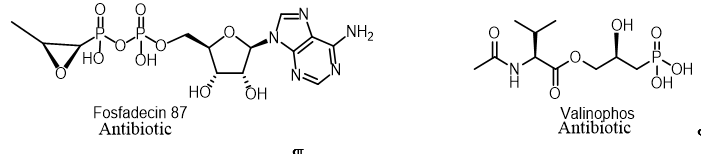
Two nucleotide antibiotics, phosphadecin and phosphocytocin, were found in the extract of the Pseudomonas viridiflava PK-5 and Pseudomonas fluorescens PK-52 cultures, respectively. These antibiotics were purified by column chromatography using adsorption, gel filtration and ion exchange resins. These antibiotics were enzymatically and chemically hydrolyzed to form fosfomycin and a new antibiotic, phosphoxacin, which are also formed in culture filtrates. They showed antibacterial activity against gram-positive and gram-negative bacteria (Figure 10)[18].
Lipids containing aminophosphonates are called phosphonolipids[19]. There are three classes of these compounds - Glycerophosphonolipids and Sphingophosphonolipids and Sphingophosphonoglycolipids (typical structures are shown in the Figure 11). Sphingolipids are a class of lipids related to aliphatic amino alcohol derivatives. Glycerophospholipids or phosphoglycerides are glycerol-based phospholipids. Sphingophosphonoglycolipids, a subtype of glycolipids containing the amino alcohol sphingosine. Phosphonolipids have been found in fractions of glycocerebrosides (lipids) of many lower marine organisms, bacterial exopolysaccharides. Amount of phosphonolipids in organisms varies depending on the species, tissue, or location of the cell. For example, vertebrates have sphingophosphonolipids as components of sphingomyelin in neural tissue, whereas at invertebrates often contain high levels of these lipids as components of the outer membrane (Figure 11).
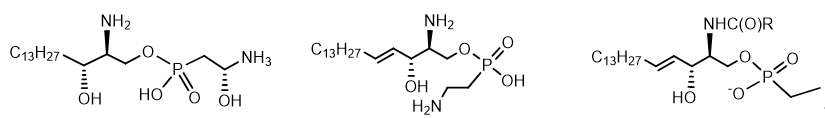
Figure 11. Sphingophosphonolipids.
Phosphonopeptides containing a P–C bond, like phosphopeptides, with a P–O–C bond, are relatively common substances in nature[20]. Currently, both phosphonopeptides isolated from natural sources and a significant amount of synthetic phosphonopeptides with a wide spectrum of biological activity are known. The peptides are thought to enhance cellular uptake through peptide permeases, masking the phosphonate antibiotic in the Trojan horse peptide, which is then hydrolyzed inside the cell by peptidases to release the phosphonate-active fragment. Dehydrophos is an antibiotic isolated from Streptomyces luridus, which is effective in vivo against Salmonella-infected chickens snd has a broad spectrum of in vitro activity. Dehydrophos consists of a glycineleucin dipeptide bound to an O-methylated aminovinylphosphonate, its dehydrophosphorylation proceeds analogously to the similar in structure alaphosphine peptide and the “Trojan horse” peptide dehydrophosphine is imported and cleaved by peptide permeases and peptidases, respectively, to release the alanine racemase inhibitor within the cell (Figure 12)
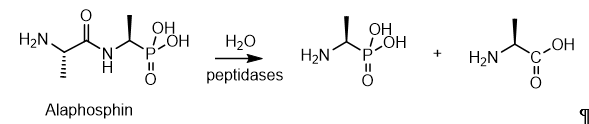
Figure 12. Hydrolytic treatment of alaphosphin peptides to obtain an alanine analog
A number of P-C antibiotics, possessing antifungal activity: rhizocticins[21], plumbemycins, phosphacetamycin, have been isolated from Bacillus subtilis and Streptomyces plumbeus. They represent di- and tripeptides containing the C-terminal (Z)-L-2-amino-5-phosphono-3-pentenoic acid, a phosphonotreonine mimetic, reminiscent of dehydrophos as a broad-spectrum antibiotic that acts against infection of Salmonella. Rhizocticin A, the main component of the antifungal, hydrophilic phosphono-oligopeptides Bacillus subtilis ATCC 6633, was used for sensitivity testing in experiments with the molecular mechanism of antibiotic action. Rhizocticins and plumbemycins are natural phosphonate antibiotics produced by the bacterial strains Bacillus subtilis ATCC 6633 and Streptomyces plumbeus, respectively. The non-proteinogenic (S,Z)-2-amino-5-phosphonopent-3-enoic acid [(S,Z)-APPA] were used for the synthesis of Rhizocticin A and Plumbemycin A (Figure 13).
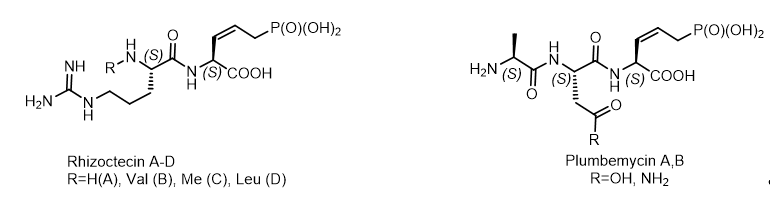
Figure 13. Antibiotic phosphonopeptides
The phosphonic tripeptides Bialaphos and Phosaline, as well as the tetrapeptide Trialaphos, are biological herbicides isolated from soil actinomycetes that attracted close attention. The Bialaphos tripeptide[22]) was isolated from the filtrates of Streptomyces viridochromogenes and Streptomyces hygroscopicus . The antibacterial activity of Bialaphos is a result of active transport of the peptide across the bacterial membrane, followed by hydrolysis of the peptide and the release of the terminal phosphonate, phosphinotricin, which inhibits glutamine synthetase. Further studies of Bialaphos led to the isolation of the tetrapeptide Trialaphos and Phosalacine from the Streptomyces hygroscopicus strain with a similar mechanism of action. Trialaphos (L-alanyl-L-alanyl-L-alanyl-phosphinothricin) is a naturally occurring tetrapeptide phosphinate composed of the combination of the three amino acids Alanine and Glufosinate. Phosalacine was isolated from the fermentation broth of actinomycete strain KA-338 during the screening of antimetabolites competing with L-glutamine as structural analogs of the catalytic intermediate glutamine synthetase. Glufosinate actively inhibits this enzyme, but has low activity as a free amino acid, probably due to poor absorption. However, the “Trojan horse” peptides derived from glufosinate are readily imported into cells, where the peptidases release the active inhibitor (Figure 14).
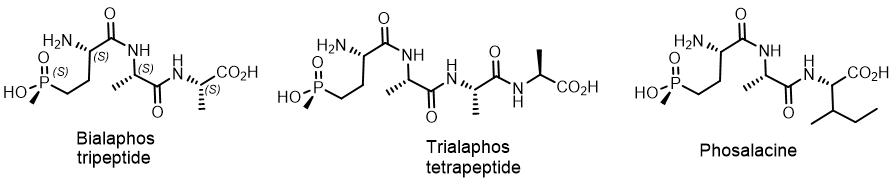
Figure 14. . Phosphonic tripeptides and tetrapeptide
Phosalacin, a herbicidal antibiotic containing phosphinothricin, was isolated from a culture filtrate of Kitasatosporia phosalacinea KA-338 soil extract. Bialaphos and phosphalacin, which are produced by soil actinomycetes, are tripeptides and contain phosphinothricin as the N-terminal amino acid. Bialaphos, Trialaphos and Phosalacine are pro-herbicides and are active only after their metabolic hydrolysis in the plant. Only the (S) –enantiomer of phosphinothricin exhibits herbicidal activity (Figure 15)[23].
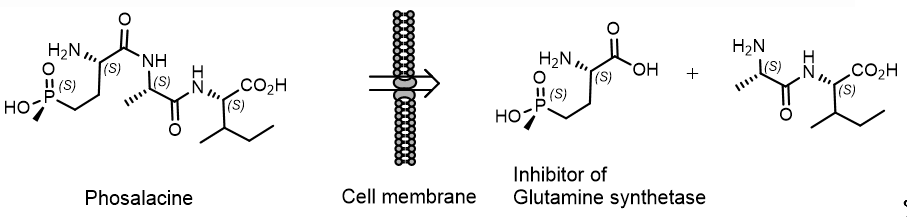
Figure 15. . Representative mechanism of action of Phosalacine.
References
- Lee, W.; Abdillah, A.; Palma, J.; Churchill, D. G. Introductory Chapter: Phosphorus - Nature’s Versatile Pentavalent and Tetrahedral Covalent Building Block and Agent for Energy, Disease and Health. In book: Contemporary Topics about Phosphorus in Biology and Materials Publisher: Intech Open, 2020, pp.1-17.
- Liu, Z.; Rossi, J.-C.; Pascal, R. How Prebiotic Chemistry and Early Life Chose Phosphate. Life 2019, 9, 26-42.
- Altstein, A.D. Origin of Biological Nucleic Acids and the First Genetic System (The Progene Hypothesis). Transcriptomics 2017, 5, 139-143.
- Schwartz, A.W. Phosphorus in prebiotic chemistry. Philos. Trans. R. Soc. B. 2006, 361, 1743–1749.
- Skulachev, V. P.; Bogachev, A. V.; Kasparinsky, F. O. Principles of Bioenergetics. Springer Science & Business Media. 2012, p. 252. ISBN 978-3-642-33430-6
- Ussery, D. W. DNA Structure: A-, B- and Z-DNA Helix Families. Encyclopedia of Life Sciences. John Wiley & Sons, Ltd. Published Online: 2002, pp.1-7.
- Wang, J.; Benedetti, E.; Bethge, L.; Vonhoff, S.; Klussmann, S.; Vasseur, J.-J.; Cossy, J.; Smietana, M.; Arseniyadis, S. DNA vs. Mirror Image DNA: A Universal Approach to Tune the Absolute Configuration in DNA Based Asymmetric Catalysis. Angew. Chem. Int. Ed. 2013, 52, 11546-11549.
- Anastasi, C.; Buchet, F.F.; Crowe, M.A.; Helliwell, M.; Raftery, J.; Sutherland, J.D. The Search for a Potentially Prebiotic Synthesis of Nucleotides via Arabinose-3-phosphate and Its Cyanamide Derivative. Chem. Eur. J. 2008, 14, 2375-2388.
- Bretes, E.; Wojdyla-Mamon, A.M.; Kowalska, J.; Jemielity, J.; Kaczmarek, R.; Baraniak, J.; Guranowski, A. Hint2, the mitochondrial nucleoside 50-phosphoramidate hydrolase; properties of the homogeneous protein from sheep (Ovis aries) liver. Acta Biochim. Pol. 2013, 60, 249–254.
- Kim, J.-G.; Park, B. K.; Kim, S.-U.; Choi, D.; Nahm, B. H.; Moon, J. S. Bases of biocontrol: Sequence predicts synthesis and mode of action of agrocin 84, the Trojan Horse antibiotic that controls crown gall. Proc. Natl. Acad. Sci. USA, 2006, 103, 8846-8851.
- Kim, J.-G.; Park, B. K.; Kim, S.-U.; Choi, D.; Nahm, B. H.; Moon, J. S. Bases of biocontrol: Sequence predicts synthesis and mode of action of agrocin 84, the Trojan Horse antibiotic that controls crown gall. Proc. Natl. Acad. Sci. USA, 2006, 103, 8846-8851. DOI: 10.1073/pnas.0602965103
- Paz-Yepes, J.; Brahamsha, B.; Palenik, B. Role of a microcin-C-like biosynthetic gene cluster in allelopathic interactions in marine Synechococcus. Proc. Natl. Acad. Sci. USA 2013,110,12030-12035.
- Cohen, P. "The origins of protein phosphorylation". Nature Cell Biology. 2002, 4, E127–130.
- Mikołajczyk, M. Phosphonate reagents and building blocks in the synthesis of bioactive compounds, natural products and medicines. Pure Appl. Chem. 2019, 91, 811-838. DOI:10.1515/pac-2018-1117
- Kafarski, P. Phosphonates: Their Natural Occurrence and Physiological Role. Contemporary Topics about Phosphorus in Biology and Materials. Eds W. Lee, A. Abdillah, J. Palma, D. G. Churchill 2020, pp.1-19.
- Kolodiazhnyi, O.I. Asymmetric synthesis of hydroxyphosphonates. Tetrahedron: Asymm. 2005, 16, 3295-3341.
- Lell, B.; Ruangweerayut, R.; Wiesner, J.; Missinou, M.A.; Schindler, A.; Baranek, T.; Hintz, M,; Hutchinson, D.; Jomaa, H.; Kremsner, P.G. Fosmidomycin, a novel chemotherapeutic agent for malaria. Antimicrob. Agents Chemother 2003, 47, 735–738.
- Kinarivala, N.; Suh, J. H.; Botros, M.; Webb, P.; Trippier, P.C. Pharmacophore elucidation of phosphoiodyn A - Potent and selective peroxisome proliferator-activated receptor β/δ agonists with neuroprotective activity. Bioorg Med Chem Lett. 2016, 26, 1889-1893.
- Mukhamedova, K. S., Glushenkova, A. I. Natural phosphonolipids. Chemistry of Natural Compound. 2000, 36, 329-341.
- Marrs, E.C.L.; Varadi, L.; Bedernjak, A. F.; Day, K. M.; Gray, M.; Jones, A. L.; Cummings, Anderson, S.P.; Anderson, R. J.; Perry, J. D. Phosphonopeptides Revisited, in an Era of Increasing Antimicrobial Resistance. Molecules 2020, 25, 1445-1455.
- Rapp, C.; Jung, G.; Kugler, M.; Loeffler, W. Rhizocticins—New phosphono- oligopeptides with antifungal activity. Eur. J. Org. Chem. 1988, 655-661.
- Blodgett, J.A.V.; Zhang, J.K.; Yu, X.; Metcalf, W.W. Conserved biosynthetic pathways for phosalacine, bialaphos and newly discovered phosphonic acid natural products. J. Antibiotics. 2016, 69, 15-25.
- Lamberth, C. Naturally occurring amino acid derivatives with herbicidal, fungicidal or insecticidal activity. Amino Acids 2016, 48, 929–940.





