Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Ioan Botiz | + 4850 word(s) | 4850 | 2021-05-19 07:43:13 | | | |
| 2 | Catherine Yang | -2 word(s) | 4848 | 2021-05-28 03:22:05 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Botiz, I. Antimicrobial Polymeric Structures. Encyclopedia. Available online: https://encyclopedia.pub/entry/10206 (accessed on 12 January 2026).
Botiz I. Antimicrobial Polymeric Structures. Encyclopedia. Available at: https://encyclopedia.pub/entry/10206. Accessed January 12, 2026.
Botiz, Ioan. "Antimicrobial Polymeric Structures" Encyclopedia, https://encyclopedia.pub/entry/10206 (accessed January 12, 2026).
Botiz, I. (2021, May 27). Antimicrobial Polymeric Structures. In Encyclopedia. https://encyclopedia.pub/entry/10206
Botiz, Ioan. "Antimicrobial Polymeric Structures." Encyclopedia. Web. 27 May, 2021.
Copy Citation
Certain natural and synthetic polymers are versatile materials that have already proved themselves to be highly suitable for the development of the next-generation of antimicrobial systems that can efficiently prevent and kill microbes in various environments.
synthetic antimicrobial polymers
assembled nanostructures
1. Introduction
Antimicrobial polymers are (biocidal) materials that can prevent and suppress the growth of various undesired microorganisms, including bacteria. Moreover, they can combat the bacterial resistance to antibiotics because, unlike conventional antibiotics, polymers exhibit antimicrobial mechanisms that cannot be outwitted by pathogens [1]. Furthermore, antimicrobial polymers can be easily adapted to applications, such as coatings and used to sterilize various surfaces, inclusively those of medical instruments. Thus, such polymers could become a good alternative to antibiotics and disinfectants and, why not, could eventually replace them in the future. This could be possible, especially because polymers come with important advantages, as they can adopt more or less complex chemical structures [2][3] that can favor their assembly and crystallization processes. These processes actually dictate the final properties of polymers in bulk, solutions, or thin-films [4][5][6][7]. Due to the potentiality to precisely control other processing parameters, such as melting, crystallization or glass-transition temperature, a polymer can display a highly tunable molecular ordering on multiple-length scales, ranging from nanometers to macroscopic dimensions that can generate a diverse landscape of nanostructures [4][8][9][10]. Further expansion of this landscape on the molecular, microscopic and macroscopic scales can be induced by favoring physical and chemical interactions of specific chain segments with their neighbors [3][4][11] or by degrading the phase purity through the addition of other (polymeric) components [12][13][14][15].
Antimicrobial polymeric systems include biopolymers and synthetic polymers (hereafter simply “polymers”). While biopolymers, such as polypeptides, polysaccharides or polynucleotides, are natural chains produced by the cells of various living organisms, polymers are human-made from precursors, such as petroleum derivatives or even biological components like peptides [16], lysine or arginine [17]. Antimicrobial polymers are designed to imitate the antimicrobial structures produced by the immune systems of various living organisms to kill microbes and can contain in their backbone or their side-chains various moieties with biocidal properties.
2. Recent Antimicrobial Structures Assembled from Polymers on Surfaces
Several well-established polymer-based antimicrobial mechanisms are challenging researchers to employ synthetic polymers to generate efficient antimicrobial structures in various environments. A prominent category of such structures is represented by drug-loaded and gel-like structures [18][19][20][21][22][23]. Another equally important category of antimicrobial structures includes those obtained from polymeric systems assembled on specific surfaces [24][25][26][27][28][29][30][31][32][33][34][35][36][37]. In this work, we further review only the most recent antimicrobial structures falling within the last category.
2.1. Polymeric Structures Preventing the Adhesion of Microbes on Surfaces
Nowadays, it is of paramount importance to prevent various microorganisms from contacting, adhering and flourishing on specific surfaces in order, for example, to keep various medical devices/facilities uncontaminated [38][39][40][41], to generate efficient implants [26], or to avoid biocorrosion of marine-related applications [42]. Polymers can be employed to engineer films and coatings with tuned surface properties that can prevent the adhesion of microorganisms. One such polymer is PEG, a polyether that can be synthesized in various molecular weights and that can display a diversity of hierarchically ordered nanostructures at multiple-length scales, especially when incorporated in block copolymers [43][44]. This ability to adopt various highly ordered intrachain conformations endows PEG with protein repellent or attractive properties at low or high compressive loads, respectively [45]. It makes it a versatile material that can be used to efficiently fight microbes when coated on surfaces [24][25][26].
Coating various surfaces with PEG can be mainly realized either by employing various direct deposition techniques [25][26] or through the grafting procedure, i.e., by covalently anchoring the polymer chains to surfaces as brushes [24]. The latter procedure has the advantage of facile tunning of the microbial adhesion through the control of polymer chain length and temperature at which experiments are performed [24] and can reduce the adhesion of microbes by several to tens of times (Figure 1a) [25]. For example, PEG can be utilized to construct multilayered films on stainless steel, nylon, titanium oxide or silicon oxide substrates, previously covered with mussel-inspired polydopamine (PDA) [25]. Moreover, to target marine biofouling (i.e., problematic accumulation of diverse microorganisms, plants and/or algae on surfaces immersed in water that damages boats or various underwater constructions and devices) on the above-mentioned surfaces, PEG catechols, obtained by coupling the amine groups of 6-arm-PEG-amine and the carboxylic group of 3,4-dihydroxyhydrocinnamic acid [46], can be crosse-linked with PDA through catechol-catechol based interactions to become insoluble. The resulting multilayered films spin-cast from PEG catechol solution is not only stable under marine environments but also exhibits high resistance towards marine A. coffeaeformis (Figure 1a) [25]. Crosslinking process can further be employed to develop anticellular and bacterial repellent PEG-based coatings on bare and sandpapered titanium surfaces [26]. More exactly, nanofibers of PEG prepared via electrospinning can be coated on titanium surfaces and then rendered insoluble through using a photo-crosslinking agent. Results have shown that titanium surfaces covered with PEG nanofibers display enhanced antiadhesive properties against fibroblastic preosteoblasts and S. epidermidis compared to their counterpart blank surfaces [26].
Another class of polymers, known for their role in impeding the adhesion of microbes on surfaces, is represented by the zwitterionic polymers, such as polysulfobetaine (PSB) [47]. Moreover, methacrylate-based PSB (PSBMA) can be grafted on glass substrates by employing an atom transfer radical polymerization (ATRP) route and can lead to 10-15 nm thick brush-like structures of specific molecular conformations. These nontoxic structures can efficiently inhibit the adhesion of marine green algae, spores, sporelings and diatoms, such as Navicula, over various periods [27]. More recently, the microbe repellent properties of zwitterionic PSBMA were extended to S. aureus and S. epidermidis through the realization of thin brush-like films of PSBMA and PCBMA on polydimethylsiloxane (PDMS) surfaces [48]. PSBMA and PCBMA were covalently attached to PDMS substrates by employing a grafting method based on the exposure to UV light of the zwitterionic monomers in the presence of photoinitiators and crosslinking agents. Resulting PSBMA and PCBMA films decreased the adhesion of the above-mentioned bacteria under dry conditions by an order of magnitude [48]. A significant bacteria antiadhesive effect was also demonstrated for zwitterionic brushes grafted on brominated stainless steel and polymerized from 2-methacryloyloxyethyl phosphorylcholine and N-(3-sulfopropyl)-N-(methacryloxyethyl)-N,N-dimethylammonium betaine [49]. The thickness of these brushes was evaluated to be 53 and 132 nm, while the static water contact angle was measured to be only 14° and 11°, respectively.
The same ATRP reaction can be utilized along with photochemical grafting to grow protein-repelling zwitterionic brushes of poly [3-(methacryloylamino)propyl]dimethyl(3-sulfopropyl)ammonium hydroxide (PMPDSAH) onto indium thin oxide substrates [50]. Interestingly, the antifouling performance of PMPDSAH coated surfaces is outperforming that of the PEG surfaces [51]. Moreover, this polymer can be functionalized by a 1.9 nm thick metal–polyphenol coating to make it attractive to proteins and cells [52]. This is particularly important when targeting the spatio-selective functionalization of a specific surface. For instance, by patterning the PMPDSAH surface with a metal–polyphenol coating using a microcontact printing technique, surfaces displaying alternating hundred micrometers sized regions with fouling and antifouling properties can be fabricated [52].
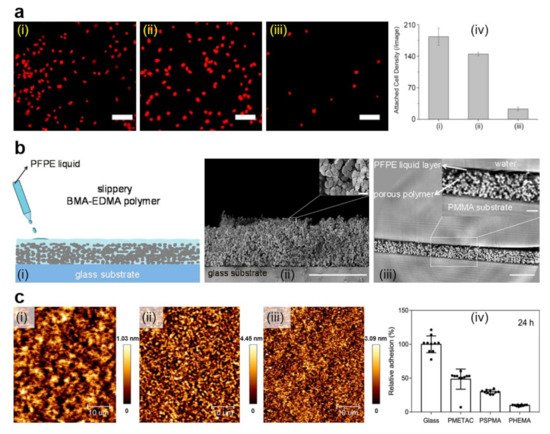
Figure 1. (a) Fluorescence images (i–iii) and quantification (iv) of A. coffeaeformis diatoms that succeeded to attach on the (i) untreated stainless steel, (ii) PDA-coated stainless steel, and (iii) PEG-based film. Each point is the mean of 60 counts on three replicate samples in (iv). Scale bars represent 50 μm. (b) Schematic representation of the fabrication of slippery P(BMA-co-EDMA) surface by infusion of the porous polymer with the PFPE fluid (i), scanning electron microscopy (SEM) images depict a cross-section and the porous morphology of the P(BMA-EDMA) surface (ii) and reconstructed X-ray propagation phase-contrast tomography image displaying the cross-section of the slippery P(BMA-EDMA) surface underwater (iii). Scale bars are 100 μm (ii,iii), 2 μm (ii) and 20 μm (iii) in the insets, respectively. (c) atomic force microscopy (AFM) height images depict PMETAC (i), PSPMA (ii), and PHEMA (iii) polymer brushes attached to the glass coverslips and their antiadhesive performance against E. coli compared to naked glass, after an incubation time of 24 h (iv). The relative adhesion of bacteria on the above substrates was normalized with respect to the adhesion on the glass substrate for 24 h. Adapted with permission from ref. [25] (a), ref. [28] (b) and ref. [53] (c).
2.2. Polymeric Structures Employed to Kill Microbes on Surfaces
Bioactive polymers exhibit their antimicrobial attributes due to their intrinsic nature and/or their decorative biocidal moieties and conjugates incorporated into their backbone and/or side-chains [54][55][56]. An example of highly biocidal conjugates is given by the short antimicrobial peptides (AMPs), i.e., highly biocidal cationic units of gene-encoded peptide antibiotics possessing a low rate in driving antimicrobial resistance [57]. Generally, AMPs can be incorporated into polymeric device coatings and released to kill bacteria. Nonetheless, the time-limited antimicrobial effect is dictated by the elution of AMPs [58]. Instead, decorating polymers with AMPs might lead to augmented efficiency in targeting and killing the pathogens and can confer longer-term antibacterial properties while reducing the toxicity of the resulting systems against mammalian cells. In earlier studies, AMPs, such as Tet-213 (KRWWKWWRRC) [54] and other similar [55] were designed and tethered to poly-(N,N-dimethylacrylamide-co-N-(3-aminopropyl)-methacrylamide hydrochloride) P(DMA-co-APMA) copolymer chains that were grafted, in various molecular conformations and densities, from titanium surfaces (Figure 2a) [54][55]. The morphology of resulting surfaces consisted of different features exhibiting a roughness of about 6 nm. Moreover, higher copolymer brush densities rendered the resulting surfaces with more crowded AMPs and, thus, with better bactericidal properties [54].
More recently, Yu and coworkers have modulated the functionality of AMPs along with that of polymer brushes to generate high antimicrobial peptide potency that could be used in developing infection-resistant implant surfaces [59]. Their results revealed that the antimicrobial activity of brush coatings tethered with AMPs depended on the polymer brush chemistry (which has an impact on changes in the secondary structure of the AMPs) as well as on the AMP molecular conformations (which determine the density of AMPs on the polymer brushes and their microstructure) [59]. The microstructure of AMPs is further important because it regulates the rather unwanted interactions between peptides and biomolecules, such as blood proteins. An approach to eliminate such interactions, and thus, to allow peptides to attach effectively to the negatively charged bacterial surface, is to actually covalently connect few units of helical antimicrobial peptides with radial amphiphilicity into short polypeptides with a hydrophobic helical core and a charged shell [60]. Consequently, the resulting polypeptides can adopt peculiar conformations and form nanostructures exhibiting one to two orders of magnitude enhancement in their antimicrobial activity against both Gram-positive and Gram-negative bacteria [60].
Enhanced antimicrobial activity was further noticed for similar spherical nanostructures made of polylysine-b-poly(2-hydroxypropyl methacrylate) (PLys-b-PHPMA) block copolymer [61]. These nanostructures were created via polymerization-induced self-assembly of HPMA by employing PLys as the macrochain transfer agent and are comprised of a PHPMA core and a PLys shell. For instance, simple vesicles or vesicles with rather few and short branched worms formed when long or shorter PHPMA polymer chains were employed, respectively (Figure 2b). The obtained spherical nanostructures exhibited significant antimicrobial properties in thin-film membranes. This was possible due to the positively charged nature of PLys chains forming the shell of the spherical particles [61].
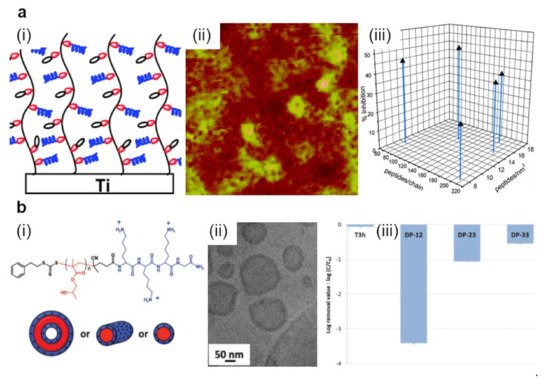
Figure 2. (a) Schematic representation (i) and AFM topography (ii) depict P(DMA-co-APMA) copolymer chains adopting brush-like conformations on titanium surfaces. Depending on the initial composition ratio between the DMA and APMA monomers and on the peptide density, P(DMA-co-APMA) copolymer exhibits more or less inhibitive effects against bacteria (iii). The size of the AFM image is 3 × 3 µm2. (b) Schematic representation (i) and a cryo-transmission electron microscopy (cryo-TEM) image (ii) depict spherical core–shell vesicles that could self-assemble from PLys-b-PHPMA block copolymer. The chemical structure of the copolymer is presented on top of (i). Experimentally observed structures are then able to act against S. epidermidis with high efficiency (iii). Log removal data were obtained after 3 h for the control reactor without any material (T3h) and the reactor with various PLys-b-PHPMA block copolymers. Adapted with permission from ref. [60] (a) and ref. [61] (b).
Another widely-used biocidal moiety that can be tethered to various polymer chains to render them antimicrobial is the quaternary ammonium (QA) salt, a disinfectant used already for many years to kill microbes, such as bacteria, yeasts or molds [62]. QA moiety can be attached to various copolymers made of polymethylhydrosiloxane (PMHS) and PDMS via a quaternization procedure based on 1-iodooctane. This procedure leads to crosslinked QA-functional polymers containing different concentrations of QA moieties that can generate highly homogeneous films. The latter can be then optimized with respect to the moisture curability and used to correlate the QA concentration with the biocidal activity toward the marine bacterium Cellulophaga lytica and algae Navicula incerta [62]. Optimized samples exhibit about 80% in biofilm retention and 90% reduction in biofilm growth for the two microbes, respectively. Similarly, QA-based antimicrobial coatings can be prepared by dip-coating the surface of interest directly into a solution made of amphiphilic poly((dopamine methacrylamide)-(methoxyethyl acrylate)-dodecyl QA) (P(DMA-MEA-DQA)) containing various amounts of antimicrobial dodecyl QA, hydrophobicity tuning methoxyethyl and immobilizing catechol groups [30]. The resulting antimicrobial polymer films display a smooth morphology comprised of dodecyl chains localized rather at the air-surface interface and with the phenyl groups of the catechols oriented with respect to the substrate surface. Instead, the most hydrophobic films made of polymers containing no methoxyethyl side-chains were comprised of polymeric domains of an average size of hundreds of nanometers that exhibited high surface roughness. All films proved themselves highly biocidal against various microbes, inclusively due to their adhesive functionality of catechol groups that have prevented the leaching of polymers [30].
QA moiety can be further attached to polymers, such as polyurethane (PU) [56] and poly(2-(dimethylamino)-ethyl methacrylate-co-methyl methacrylate) (P(DMEMA-co-MMA)) [63]. In the first case, QA salt moieties and hydroxyl groups are introduced to the backbone of the soybean oil-based polyols and then are reacted with diisocyanate monomers to obtain PUs. PU coatings containing more QA salt moieties exhibit the best antibacterial activity by killing about 95% of bacteria [56]. In the second case, a solution of partially quaternized P(DMEMA-co-MMA) mixed with ethylene glycol dimethacrylate (EGDMA) and photoinitiator 2-hydroxy-4-(2-hydroxyethoxy)-2-methylpropiophenone (HHMP) is spin-cast on glass slides and cured under UV light to generate a semi-interpenetrating network (SIPN) of P(DMEMA-co-MMA) and polymerized EGDMA [63]. This QA-garnished polymer-based coating not only displays strong bacteria-killing properties but can prevent the biocidal QA moieties from leaching.
The fact that ammonium-based polymers are capable to efficiently kill bacteria was recently further demonstrated by Sanches and coworkers, who generated core–shell NPs by decorating the poly(methyl methacrylate) (PMMA) NPs with antimicrobial poly(diallyldimethylammonium chloride) (PDDA) via emulsion polymerization reaction (Figure 3a) [64]. Resulting cationic NPs, self-assembled under low ionic strength conditions (Figure 3b), can access the inner layers of the cell and its membrane through the antimicrobial action of the PDDA shell and, thus, can kill microbes very efficiently (Figure 3c). The killing efficiency is nonetheless depending on the hydrophobic-hydrophilic balance of PDDA, as well as on the type of microbe, possibly due to the microstructural differences of the microbial cell walls [64]. More recently, PDDA/PMMA core–shell NPs were further optimized and utilized to fabricate antimicrobial coatings on substrates of interest by drop or spin casting [65]. Deposited hydrophilic coatings exhibited contact angles depending proportionally on the amount of PDDA in the NPs and reduced bacteria by about 7 logs.
A distinguished class of polymers that can be successfully used to generate antimicrobial structures on surfaces is represented by the cationic polymers. The main representants of this class of polymers are polyethylenimines (PEIs). The antimicrobial efficiency of PEIs, deriving from their cationic character, depends on optimizing their chemical structure [66]. For example, to enlarge their capability to perforate the hydrophobic membrane of bacteria, linear or branched PEIs with different molecular weights need to be synthesized [67]. While both types of PEIs possess enhanced antibacterial activity against S. aureus, only linear PEIs can induce depolarization of the bacterial membrane. Furthermore, PEIs can be N-alkylated with quaternary amino functional groups, such as hexyl, octadecyl or dodecyl, just to name a few, and rendered antimicrobial when deposited on surfaces or incorporated into NPs [66]. Besides N-alkyl, benzophenone can also be used to decorate PEIs. N-alkylated and benzophenone-based PEIs synthesized from poly(2-ethyl-2-oxazoline) (PEOX) can be then attached by photo-crosslinking to various surfaces, such as cotton, silicon oxide, or various other polymers, to fabricate leaching-free antimicrobial coatings for textile and plastic materials (Figure 3d) [31]. Exhibiting a morphology comprised of random, tens of nanometers sized features of the roughness of less than one nanometer (Figure 3e), resulting PEI-based coatings are capable to kill more than 98% of S. aureus or E. coli. (Figure 3f).
Besides PEIs, various other cationic polymers exhibiting important antimicrobial properties were reported. They were recently thoroughly reviewed by Alfei and Schito, and therefore, additional information on this topic can be found elsewhere [68]. We would only like to additionally emphasize the existence of a new water-soluble cationic copolymer synthesized from 4-ammoniumbuthylstyrene hydrochloride and reported only very recently [69]. This copolymer was shown to be able to display a rapid non-lytic bactericidal activity and thus, to be capable of acting against several bacteria, including E. coli, Acinetobacter baumannii and Stenotrophomonas maltophilia.
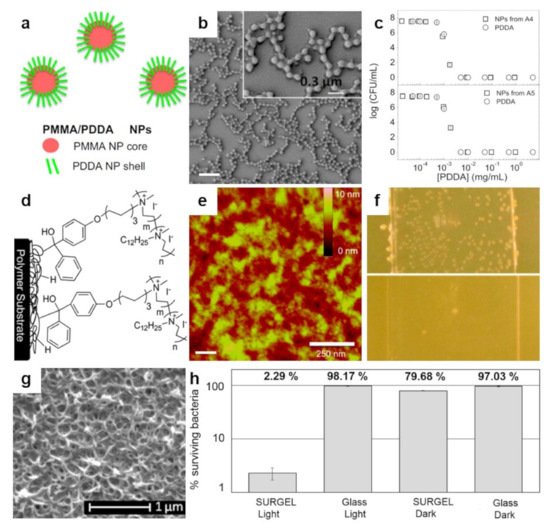
Figure 3. (a,b) Schematic representation (a) and SEM morphology (b) of the core–shell PMMA/PDDA NPs assembled at low ionic strength. The scale bar represents 1 µm. (c) Antimicrobial activity of NPs against E. coli. Representation of cell viability (log10 of colony-forming units CFU/mL) for E. coli with respect to the concentration of free PDDA or PDDA in the PMMA/PDDA NPs exhibiting sizes of 112 nm and 164 nm in diameter, respectively. (d) Schematics depict the covalent attachment of the benzophenone-based PEI copolymer to various surfaces and plastics. (e) Tapping-mode AFM height image depicts the surface of a thin-film of benzophenone-based PEI copolymer after sonication. (f) Digital images of a control glass substrate (top) and of a glass substrate modified with benzophenone-based PEI copolymer (bottom) sprayed with S. aureus and incubated for 24 h. (g) SEM micrograph depicts the surface of a porphyrin-based SURMOF after crosslinking and subsequent treatment with EDTA solution. (h) Antibacterial activity of the porphyrin SURGEL thin-films against E. coli using the LIVE/DEAD BacLight bacterial viability kit. Adapted with permission from ref. [64] (a–c), ref. [31] (d,e) and ref. [70] (g,h).
Antimicrobial polymers can be further synthesized by incorporating in their chemical structure the N-halamine, a biocidal moiety capable of almost instant and total sterilization over a broad spectrum of microorganisms [71][72]. Importantly, N-halamine polymers do not form toxic products and do not release halogen unless they are in contact with bacteria [73]. The synthesis of N-halamine-based polymers and grafted copolymers are cheap and rely on employing 4-(alkyl acryloxymethyl)-4-ethyl-2-oxazolidinones and commercial monomers or polymers (the latter are well-known for their efficacy to kill bacteria in both granular forms and as surface, coatings covering glass or plastics [74]). Alternatively, any inert polymer, including bilayers of PS functionalized with a top surface layer of poly(styrene-b-tert-butyl acrylate) (PS-b-PtBA), can be employed to generate the desired density of chemical groups containing amine bonds grafted from PS surfaces (i.e., PS/PS-PAA) and can be further chlorinated to N-halamine [75]. Resulting N-halamine polymeric surfaces are highly biocidal against S. aureus and E. coli. Unfortunately, such antimicrobial systems are not very stable and need re-chlorination, as a significant part of the chlorine is lost upon UV irradiation [75].
Other N-halamine polymer precursors of cationic homopolymer poly((3-acrylamidopropyl) trimethylammonium chloride) (PCHP) and of anionic homopolymer poly(2-acrylamido-2-methylpropane sulfonic acid sodium salt) (PAHP) [32] or of poly[5,5-dimethyl-3-(3′-triethoxysilylpropyl)-hydantoin] (PSPH) [76] can be synthesized and coated onto cotton fabrics or mesoporous molecular sieves via LbL deposition or grafting techniques. The resulting N-halamine coatings can be further rendered biocidal upon their exposure to household bleach. Both concepts lead to chlorinated coatings of well-defined roughness that can inactivate 100% of S. aureus and E. coli [32][76], with significant log reductions within the first minute of contact [76]. Again, washing of the fabrics coated with N-halamine polymers is accompanied by a reduction of chlorine. This drawback is compensated by the fact that coated fabrics produce no irritations to rabbit skin, displaying thus important potential towards future biomedical applications [32]. More recently, the process of chlorine bleaching at different bleach concentrations on tape was further employed to transform polypyrrole (PPy) into N-halamines and to develop highly efficient antimicrobial coatings on stainless steel by taking advantage of the electrochemical deposition process [77].
At the end of this section, we note other peculiar polymers, such as porphyrins, which can be integrated as constituents into a surface anchored metal–organic framework (MOF). The resulting composition can be deposited on a substrate and crosslinked, leading to SURMOF (Figure 3g). The latter can be transformed by a treatment with ethylenediaminetetraacetic acid (EDTA) solution into a metal-free antimicrobial polymer-based coating abbreviated SURGEL that demonstrates significant antimicrobial activity by killing more than 97% of some bacteria (Figure 3h). This is possible due to ROS generation when thin polymer films based on porphyrin are exposed to visible light [70].
2.3. Polymeric Surface Structures Exhibiting Microbe Antiadhesive and Killing Properties
An interesting strategy against the accumulation of microbes on surfaces relies on combining the antiadhesive and antimicrobial properties of polymers to develop more complex bifunctional surface systems capable of both repelling and killing microbes. This strategy can be implemented by conjugating antiadhesive polymers with various antimicrobial (biocidal) moieties or even polymers. A relevant example was given a decade ago by Muszanska and coworkers, who have synthesized a triblock copolymer with a central polypropylene oxide (PPO) block and two terminal antiadhesive PEG segments under the name of Pluronic F-127 (PF-127) [78]. At the telechelic groups of the PEG chains, they have further covalently attached the antimicrobial enzyme lysozyme conjugate. This triblock copolymer led to structures comprised of one or two lysozyme molecules per each PF-127 polymer chain that could adsorb on a hydrophobic surface by adopting a brush-like molecular conformation. Surfaces coated with such brushes showed both antiadhesive and antimicrobial properties. Intriguingly, the structures with less lysozyme coverage obtained from a mixture of unconjugated PF-127 and PF-127-lysozyme conjugates were more bactericidal than brushes realized only from PF-127-lysozyme conjugates [78].
Bifunctional brushes adopting a bottle-like conformation were further designed and developed from a block copolymer obtained through the conjugation of antimicrobial polyhexanide (PHMB) with allyloxy PEG of both low and higher molecular weight. APEG1200-PHMB and APEG2400–PHMB copolymers assembled into 25 nm-thick bottlebrush nanostructures when grafted, via surface-initiated polymerization, from silicone rubber surfaces [79]. Nanostructures assembled from both copolymers showed excellent antimicrobial properties against Gram-negative and Gram-positive bacteria, with the emphasis that the APEG2400–PHMB coating exhibited improved antiadhesive properties, most probably due to more abundant PEG units incorporated in its chemical structure [79]. A similar strategy was used to synthesize block copolymers with the same amount of PEG units but attached to an antimicrobial polyhexamethylene guanidine (PHMG) block [80]. As expected, the nanostructures obtained by grafting these block copolymers from silicone surfaces are bifunctional 20 nm thick bottlebrushes displaying a “crinkled” morphology. In this case, too, the APEG2400–PHMG coating could inhibit the adsorption of proteins and kill bacteria more efficiently than its counterpart (Figure 4) [80].
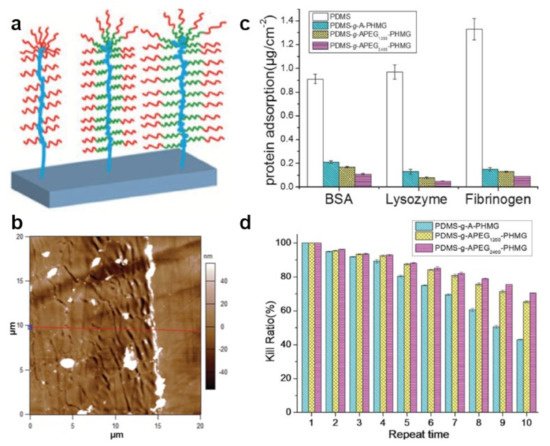
Figure 4. (a) Schematics of the APEG2400–PHMG bottlebrushes grafted from a polymer-covered substrate. (b) AFM topography image depicts the surface morphology of the APEG2400–PHMG coating (on the left side) and of pristine PDMS (on the right side). The red line corresponds to a height cross-section used to evaluate surface roughness. (c,d) Protein adsorption (c) and long-term reusable antibacterial properties (d) of pristine PDMS and of PDMS surfaces coated with allyl terminated PHMG or with APEG–PHMG. The antibacterial properties were tested against P. aeruginosa. Adapted with permission from ref. [80].
Antiadhesive PEG can further be used, along with a cationic PC combined either with tethering or with an adhesive functional block, to synthesize V- and S-shaped triblock copolymers by placing the tethering block centrally or at the end, respectively [81]. While the surfaces coated with V-shaped polymer exhibited antibacterial properties but without being able to prevent microbial adhesion, the surfaces coated with S-shaped polymer exhibited strong antibacterial and antiadhesive attributes [81]. In comparison, linear PEG-b-PC diblock copolymers were also reported to exhibit both antiadhesive and antimicrobial properties when grafted onto PDA-covered silicone rubber surfaces [82]. Furthermore, if PC is replaced with cationic antimicrobial polypeptides, PEG-b-polypeptide diblock amphiphilic polymer chains with both antimicrobial and antiadhesive segments can be obtained via ring-opening polymerization (ROP) of N-carboxyanhydrides [83]. These polymers can be then grafted onto the PDMS surface via surface-induced polymerization to form bottlebrush nanostructures able to repel and kill microbes, such as E. coli, P. aeruginosa or S. aureus.
An efficient approach to combine antiadhesive and antimicrobial (polymeric) entities is based on the LbL deposition technique. For example, as synthesized hydantoinyl acrylamide-co-trimethyl-2-methacryloxyethylammonium chloride and hydantoinyl acrylamide-co-2-acrylamido-2-methyl-1-propanesulfonic acid polyelectrolytes can be deposited one layer at a time onto polypropylene (PP) fabrics either as single or as multilayers [84]. Resulting copolymer-based layered structures, when embedded into dilute sodium hypochlorite solution, can reduce microbes by about 6 logs within the first two minutes of contact [84]. To increase the stability of multilayered polyelectrolytes, it looks more appealing to “click” the antiadhesive polymer with another antimicrobial polymer. For instance, Yang and coworkers combined antiadhesive azido-functionalized polyethylene glycol methyl ether methacrylate-based (PEGMA) polymer chains with antimicrobial alkynyl-functionalized 2-(methacryloyloxy)ethyl trimethyl ammonium chloride-based (PMETA) polymer system via a click-based LbL technique [33]. Practically, by repetitive deposition of a layer of the antiadhesive polymer on top of a layer of the antimicrobial polymer, multilayered polymeric coatings were obtained. These coatings were demonstrated to be not only resistant to bacterial adhesion but also bactericidal to marine microorganisms. One advantage of such multilayered structures is given by their tunable antimicrobial efficiency that depends on the number of polymer layers [33]. Interestingly, AFM studies conducted on the topography of such polymeric films revealed that the surface roughness decreased by almost 100% when increasing the number of polymer bilayers from 1 to 11, indicating a more compact coating structure for the thicker films [33].
Coatings with compact structures simultaneously exhibiting antiadhesive and antimicrobial properties can also be obtained when spin casting, on top of PP/PP-graft-maleic anhydride hot-pressed coupons, branched PEI and styrene-maleic anhydride (SMA) copolymer in a PEI/SMA/PEI configuration [85]. The resulting structure exhibiting pores of about 100 nm in diameters (Figure 5a) was formed from hydrophobic styrene subunits, cationic primary amine groups with intrinsic antimicrobial properties and chlorinated N-halamine-based groups exhibiting enhanced antimicrobial attributes (Figure 5b). Experiments have revealed no evidence of E. coli adhesion on the PEI/SMA/PEI-coated surface [85].
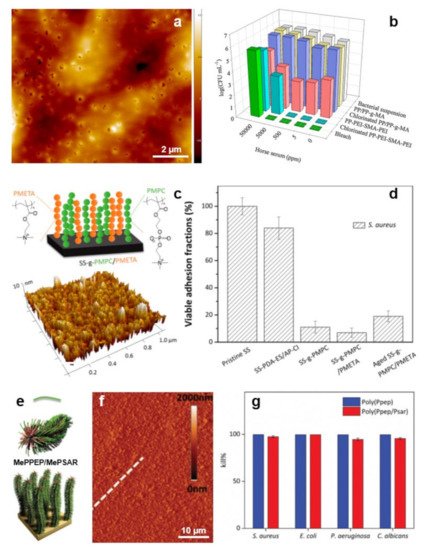
Figure 5. (a) AFM topography image depicts the morphology of the as-prepared PEI/SMA/PEI surface. (b) Antimicrobial properties of non-chlorinated and chlorinated PEI/SMA/PEI systems with respect to their analogs. (c) Schematic representation (top) and surface morphology, as revealed by AFM (bottom), of the PMPC/a-PMETA binary polymer brushes. (d) Percentage of the adhered S. aureus cells on the pristine and PMPC/a-PMETA surfaces compared to their analogs before and after aging, after exposure to the bacterial suspension in artificial seawater for 4 h. (e,f) Schematics (e) and AFM topography image (f) depict MePPEP/MePSAR binary brushes on a solid surface. White broken line in (f) was used to estimate the film roughness. (g) Contact bactericidal activity of the MePPEP and MePPEP/MePSAR coatings against various microbes. Adapted with permission from ref. [85] (a,b), ref. [86] (c,d) and ref. [87] (e–g).
Often, the bilayer/multilayer film configurations are unable to allow full percolation on the top surface of both the antiadhesive and antimicrobial polymer components. This inconvenience can be avoided by using peculiar grafting techniques. Here, polymer systems with antiadhesive properties can be grafted from a brush-like substrate displaying antimicrobial properties [88], or both antiadhesive and antimicrobial polymer components can be grafted from the same substrate, leading to surfaces comprised of the mixed brush “forests” [86]. The first approach can deliver bifunctional materials comprised of antiadhesive poly(oxonorbornene)-based zwitterions grafted onto the brush-like polymer network of antimicrobial cationic poly(oxonorbornene). The resulting structures are 30–40 nm thick while displaying a roughness of 3–4 nm. Their morphology consists of 5 nm deep pores randomly and homogeneously distributed over the whole surface [88] (more details on how polyzwitterions can be grafted onto a carpet of polycationic antimicrobial polymers can be found elsewhere [89]). The second approach, based on specific surface modifications, favors the assembly of zwitterionic poly(2-methacryloyloxyethyl phosphorylcholine) (PMPC) and alkynyl-modified cationic poly(2-(methacryloyloxy) ethyl trimethylammonium chloride) (a-PMETA) binary polymer brushes onto PDA-anchored stainless steel surfaces through thiol-ene and azide-alkyne graft polymerizations, respectively (Figure 5c). Resulting PMPC/a-PMETA binary polymer brushes not only display a smooth surface with a roughness of ~1.2 nm but are also highly stable in seawater. More important, they endow the stainless steel surfaces with antiadhesive and antimicrobial attributes, especially against Gram-positive S. aureus (Figure 5d) and Gram-negative Pseudomonas sp. bacteria [86].
Antimicrobial a-PMETA can also be coupled with antiadhesive alkyne-functionalized poly(N-hydroxyethyl acrylamide) (a-PHEAA) and then further grafted from stainless steel to obtain bifunctional brushes able to fight against Gram-negative E. coli and Gram-positive S. epidermidis [90]. Similarly, antiadhesive methacrylate-ended polysarcosine (MePSAR) and antimicrobial cationic methacrylate-ended polypeptides (MePPEP), synthesized via ROP of N-carboxyanhydrides, can be assembled on PDA coated substrates via grafting initiated under UV irradiation. The resulting MePPEP/MePSAR binary brushes can display, on flat surfaces, a roughness as high as 44 nm and can exhibit highly antiadhesive and antimicrobial properties against several microbes (Figure 5e–g) [87].
References
- Muñoz-Bonilla, A.; Fernández-García, M. Polymeric materials with antimicrobial activity. Prog. Polym. Sci. 2012, 37, 281–339.
- Milner, S.T.; McLeish, T.C.B. Reptation and Contour-Length Fluctuations in Melts of Linear Polymers. Phys. Rev. Lett. 1998, 81, 725–728.
- Botiz, I.; Grozev, N.; Schlaad, H.; Reiter, G. The influence of protic non-solvents present in the environment on structure formation of poly(γ-benzyl-L-glutamate in organic solvents. Soft Matter. 2008, 4, 993–1002.
- Jahanshahi, K.; Botiz, I.; Reiter, R.; Scherer, H.; Reiter, G. Reversible Nucleation, Growth, and Dissolution of Poly(γ-benzyl l-glutamate) Hexagonal Columnar Liquid Crystals by Addition and Removal of a Nonsolvent. Cryst. Growth Des. 2013, 13, 4490–4494.
- Strobl, G.; Cho, T.Y. Growth kinetics of polymer crystals in bulk. Eur. Phys. J. E 2007, 23, 55–65.
- Botiz, I.; Codescu, M.-A.; Farcau, C.; Leordean, C.; Astilean, S.; Silva, C.; Stingelin, N. Convective self-assembly of π-conjugated oligomers and polymers. J. Mater. Chem. C 2017, 5, 2513–2518.
- Rahimi, K.; Botiz, I.; Stingelin, N.; Kayunkid, N.; Sommer, M.; Koch, F.P.V.; Nguyen, H.; Coulembier, O.; Dubois, P.; Brinkmann, M.; et al. Controllable Processes for Generating Large Single Crystals of Poly(3-hexylthiophene). Angew. Chem. Int. Ed. 2012, 51, 11131–11135.
- Jahanshahi, K.; Botiz, I.; Reiter, R.; Thomann, R.; Heck, B.; Shokri, R.; Stille, W.; Reiter, G. Crystallization of Poly(γ-benzyl L-glutamate) in Thin Film Solutions: Structure and Pattern Formation. Macromolecules 2013, 46, 1470–1476.
- Botiz, I.; Schlaad, H.; Reiter, G. Processes of Ordered Structure Formation in Polypeptide Thin Film Solutions. In Advances in Polymer Science: Self-Organized Nanostructures of Amphiphilic Block Copolymers; Springer: Berlin/Heidelberg, Germany, 2011; Volume 242, pp. 117–149.
- Marsh, H.S.; Reid, O.G.; Barnes, G.; Heeney, M.; Stingelin, N.; Rumbles, G. Control of polythiophene film microstructure and charge carrier dynamics through crystallization temperature. J. Polym. Sci. B Polym. Phys. 2014, 52, 700–707.
- Botiz, I.; Freyberg, P.; Leordean, C.; Gabudean, A.-M.; Astilean, S.; Yang, A.C.-M.; Stingelin, N. Emission properties of MEH-PPV in thin films simultaneously illuminated and annealed at different temperatures. Synth. Met. 2015, 199, 33–36.
- Babel, A.; Zhu, Y.; Cheng, K.-F.; Chen, W.-C.; Jenekhe, S.A. High Electron Mobility and Ambipolar Charge Transport in Binary Blends of Donor and Acceptor Conjugated Polymers. Adv. Funct. Mater. 2007, 17, 2542–2549.
- Alam, M.M.; Tonzola, C.J.; Jenekhe, S.A. Nanophase-Separated Blends of Acceptor and Donor Conjugated Polymers. Efficient Electroluminescence from Binary Polyquinoline/Poly(2-methoxy-5-(2‘-ethylhexyloxy)-1,4-phenylenevinylene) and Polyquinoline/Poly(3-octylthiophene) Blends. Macromolecules 2003, 36, 6577–6587.
- Todor-Boer, O.; Petrovai, I.; Tarcan, R.; David, L.; Astilean, S.; Botiz, I. Control of microstructure in polymer: Fullerene active films by convective self-assembly. Thin Solid Films 2020, 697, 137780.
- Todor-Boer, O.; Petrovai, I.; Tarcan, R.; Vulpoi, A.; David, L.; Astilean, S.; Botiz, I. Enhancing Photoluminescence Quenching in Donor–Acceptor PCE11:PPCBMB Films through the Optimization of Film Microstructure. Nanomaterials 2019, 9, 1757.
- Kukula, H.; Schlaad, H.; Tauer, K. Linear and Star-Shaped Polystyrene-block-poly(sodium glutamate)s as Emulsifiers in the Heterophase Polymerization of Styrene. Macromolecules 2002, 35, 2538–2544.
- Arora, A.; Mishra, A. Antibacterial Polymers—A Mini Review. Mater. Today Proc. 2018, 5, 17156–17161.
- Lam, S.J.; Wong, E.H.H.; Boyer, C.; Qiao, G.G. Antimicrobial polymeric nanoparticles. Prog. Polym. Sci. 2018, 76, 40–64.
- Hisey, B.; Ragogna, P.J.; Gillies, E.R. Phosphonium-Functionalized Polymer Micelles with Intrinsic Antibacterial Activity. Biomacromolecules 2017, 18, 914–923.
- Ayres Cacciatore, F.; Dalmás, M.; Maders, C.; Ataíde Isaía, H.; Brandelli, A.; da Silva Malheiros, P. Carvacrol encapsulation into nanostructures: Characterization and antimicrobial activity against foodborne pathogens adhered to stainless steel. Food Res. Int. 2020, 133, 109143.
- Rahman, M.A.; Jui, M.S.; Bam, M.; Cha, Y.; Luat, E.; Alabresm, A.; Nagarkatti, M.; Decho, A.W.; Tang, C. Facial Amphiphilicity-Induced Polymer Nanostructures for Antimicrobial Applications. ACS Appl. Mater. Interfaces 2020, 12, 21221–21230.
- Su, Y.-R.; Yu, S.-H.; Chao, A.-C.; Wu, J.-Y.; Lin, Y.-F.; Lu, K.-Y.; Mi, F.-L. Preparation and properties of pH-responsive, self-assembled colloidal nanoparticles from guanidine-containing polypeptide and chitosan for antibiotic delivery. Colloids Surf. A Physicochem. Eng. Asp. 2016, 494, 9–20.
- Hou, Z.; Shankar, Y.V.; Liu, Y.; Ding, F.; Subramanion, J.L.; Ravikumar, V.; Zamudio-Vázquez, R.; Keogh, D.; Lim, H.; Tay, M.Y.F.; et al. Nanoparticles of Short Cationic Peptidopolysaccharide Self-Assembled by Hydrogen Bonding with Antibacterial Effect against Multidrug-Resistant Bacteria. ACS Appl. Mater. Interfaces 2017, 9, 38288–38303.
- Roosjen, A.; van der Mei, H.C.; Busscher, H.J.; Norde, W. Microbial Adhesion to Poly(ethylene oxide) Brushes: Influence of Polymer Chain Length and Temperature. Langmuir 2004, 20, 10949–10955.
- Kim, S.; Gim, T.; Jeong, Y.; Ryu, J.H.; Kang, S.M. Facile Construction of Robust Multilayered PEG Films on Polydopamine-Coated Solid Substrates for Marine Antifouling Applications. ACS Appl. Mater. Interfaces 2018, 10, 7626–7631.
- Şimşek, M.; Aldemir, S.D.; Gümüşderelioğlu, M. Anticellular PEO coatings on titanium surfaces by sequential electrospinning and crosslinking processes. Emerg. Mater. 2019, 2, 169–179.
- Zhang, Z.; Finlay, J.A.; Wang, L.; Gao, Y.; Callow, J.A.; Callow, M.E.; Jiang, S. Polysulfobetaine-Grafted Surfaces as Environmentally Benign Ultralow Fouling Marine Coatings. Langmuir 2009, 25, 13516–13521.
- Li, J.; Kleintschek, T.; Rieder, A.; Cheng, Y.; Baumbach, T.; Obst, U.; Schwartz, T.; Levkin, P.A. Hydrophobic Liquid-Infused Porous Polymer Surfaces for Antibacterial Applications. ACS Appl. Mater. Interfaces 2013, 5, 6704–6711.
- He, T.; Jańczewski, D.; Jana, S.; Parthiban, A.; Guo, S.; Zhu, X.; Lee, S.S.-C.; Parra-Velandia, F.J.; Teo, S.L.-M.; Vancso, G.J. Efficient and robust coatings using poly(2-methyl-2-oxazoline) and its copolymers for marine and bacterial fouling prevention. J. Polym. Sci. A Polym. Chem. 2016, 54, 275–283.
- Han, H.; Wu, J.; Avery, C.W.; Mizutani, M.; Jiang, X.; Kamigaito, M.; Chen, Z.; Xi, C.; Kuroda, K. Immobilization of Amphiphilic Polycations by Catechol Functionality for Antimicrobial Coatings. Langmuir 2011, 27, 4010–4019.
- Dhende, V.P.; Samanta, S.; Jones, D.M.; Hardin, I.R.; Locklin, J. One-Step Photochemical Synthesis of Permanent, Nonleaching, Ultrathin Antimicrobial Coatings for Textiles and Plastics. ACS Appl. Mater. Interfaces 2011, 3, 2830–2837.
- Liu, Y.; Li, J.; Cheng, X.; Ren, X.; Huang, T.S. Self-assembled antibacterial coating by N-halamine polyelectrolytes on a cellulose substrate. J. Mater. Chem. B 2015, 3, 1446–1454.
- Yang, W.J.; Pranantyo, D.; Neoh, K.-G.; Kang, E.-T.; Teo, S.L.-M.; Rittschof, D. Layer-by-Layer Click Deposition of Functional Polymer Coatings for Combating Marine Biofouling. Biomacromolecules 2012, 13, 2769–2780.
- Tejero, R.; Gutiérrez, B.; López, D.; López-Fabal, F.; Gómez-Garcés, J.L.; Muñoz-Bonilla, A.; Fernández-García, M. Tailoring Macromolecular Structure of Cationic Polymers towards Efficient Contact Active Antimicrobial Surfaces. Polymers 2018, 10, 241.
- Turalija, M.; Bischof, S.; Budimir, A.; Gaan, S. Antimicrobial PLA films from environment friendly additives. Compos. Part B Eng. 2016, 102, 94–99.
- Demchenko, V.; Riabov, S.; Rybalchenko, N.; Goncharenko, L.; Kobylinskyi, S.; Shtompel, V. X-ray study of structural formation, thermomechanical and antimicrobial properties of copper-containing polymer nanocomposites obtained by the thermal reduction method. Eur. Polym. J. 2017, 96, 326–336.
- Mural, P.K.S.; Banerjee, A.; Rana, M.S.; Shukla, A.; Padmanabhan, B.; Bhadra, S.; Madras, G.; Bose, S. Polyolefin based antibacterial membranes derived from PE/PEO blends compatibilized with amine terminated graphene oxide and maleated PE. J. Mater. Chem. A 2014, 2, 17635–17648.
- Zander, Z.K.; Becker, M.L. Antimicrobial and Antifouling Strategies for Polymeric Medical Devices. ACS Macro Lett. 2018, 7, 16–25.
- Kazemzadeh-Narbat, M.; Cheng, H.; Chabok, R.; Alvarez, M.M.; de la Fuente-Nunez, C.; Phillips, K.S.; Khademhosseini, A. Strategies for antimicrobial peptide coatings on medical devices: A review and regulatory science perspective. Crit. Rev. Biotechnol. 2021, 41, 94–120.
- Chamsaz, E.A.; Mankoci, S.; Barton, H.A.; Joy, A. Nontoxic Cationic Coumarin Polyester Coatings Prevent Pseudomonas aeruginosa Biofilm Formation. ACS Appl. Mater. Interfaces 2017, 9, 6704–6711.
- Zuniga, J.M.; Cortes, A. The role of additive manufacturing and antimicrobial polymers in the COVID-19 pandemic. Expert Rev. Med. Devices 2020, 17, 477–481.
- Neoh, K.G.; Kang, E.T. Combating Bacterial Colonization on Metals via Polymer Coatings: Relevance to Marine and Medical Applications. ACS Appl. Mater. Interfaces 2011, 3, 2808–2819.
- Grozev, N.; Botiz, I.; Reiter, G. Morphological instabilities of polymer crystals. Eur. Phys. J. E 2008, 27, 63–71.
- Darko, C.; Botiz, I.; Reiter, G.; Breiby, D.W.; Andreasen, J.W.; Roth, S.V.; Smilgies, D.M.; Metwalli, E.; Papadakis, C.M. Crystallization in diblock copolymer thin films at different degrees of supercooling. Phys. Rev. E 2009, 79, 041802.
- Sheth, S.R.; Leckband, D. Measurements of attractive forces between proteins and end-grafted poly(ethylene glycol) chains. Proc. Natl. Acad. Sci. USA 1997, 94, 8399–8404.
- Jeong, J.-H.; Hong, S.W.; Hong, S.; Yook, S.; Jung, Y.; Park, J.-B.; Khue, C.D.; Im, B.-H.; Seo, J.; Lee, H.; et al. Surface camouflage of pancreatic islets using 6-arm-PEG-catechol in combined therapy with tacrolimus and anti-CD154 monoclonal antibody for xenotransplantation. Biomaterials 2011, 32, 7961–7970.
- Ladd, J.; Zhang, Z.; Chen, S.; Hower, J.C.; Jiang, S. Zwitterionic Polymers Exhibiting High Resistance to Nonspecific Protein Adsorption from Human Serum and Plasma. Biomacromolecules 2008, 9, 1357–1361.
- Shen, N.; Cheng, E.; Whitley, J.W.; Horne, R.R.; Leigh, B.; Xu, L.; Jones, B.D.; Guymon, C.A.; Hansen, M.R. Photograftable Zwitterionic Coatings Prevent Staphylococcus aureus and Staphylococcus epidermidis Adhesion to PDMS Surfaces. ACS Appl. Bio Mater. 2021.
- Pranantyo, D.; Xu, L.Q.; Neoh, K.-G.; Kang, E.-T.; Ng, Y.X.; Teo, S.L.-M. Tea Stains-Inspired Initiator Primer for Surface Grafting of Antifouling and Antimicrobial Polymer Brush Coatings. Biomacromolecules 2015, 16, 723–732.
- Li, Y.; Giesbers, M.; Gerth, M.; Zuilhof, H. Generic Top-Functionalization of Patterned Antifouling Zwitterionic Polymers on Indium Tin Oxide. Langmuir 2012, 28, 12509–12517.
- Cho, W.K.; Kong, B.; Park, H.J.; Kim, J.; Chegal, W.; Choi, J.S.; Choi, I.S. Long-term stability of cell micropatterns on poly((3-(methacryloylamino)propyl)-dimethyl(3-sulfopropyl)ammonium hydroxide)-patterned silicon oxide surfaces. Biomaterials 2010, 31, 9565–9574.
- Kim, S.; Kwak, S.; Lee, S.; Cho, W.K.; Lee, J.K.; Kang, S.M. One-step functionalization of zwitterionic poly[(3-(methacryloylamino)propyl)dimethyl(3-sulfopropyl)ammonium hydroxide] surfaces by metal–polyphenol coating. Chem. Commun. 2015, 51, 5340–5342.
- Oh, Y.J.; Khan, E.S.; Campo, A.D.; Hinterdorfer, P.; Li, B. Nanoscale Characteristics and Antimicrobial Properties of (SI-ATRP)-Seeded Polymer Brush Surfaces. ACS Appl. Mater. Interfaces 2019, 11, 29312–29319.
- Gao, G.; Yu, K.; Kindrachuk, J.; Brooks, D.E.; Hancock, R.E.W.; Kizhakkedathu, J.N. Antibacterial Surfaces Based on Polymer Brushes: Investigation on the Influence of Brush Properties on Antimicrobial Peptide Immobilization and Antimicrobial Activity. Biomacromolecules 2011, 12, 3715–3727.
- Gao, G.; Lange, D.; Hilpert, K.; Kindrachuk, J.; Zou, Y.; Cheng, J.T.J.; Kazemzadeh-Narbat, M.; Yu, K.; Wang, R.; Straus, S.K.; et al. The biocompatibility and biofilm resistance of implant coatings based on hydrophilic polymer brushes conjugated with antimicrobial peptides. Biomaterials 2011, 32, 3899–3909.
- Bakhshi, H.; Yeganeh, H.; Mehdipour-Ataei, S.; Shokrgozar, M.A.; Yari, A.; Saeedi-Eslami, S.N. Synthesis and characterization of antibacterial polyurethane coatings from quaternary ammonium salts functionalized soybean oil based polyols. Mater. Sci. Eng. C 2013, 33, 153–164.
- Sun, H.; Hong, Y.; Xi, Y.; Zou, Y.; Gao, J.; Du, J. Synthesis, Self-Assembly, and Biomedical Applications of Antimicrobial Peptide–Polymer Conjugates. Biomacromolecules 2018, 19, 1701–1720.
- Sinclair, K.D.; Pham, T.X.; Farnsworth, R.W.; Williams, D.L.; Loc-Carrillo, C.; Horne, L.A.; Ingebretsen, S.H.; Bloebaum, R.D. Development of a broad spectrum polymer-released antimicrobial coating for the prevention of resistant strain bacterial infections. J. Biomed. Mater. Res. A 2012, 100A, 2732–2738.
- Yu, K.; Lo, J.C.Y.; Mei, Y.; Haney, E.F.; Siren, E.; Kalathottukaren, M.T.; Hancock, R.E.W.; Lange, D.; Kizhakkedathu, J.N. Toward Infection-Resistant Surfaces: Achieving High Antimicrobial Peptide Potency by Modulating the Functionality of Polymer Brush and Peptide. ACS Appl. Mater. Interfaces 2015, 7, 28591–28605.
- Xiong, M.; Lee, M.W.; Mansbach, R.A.; Song, Z.; Bao, Y.; Peek, R.M.; Yao, C.; Chen, L.-F.; Ferguson, A.L.; Wong, G.C.L.; et al. Helical antimicrobial polypeptides with radial amphiphilicity. Proc. Natl. Acad. Sci. USA 2015, 112, 13155–13160.
- Luppi, L.; Babut, T.; Petit, E.; Rolland, M.; Quemener, D.; Soussan, L.; Moradi, M.A.; Semsarilar, M. Antimicrobial polylysine decorated nano-structures prepared through polymerization induced self-assembly (PISA). Polym. Chem. 2019, 10, 336–344.
- Majumdar, P.; Lee, E.; Patel, N.; Stafslien, S.J.; Daniels, J.; Chisholm, B.J. Development of environmentally friendly, antifouling coatings based on tethered quaternary ammonium salts in a crosslinked polydimethylsiloxane matrix. J. Coat. Technol. Res. 2008, 5, 405–417.
- Zhao, J.; Ma, L.; Millians, W.; Wu, T.; Ming, W. Dual-Functional Antifogging/Antimicrobial Polymer Coating. ACS Appl. Mater. Interfaces 2016, 8, 8737–8742.
- Sanches, L.M.; Petri, D.F.S.; de Melo Carrasco, L.D.; Carmona-Ribeiro, A.M. The antimicrobial activity of free and immobilized poly (diallyldimethylammonium) chloride in nanoparticles of poly (methylmethacrylate). J. Nanobiotechnol. 2015, 13, 58.
- Galvão, C.N.; Sanches, L.M.; Mathiazzi, B.I.; Ribeiro, R.T.; Petri, D.F.S.; Carmona-Ribeiro, A.M. Antimicrobial Coatings from Hybrid Nanoparticles of Biocompatible and Antimicrobial Polymers. Int. J. Mol. Sci. 2018, 19, 2965.
- Lin, J.; Qiu, S.; Lewis, K.; Klibanov, A.M. Bactericidal Properties of Flat Surfaces and Nanoparticles Derivatized with Alkylated Polyethylenimines. Biotechnol. Prog. 2002, 18, 1082–1086.
- Gibney, K.A.; Sovadinova, I.; Lopez, A.I.; Urban, M.; Ridgway, Z.; Caputo, G.A.; Kuroda, K. Poly(ethylene imine)s as Antimicrobial Agents with Selective Activity. Macromol. Biosci. 2012, 12, 1279–1289.
- Alfei, S.; Schito, A.M. Positively Charged Polymers as Promising Devices against Multidrug Resistant Gram-Negative Bacteria: A Review. Polymers 2020, 12, 1195.
- Alfei, S.; Piatti, G.; Caviglia, D.; Schito, A.M. Synthesis, Characterization, and Bactericidal Activity of a 4-Ammoniumbuthylstyrene-Based Random Copolymer. Polymers 2021, 13, 1140.
- Zhou, W.; Begum, S.; Wang, Z.; Krolla, P.; Wagner, D.; Bräse, S.; Wöll, C.; Tsotsalas, M. High Antimicrobial Activity of Metal–Organic Framework-Templated Porphyrin Polymer Thin Films. ACS Appl. Mater. Interfaces 2018, 10, 1528–1533.
- Hui, F.; Debiemme-Chouvy, C. Antimicrobial N-Halamine Polymers and Coatings: A Review of Their Synthesis, Characterization, and Applications. Biomacromolecules 2013, 14, 585–601.
- Jang, J.; Kim, Y. Fabrication of monodisperse silica–polymer core–shell nanoparticles with excellent antimicrobial efficacy. Chem. Commun. 2008, 4016–4018.
- Dong, A.; Huang, J.; Lan, S.; Wang, T.; Xiao, L.; Wang, W.; Zhao, T.; Zheng, X.; Liu, F.; Gao, G.; et al. Synthesis ofN-halamine-functionalized silica–polymer core–shell nanoparticles and their enhanced antibacterial activity. Nanotechnology 2011, 22, 295602.
- Eknoian, M.W.; Worley, S.D. New N-Halamine Biocidal Polymers. J. Bioact. Compat. Polym. 1998, 13, 303–314.
- Chen, Y.; Han, Q. Designing N-halamine based antibacterial surface on polymers: Fabrication, characterization, and biocidal functions. Appl. Surf. Sci. 2011, 257, 6034–6039.
- Wang, Y.; Li, L.; Liu, Y.; Ren, X.; Liang, J. Antibacterial mesoporous molecular sieves modified with polymeric N-halamine. Mater. Sci. Eng. C 2016, 69, 1075–1080.
- Nautiyal, A.; Ren, M.Q.T.; Huang, T.-S.; Zhang, X.; Cook, J.; Bozack, M.J.; Farag, R. High-performance Engineered Conducting Polymer Film towards Antimicrobial/Anticorrosion Applications. Eng. Sci. 2018, 4, 70–78.
- Muszanska, A.K.; Busscher, H.J.; Herrmann, A.; van der Mei, H.C.; Norde, W. Pluronic–lysozyme conjugates as anti-adhesive and antibacterial bifunctional polymers for surface coating. Biomaterials 2011, 32, 6333–6341.
- Zhi, Z.; Su, Y.; Xi, Y.; Tian, L.; Xu, M.; Wang, Q.; Pandidan, S.; Li, P.; Huang, W. Dual-Functional Polyethylene Glycol-b-polyhexanide Surface Coating with in Vitro and in Vivo Antimicrobial and Antifouling Activities. ACS Appl. Mater. Interfaces 2017, 9, 10383–10397.
- Su, Y.; Zhi, Z.; Gao, Q.; Xie, M.; Yu, M.; Lei, B.; Li, P.; Ma, P.X. Autoclaving-Derived Surface Coating with In Vitro and In Vivo Antimicrobial and Antibiofilm Efficacies. Adv. Healthc. Mater. 2017, 6, 1601173.
- Voo, Z.X.; Khan, M.; Narayanan, K.; Seah, D.; Hedrick, J.L.; Yang, Y.Y. Antimicrobial/Antifouling Polycarbonate Coatings: Role of Block Copolymer Architecture. Macromolecules 2015, 48, 1055–1064.
- Ding, X.; Yang, C.; Lim, T.P.; Hsu, L.Y.; Engler, A.C.; Hedrick, J.L.; Yang, Y.-Y. Antibacterial and antifouling catheter coatings using surface grafted PEG-b-cationic polycarbonate diblock copolymers. Biomaterials 2012, 33, 6593–6603.
- Gao, Q.; Yu, M.; Su, Y.; Xie, M.; Zhao, X.; Li, P.; Ma, P.X. Rationally designed dual functional block copolymers for bottlebrush-like coatings: In vitro and in vivo antimicrobial, antibiofilm, and antifouling properties. Acta Biomater. 2017, 51, 112–124.
- Cerkez, I.; Worley, S.D.; Broughton, R.M.; Huang, T.S. Antimicrobial surface coatings for polypropylene nonwoven fabrics. React. Funct. Polym. 2013, 73, 1412–1419.
- Bastarrachea, L.J.; Goddard, J.M. Self-healing antimicrobial polymer coating with efficacy in the presence of organic matter. Appl. Surf. Sci. 2016, 378, 479–488.
- Xu, G.; Liu, P.; Pranantyo, D.; Xu, L.; Neoh, K.-G.; Kang, E.-T. Antifouling and Antimicrobial Coatings from Zwitterionic and Cationic Binary Polymer Brushes Assembled via “Click” Reactions. Ind. Eng. Chem. Res. 2017, 56, 14479–14488.
- Gao, Q.; Li, P.; Zhao, H.; Chen, Y.; Jiang, L.; Ma, P.X. Methacrylate-ended polypeptides and polypeptoids for antimicrobial and antifouling coatings. Polym. Chem. 2017, 8, 6386–6397.
- Zou, P.; Hartleb, W.; Lienkamp, K. It takes walls and knights to defend a castle—Synthesis of surface coatings from antimicrobial and antibiofouling polymers. J. Mater. Chem. 2012, 22, 19579–19589.
- Hartleb, W.; Saar, J.S.; Zou, P.; Lienkamp, K. Just Antimicrobial is not Enough: Toward Bifunctional Polymer Surfaces with Dual Antimicrobial and Protein-Repellent Functionality. Macromol. Chem. Phys. 2016, 217, 225–231.
- Yang, W.J.; Cai, T.; Neoh, K.-G.; Kang, E.-T.; Teo, S.L.-M.; Rittschof, D. Barnacle Cement as Surface Anchor for “Clicking” of Antifouling and Antimicrobial Polymer Brushes on Stainless Steel. Biomacromolecules 2013, 14, 2041–2051.
More
Information
Subjects:
Polymer Science
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.2K
Revisions:
2 times
(View History)
Update Date:
28 May 2021
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




