
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Tadao Maeda | + 2052 word(s) | 2052 | 2021-05-19 06:21:02 | | | |
| 2 | Peter Tang | Meta information modification | 2052 | 2021-05-31 04:35:53 | | |
Video Upload Options
Age-related macular degeneration (AMD) is a highly prevalent irreversible impairment in the elderly population worldwide. Stem cell therapies have been considered potentially viable for treating AMD through the direct replacement of degenerated cells or secretion of trophic factors that facilitate the survival of existing cells.
1. Introduction
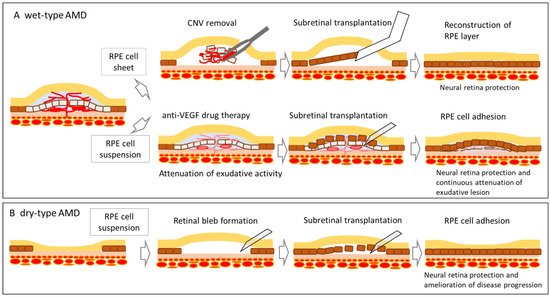
2. History of RPE Cell Therapy for Age-Related Macular Degeneration
3. Cell Therapy for Age-Related Macular Degeneration Using Pluripotent Stem Cell-Derived RPE Cells
|
No. |
Study Title |
Sponsor/Collaborators |
Intervention |
Age |
Phases |
No. of Subjects |
Start/Completion Date |
Status |
Study ID |
|---|---|---|---|---|---|---|---|---|---|
|
1 |
A Study of transplantation of autologous induced pluripotent stem cell (iPSC) derived retinal pigment epithelium (RPE) cell sheet in subjects with exudative age-related macular degeneration |
the Laboratory for Retinal Regeneration, RIKEN Center for Developmental Biology |
autologous hiPSC derived RPE cell sheet |
50 years and older |
P1 |
1 |
October 2013 /September 2018 |
completed |
UMIN000011929 |
|
2 |
Autologous Transplantation of Induced Pluripotent Stem Cell-Derived Retinal Pigment Epithelium for Geographic Atrophy Associated With Age-Related Macular Degeneration |
National Institutes of Health Clinical Center, Bethesda, Maryland, U.S. |
Combination Product: hiPSC-derived RPE/PLGA scaffold |
55 years and older |
P1 |
20 |
July 2020 /March 2029 |
Recruiting |
NCT04339764 |
|
3 |
A Study Of Implantation Of Retinal Pigment Epithelium In Subjects With Acute Wet Age Related Macular Degeneration |
Moorfields Eye Hospital NHS Foundation Trust, London, U.K. |
PF-05206388: RPE living tissue equivalent for intraocular use in the form of a monolayer of RPE cells immobilized on a polyester membrane. |
60 years and older |
P1 |
2 |
July 2020 /March 2029 |
Recruiting |
NCT04339764 |
|
4 |
Study of Subretinal Implantation of Human Embryonic Stem Cell-Derived RPE Cells in Advanced Dry AMD |
Retinal Arizona LTD, Phoenix, Arizona, U.S./Retina-Vitreous Associates Medical Group, Beverly Hills, California, U.S. and others |
CPCB-RPE1 (Human Embryonic Stem Cell-Derived RPE Cells Seeded on a Polymeric Substrate) |
55 years to 85 years |
P1/2a |
16 |
July 2019 /June 2023 |
Active, not recruiting |
NCT02590692 |
|
5 |
A Study of transplantation of allogenic induced pluripotent stem cell (iPSC) derived retinal pigment epithelium (RPE) cell suspension in subjects with neovascular age related macular degeneration |
the Laboratory for Retinal Regeneration, RIKEN Center for Developmental Biology, Kobe, Japan/ Kobe City Medical Center General Hosital, Kobe, Japan |
Subretinal transplantation of allogenic hiPSC derived RPE cells |
50 years to 85 years |
P1 |
5 |
February 2017 /October 2021 |
Active, not recruiting |
UMIN000026003 |
|
6 |
Stem Cell Therapy for Outer Retinal Degenerations |
Federal University of Sao Paulo, Sao Paulo, Brazil |
injection of hESC derived RPE in suspension/Procedure: injection hESC derived RPE seeded in a substrate |
18 years to 90 years |
P1/2 |
15 |
September 2016 /July 2020 |
Completed |
NCT02903576 |
|
7 |
Subretinal Transplantation of Retinal Pigment Epitheliums in Treatment of Age-related Macular Degeneration Diseases |
Chinese Academy of Sciences/Beijing Tongren Hospital, China |
hESC derived RPE |
55 years and older |
P1/2 |
10 |
January 2018 /December 2020 |
Recruiting |
NCT02755428 |
|
8 |
Safety and Efficacy of Subretinal Transplantation of Clinical Human Embryonic Stem Cell Derived Retinal Pigment Epitheliums in Treatment of Retinitis Pigmentosa |
Qi Zhou, Chinese Academy of Sciences |
hESC derived RPE |
18 years and older |
P1 |
10 |
May 2020 /December 2021 |
Recruiting |
NCT03944239 |
|
9 |
Treatment of Dry Age Related Macular Degeneration Disease With Retinal Pigment Epithelium Derived From Human Embryonic Stem Cells |
Chinese Academy of Sciences/ The First Affiliated Hospital of Zhengzhou University, China |
hESC derived RPE |
55 years and older |
P1/2 |
15 |
September 2017 /December 2020 |
Recruiting |
NCT03046407 |
|
10 |
Safety and Tolerability of Sub-retinal Transplantation of Human Embryonic Stem Cell Derived Retinal Pigmented Epithelial (hESC-RPE) Cells in Patients With Stargardt’s Macular Dystrophy (SMD) |
Astellas Institute for Regenerative Medicine/Astellas Pharma Inc., U.S. |
hESC derived RPE (MA09-hRPE) |
18 years and older |
P1/2 |
15 |
November 2011 /September 2015 |
completed |
NCT01469832 |
|
11 |
A Follow up Study to Determine the Safety and Tolerability of Sub-retinal Transplantation of Human Embryonic Stem Cell Derived Retinal Pigmented Epithelial (hESC-RPE) Cells in Patients With Stargardt’s Macular Dystrophy (SMD) |
Astellas Institute for Regenerative Medicine/Astellas Pharma Inc., U.S. |
hESC derived RPE (MA09-hRPE) |
18 years and older |
12 |
January 2013 /October 2019 |
completed |
NCT02941991 |
|
|
12 |
Sub-retinal Transplantation of hESC Derived RPE(MA09-hRPE) Cells in Patients With Stargardt’s Macular Dystrophy |
Astellas Institute for Regenerative Medicine/Astellas Pharma Inc., U.S. |
hESC derived RPE (MA09-hRPE) |
18 years and older |
P1/2 |
13 |
April 2011 /August 2015 |
completed |
NCT01345006 |
|
13 |
Safety and Tolerability of Sub-retinal Transplantation of hESC Derived RPE (MA09-hRPE) Cells in Patients With Advanced Dry Age Related Macular Degeneration |
Astellas Institute for Regenerative Medicine/Astellas Pharma Inc., U.S. |
hESC derived RPE (MA09-hRPE) |
55 years and older |
P1/2 |
13 |
April 2011 /August 2015 |
completed |
NCT01344993 |
|
14 |
Long Term Follow Up of Sub-retinal Transplantation of hESC Derived RPE Cells in Stargardt Macular Dystrophy Patients |
Astellas Institute for Regenerative Medicine/Astellas Pharma Inc., U.S. |
hESC derived RPE (MA09-hRPE) |
18 years and older |
P1 |
13 |
July 2012 /June 2019 |
completed |
NCT02445612 |
|
15 |
Long Term Follow Up of Sub-retinal Transplantation of hESC Derived RPE Cells in Patients With AMD |
Astellas Institute for Regenerative Medicine/Astellas Pharma Inc., U.S. |
hESC derived RPE (MA09-hRPE) |
18 years and older |
11 |
February 2013 /August 2019 |
completed |
NCT02463344 |
|
|
16 |
A Phase I/IIa, Open-Label, Single-Center, Prospective Study to Determine the Safety and Tolerability of Sub-retinal Transplantation of Human ES Cell Derived RPE (MA09-hRPE) Cells in Patients With Advanced Dry Age-related Macular Degeneration (AMD) |
Astellas Institute for Regenerative Medicine/Astellas Pharma Inc., U.S. |
hESC derived RPE (MA09-hRPE) |
55 years and older |
P1/2a |
12 |
September 2012 /June 2020 |
Active, not recruiting |
NCT01674829 |
|
17 |
A Safety Surveillance Study in Subjects With Macular Degenerative Disease Treated With Human Embryonic Stem Cell-derived Retinal Pigment Epithelial Cell Therapy |
Astellas Institute for Regenerative Medicine/Astellas Pharma Inc., U.S. |
hESC derived RPE (MA09-hRPE) |
18 years and older |
P1/2 |
36 |
January 2018 /December 2029 |
Enrolling by invitation |
NCT03167203 |
|
18 |
Retinal Pigment Epithelium Safety Study For Patients In B4711001 |
Moorfields Eye Hospital NHS Foundation Trust, U.K. |
hESC derived RPE |
60 years and older |
2 |
September 2016 /October 2020 |
Active, not recruiting |
NCT03102138 |
|
|
19 |
Safety and Efficacy Study of OpRegen for Treatment of Advanced Dry-Form Age-Related Macular Degeneration |
Lineage Cell Therapeutics, Inc./CellCure Neurosciences Ltd., Israel |
OpRegen |
50 years and older |
P1/2 |
24 |
August 2015 /December 2024 |
Recruiting |
NCT02286089 |
References
- Wong, W.L.; Su, X.; Li, B.X.; Cheung, C.M.G.; Klein, B.E.; Cheng, C.-Y.; Wong, T.Y. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: A systematic review and meta-analysis. Lancet Glob. Health 2014, 2, e106–e116.
- Singh, M.S.; Park, S.S.; Albini, T.A.; Canto-Soler, M.V.; Klassen, H.; MacLaren, R.E.; Takahashi, M.; Nagiel, A.; Schwartz, S.D.; Bharti, K. Retinal stem cell transplantation: Balancing safety and potential. Prog. Retin. Eye Res. 2020, 75, 100779.
- Wang, Y.; Tang, Z.; Gu, P. Stem/progenitor cell-based transplantation for retinal degeneration: A review of clinical trials. Cell Death Dis. 2020, 11, 1–14.
- Maeda, A.; Mandai, M.; Takahashi, M. Gene and Induced Pluripotent Stem Cell Therapy for Retinal Diseases. Annu. Rev. Genom. Hum. Genet. 2019, 20, 201–216.
- Scholl, H.P.N.; Strauss, R.W.; Singh, M.S.; Dalkara, D.; Roska, B.; Picaud, S.; Sahel, J.-A. Emerging therapies for inherited retinal degeneration. Sci. Transl. Med. 2016, 8, 368rv6.
- Mandai, M.; Watanabe, A.; Kurimoto, Y.; Hirami, Y.; Morinaga, C.; Daimon, T.; Fujihara, M.; Akimaru, H.; Sakai, N.; Shibata, Y.; et al. Autologous Induced Stem-Cell–Derived Retinal Cells for Macular Degeneration. N. Engl. J. Med. 2017, 376, 1038–1046.
- Da Cruz, L.; Fynes, K.; Georgiadis, O.; Kerby, J.; Luo, Y.H.; Ahmado, A.; Vernon, A.; Daniels, J.T.; Nommiste, B.; Hasan, S.M.; et al. Phase 1 clinical study of an embryonic stem cell–derived retinal pigment epithelium patch in age-related macular degeneration. Nat. Biotechnol. 2018, 36, 328–337.
- Kashani, A.H.; Lebkowski, J.S.; Rahhal, F.M.; Avery, R.L.; Salehi-Had, H.; Dang, W.; Lin, C.-M.; Mitra, D.; Zhu, D.; Thomas, B.B.; et al. A bioengineered retinal pigment epithelial monolayer for advanced, dry age-related macular degeneration. Sci. Transl. Med. 2018, 10, eaao4097.
- Sugita, S.; Mandai, M.; Hirami, Y.; Takagi, S.; Maeda, T.; Fujihara, M.; Matsuzaki, M.; Yamamoto, M.; Iseki, K.; Hayashi, N.; et al. HLA-Matched Allogeneic iPS Cells-Derived RPE Transplantation for Macular Degeneration. J. Clin. Med. 2020, 9, 2217.
- Schwartz, S.D.; Regillo, C.D.; Lam, B.L.; Eliott, D.; Rosenfeld, P.J.; Gregori, N.Z.; Hubschman, J.-P.; Davis, J.L.; Heilwell, G.; Spirn, M.; et al. Human embryonic stem cell-derived retinal pigment epithelium in patients with age-related macular degeneration and Stargardt’s macular dystrophy: Follow-up of two open-label phase 1/2 studies. Lancet 2015, 385, 509–516.
- Schwartz, S.D.; Hubschman, J.-P.; Heilwell, G.; Franco-Cardenas, V.; Pan, C.K.; Ostrick, R.M.; Mickunas, E.; Gay, R.; Klimanskaya, I.; Lanza, R. Embryonic stem cell trials for macular degeneration: A preliminary report. Lancet 2012, 379, 713–720.
- Takagi, S.; Mandai, M.; Gocho, K.; Hirami, Y.; Yamamoto, M.; Fujihara, M.; Sugita, S.; Kurimoto, Y.; Takahashi, M. Evaluation of Transplanted Autologous Induced Pluripotent Stem Cell-Derived Retinal Pigment Epithelium in Exudative Age-Related Macular Degeneration. Ophthalmol. Retin. 2019, 3, 850–859.
- Mehat, M.S.; Sundaram, V.; Ripamonti, C.; Robson, A.G.; Smith, A.J.; Borooah, S.; Robinson, M.; Rosenthal, A.N.; Innes, W.; Weleber, R.G.; et al. Transplantation of Human Embryonic Stem Cell-Derived Retinal Pigment Epithelial Cells in Macular Degeneration. Ophthalmology 2018, 125, 1765–1775.
- Sung, Y.; Lee, M.J.; Choi, J.; Jung, S.Y.; Chong, S.Y.; Sung, J.H.; Shim, S.H.; Song, W.K. Long-term safety and tolerability of subretinal transplantation of embryonic stem cell-derived retinal pigment epithelium in Asian Stargardt disease patients. Br. J. Ophthalmol. 2020, 10, 1136.
- Song, W.K.; Park, K.-M.; Kim, H.-J.; Lee, J.H.; Choi, J.; Chong, S.Y.; Shim, S.H.; Del Priore, L.V.; Lanza, R. Treatment of Macular Degeneration Using Embryonic Stem Cell-Derived Retinal Pigment Epithelium: Preliminary Results in Asian Patients. Stem Cell Rep. 2015, 4, 860–872.
- Gouras, P.; Flood, M.T.; Kjeldbye, H.; Bilek, M.K.; Eggers, H. Transplantation of cultured human retinal epithelium to Bruch’s membrane of the owl monkey’s eye. Curr. Eye Res. 1985, 4, 253–265.
- Peyman, A.G.; Blinder, K.J.; Paris, C.L.; Alturki, W.; Nelson, J.N.C.; Desai, U. A Technique for Retinal Pigment Epithelium Transplantation for Age-Related Macular Degeneration Secondary to Extensive Subfoveal Scarring. Ophthalmic Surg. Lasers Imaging Retin. 1991, 22, 102–108.
- Algvere, P.V.; Berglin, L.; Gouras, P.; Sheng, Y. Transplantation of fetal retinal pigment epithelium in age-related macular degeneration with subfoveal neovascularization. Graefe’s Arch. Clin. Exp. Ophthalmol. 1994, 232, 707–716.
- Algvere, P.V.; Berglin, L.; Gouras, P.; Sheng, Y. Transplantation of RPE in age-related macular degeneration: Observations in disciform lesions and dry RPE atrophy. Graefe’s Arch. Clin. Exp. Ophthalmol. 1997, 235, 149–158.
- Weisz, J.M.; Humayun, M.S.; De Juan, E.; Del Cerro, M.; Sunness, J.S.; Dagnelie, G.; Soylu, M.; Rizzo, L.; Nussenblatt, R.B. Allogenic fetal retinal pigment epithelial cell transplant in a patient with geographic atrophy. Retina 1999, 19, 540–545.
- Del Priore, L.V.; Kaplan, H.J.; Tezel, T.H.; Hayashi, N.; Berger, A.S.; Green, W. Retinal pigment epithelial cell transplantation after subfoveal membranectomy in age-related macular degeneration. Am. J. Ophthalmol. 2001, 131, 472–480.
- Joussen, A.M.; Heussen, F.M.; Joeres, S.; Llacer, H.; Prinz, B.; Rohrschneider, K.; Maaijwee, K.J.; Van Meurs, J.; Kirchhof, B. Autologous Translocation of the Choroid and Retinal Pigment Epithelium in Age-related Macular Degeneration. Am. J. Ophthalmol. 2006, 142, 17–30.
- MacLaren, R.E.; Uppal, G.S.; Balaggan, K.S.; Tufail, A.; Munro, P.M.; Milliken, A.B.; Ali, R.R.; Rubin, G.S.; Aylward, G.W.; Da Cruz, L. Autologous Transplantation of the Retinal Pigment Epithelium and Choroid in the Treatment of Neovascular Age-Related Macular Degeneration. Ophthalmology 2007, 114, 561–570.
- Maaijwee, K.; Heimann, H.; Missotten, T.; Mulder, P.; Joussen, A.; Van Meurs, J. Retinal pigment epithelium and choroid translocation in patients with exudative age-related macular degeneration: Long-term results. Graefe’s Arch. Clin. Exp. Ophthalmol. 2007, 245, 1681–1689.
- Haruta, M.; Sasai, Y.; Kawasaki, H.; Amemiya, K.; Ooto, S.; Kitada, M.; Suemori, H.; Nakatsuji, N.; Ide, C.; Honda, Y.; et al. In vitro and in vivo characterization of pigment epithelial cells differentiated from primate embryonic stem cells. Investig. Opthalmol. Vis. Sci. 2004, 45, 1020–1025.
- Osakada, F.; Ikeda, H.; Sasai, Y.; Takahashi, M. Stepwise differentiation of pluripotent stem cells into retinal cells. Nat. Protoc. 2009, 4, 811–824.
- Hirami, Y.; Osakada, F.; Takahashi, K.; Okita, K.; Yamanaka, S.; Ikeda, H.; Yoshimura, N.; Takahashi, M. Generation of retinal cells from mouse and human induced pluripotent stem cells. Neurosci. Lett. 2009, 458, 126–131.





