
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Ali Demirci | + 2548 word(s) | 2548 | 2021-05-27 11:11:01 | | | |
| 2 | Camila Xu | Meta information modification | 2548 | 2021-05-28 03:53:19 | | |
Video Upload Options
Lignin is the most abundant material, and it can be used to produce value-added products such as lignin-modifying enzymes (LMEs), polyhydroxyalkanoates (PHAs), microbial lipids, vanillin, muconic acid, and many others.
1. Introduction
The massive consumption of fossil fuels and their limited availability presented humankind with the ever-growing problems of decreasing environmental quality and an increasing need for sustainability and energy equality. Therefore, alternative energy resources such as lignocellulosic biomass are being analyzed for their potential to meet the increasing demands of energy stability and equity. Many developed countries are trying to set forth the legislation to utilize lignocellulosic biomass. The U.S. Renewable Fuels Standard (RFS) plans to produce at least 16 billion gallons of biofuels from lignocellulosic biomass by 2022 [1]. On the other hand, European Union is trying to increase the total number of biorefineries. All such efforts entail increases in lignin production, and thus, the need for utilization of lignin in a more environmentally friendly manner [1].
Lignocellulosic biomass is one of the most abundant renewable resources on this planet. The three main components of this biomass are cellulose, hemicellulose, and lignin [2]. Lignin is the second most abundant organic material after cellulose [3]. Currently, 50–70 million metric tons of lignin are produced worldwide every year [4]. The most common sources of industrial lignin are the pulp and paper industries. The renewable fuel standard (RFS) program mandated that at least 60 billion gallons of biofuels should be produced, and this amount requires at least 0.75 billion tons of lignocellulosic biomass. Therefore, the conversion of lignocellulosic biomass will generate at least 0.225 billion tons of lignin as a byproduct [4].
Lignin provides necessary structural integrity and mechanical strength to the plant [5]; however, this material is also seen as the barrier to the effective usage of lignocellulosic biomass in different industrial sectors. Lignin is made from three different monomer alcohols: coniferyl alcohol, sinapyl alcohol, and coumaryl alcohol (as illustrated in Figure 1). The cellulose and hemicellulose components of lignocellulosic biomass are already in use in value-added products including bioethanol. Cellulose and hemicellulose are polymers of sugar monomers that can be fermented by the microbial species to make bioethanol. However, the natural structure of lignocellulosic biomass is so complex and versatile not only because of the plant species, but also the different formation mechanisms and structural arrangements of cellulose, hemicellulose, and lignin [6][7]. For example, the lignin component in softwood can be up to 25–30% (wt/wt), and it is usually formed by the chemical polymerization and oxidative coupling of coniferyl alcohol, one of the three main building blocks of lignin. On the other hand, hardwood species contain lower amounts (20–25%) of lignin, with variable proportions of both coniferyl alcohol and sinapyl alcohol [6][7].
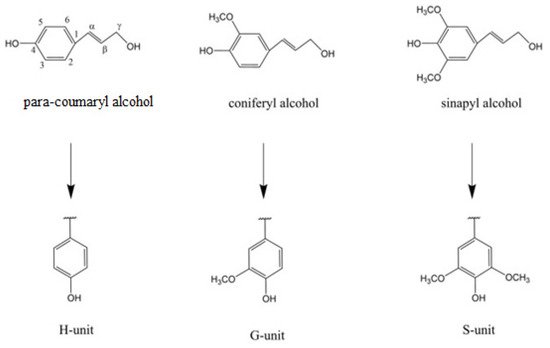
Figure 1. Structure of three lignin monomer alcohols. Numbers represent position of carbon in benzene ring while Greek letters represent distance of carbon atoms in side chain from ring C1 [2].
In most modern refineries, the main desired components are cellulose and hemicellulose, while lignin is considered a byproduct or waste material that ends up as low-quality burning fuel [8]. In the plant cell wall, three components are chemically linked to a complex web in which lignin is predominantly linked to hemicellulose, which is also diverse in its basic chemical composition (e.g., glucomannan, xylan, etc.). Therefore, no simple extraction method is available for the separation of lignin from the other two parts of the cell wall. In modern biorefineries as well as pulp and paper industries, lignin has to be removed from the cellulose and hemicellulose, which is done with different combinations of hydrothermal, chemical, or biological methods [9]. As a result, large quantities of lignin are generated worldwide as a byproduct.
While large quantities of lignin are produced, it is considered a low-value byproduct and is burned to generate heat, which is not the best way nor the most environment-friendly usage of this important organic compound. Currently, various thermochemical techniques are being developed and used at a small scale to generate value-added products from lignin. However, these techniques are not environmentally friendly, and a large amount of energy is needed for the processing of lignin, so these methods are not ideal at an industrial scale. In recent years, a more environment-friendly and effective way of lignin utilization was proposed in the literature. Some examples of such methods are biological or enzymatic conversions of lignin into value-added products [9]. While lignin is complex and diverse in its chemical composition, various microbial species were discovered that can utilize lignin by degrading it into simpler lignin monomers. As a result, various aromatic compounds are formed, which after further microbial processing could be used in the microbial fermentation processes. Therefore, utilization of lignin as a microbial substrate (carbon source) was proposed to increase its overall industrial value.
The lignin-modifying enzymes (LMEs) are the key players in making lignin an effective carbon source for use in fermentation processes. These enzymes not only degrade lignin to various specificity and effectiveness levels, but they can also be produced using lignin as the fermentation media. LMEs also have a large number of applications in various industries. In addition to LMEs, lignin degradation can also result in the production of other value-added products such as microbial lipids, vanillin, and muconic acid. All such products have various applications in food, energy, feed, and pharmaceutical industries. However, the use of lignin as the microbial media is currently in its early stages and should be researched extensively to make it more feasible at an industrial scale.
2. Types of Industrial Lignins and their Production Chemistry
Lignin is the second most abundant organic compound in the world after cellulose [3]. However, unlike cellulose, its production and chemical nature can vary greatly depending on the industrial process used to extract it from the lignocellulosic biomass. Currently, there are several methods to produce lignin from the lignocellulosic biomass, and most are collectively comprised of an industrial process known as pulping. While the three major subtypes of pulping can be categorized into chemical, semimechanical, and mechanical pulping, most of these methods are combined to get a specific type of product [10]. Mechanical pulping is used in the low-cost paper industries. Usually, nonresinous softwoods and some types of hardwoods are used in such industries, and the resultant lignin is yellowish. The mechanical pulping does not involve any type of chemicals other than water or steam. The combination of chemical and mechanical pulping is used for hardwoods that usually give a low-quality pulp. Chemical pulping can create different types of technical lignin such as kraft lignin, soda lignin, and lignosulphonates, which are available in the paper industry in bulk amounts [11]. On the other hand, other lignin types such as hydrolysis lignin, ionic liquid lignin, and organosolv are produced in relatively lower amounts. These processes are summarized as follow:
(i) Kraft Lignin
Kraft lignin is produced from a process known as sulfate or kraft cooking process. In this process, lignin in the wood is dissolved in the aqueous solution of sodium sulfide and sodium hydroxide [11]. As a result, lignin is broken down into fragments of varying sizes, which can then be dissolved into alkaline solutions. The generated solution is a deep brown liquid, which is known as spent liquor. Approximately 80% of the world’s lignin is produced through this method and is known as kraft lignin [11]. However, only a very small proportion (~1–2%) of this lignin is used to make value-added products, while 98–99% is incinerated to produce steam and energy [12][13]. The major portion of it is used to make steam and energy by burning. A distinct feature of kraft lignin’s chemical nature is the presence of a large number of phenolic hydroxyl groups and biphenyl structures [14]. The increasingly condensed structure is dependent on the duration of cooking. The ash content of kraft lignin can be up to 30% if it is not chemically removed from the end-product.
(ii) Soda Lignin
Soda pulping is used for straws and some hardwoods; the pulping is done with soda and anthraquinone [15]. The main difference between soda lignin and kraft lignin is that soda pulping is done in a sulfur-free solution [11]. Anthraquinone is good to decrease carbohydrate degradation [16]. The applications of soda lignin are in phenolic resins, dispersants, and animal nutrients [17]. These applications need the high purity of the lignin while potential toxic compounds in the animal nutrient can be dangerous for animal health [18].
(iii) Lignosulphonates
Lignosulphonates are the byproduct of sulfite cooking and contain a large number of charged particles. The main delignification process is performed with sulfate ions [19]. After sulfonation, lignin is degraded and solubilized [11]. The lignosulphonates have numerous useful chemical compounds, such as carboxylic groups, phenolic hydroxyl groups, and sulfur-containing groups. All of them are good for colloidal and dispersing properties [19].
3. Biochemistry of Lignin Degradation
Lignin is a polymer of mainly three aromatic structures: hydroxyphenyl (H), syringyl (S), and guaiacyl (G) [20]. The relative amounts of each of these structures depend on the plant species. The formation starts with the help of an electro-abstracting enzyme such as laccase. The lignin biosynthesis is then achieved through free radical coupling [21]. Just like many other lignocellulosic components, lignin should also be depolymerized before it can be used in various industrial applications. There are two different types of lignin depolymerization techniques. The first and most common in industrial settings is thermochemical depolymerization, which is achieved at high temperatures and chemical additives, catalysts, and other compounds. The second method which is more environmentally friendly and slower than thermochemical process is biological depolymerization. Various microorganisms, mainly fungi and bacteria, are capable of degrading lignin with the help of LMEs [22].
Microbial degradation and conversion of lignin occur through a complex enzymatic system including many different types of enzymes and their intermediate and final products. These enzymes work in synergy to convert lignin into smaller molecules of a different chemical nature. Different types of C–C linkages in the phenylpropane units make lignin difficult to degrade [23]. The source, pulping process, and processing conditions can make lignin different from each other in terms of chemical structure. LMEs act by an oxidative mechanism, not by hydrolytic mechanisms. As such, they were classified into various subclasses based on their specific mechanism of action. Depending on their role in lignin degradation, the ligninolytic enzymes can be divided into two major classes: LMEs and auxiliary enzymes that assist degradation by providing necessary molecules and ions to the LMEs. Some types of LMEs are also known as ligninolytic oxidative enzymes, as they oxidize oxidative cleavage [23]. The enzymes are also differentiated according to their microbial species; both fungal and bacterial lignin-degrading enzymes are identified in the literature [24].
Various fungal species are best known for producing three different types of heme peroxidases. These are lignin peroxidase (LiP), manganese-dependent peroxidase (MnP), and versatile peroxidase (VP). A detailed description of their mechanism of action with the applications and EC numbers is given in Table 1 and Figure 2. On the other hand, phenol oxidases belong to the multicopper oxidase family, and among them, laccase is primary in terms of industrial applications [25]. The dye-decolorizing peroxidase (DyP) is a new group of heme-containing peroxidases that are present in both fungi and bacteria [23]. These enzymes are different from other heme peroxidases such as LiP, MnP, and VP. There are many different types of auxiliary enzymes that help the heme peroxidases and laccases in the complete degradation of lignin. These are known as oxidoreductases, and some examples include glyoxal oxidase, aryl alcohol oxidase (veratryl alcohol oxidase), pyranose 2-oxidase (glucose 1-oxidase), and cellobiose/quinone oxidoreductase [26].
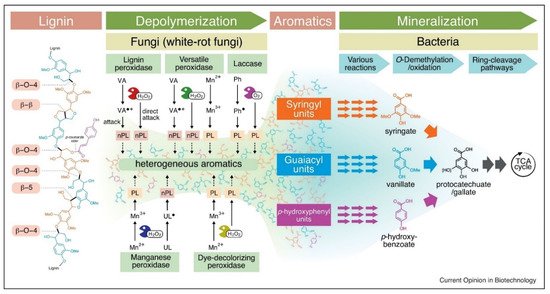
Figure 2. Different enzymatic pathways of microbial lignin degradation [27]. Copyright 2021 by Elsevier.
Table 1. Lignin-modifying enzymes (LMEs) and their applications.
| Enzyme Class | Mechanism of Action | EC Number | Applications | References |
|---|---|---|---|---|
| Lignin peroxidases (LiPs) | H2O2-dependent oxidative depolymerization of lignin | EC 1.11.1.14 | Delignification in pulp industry, bioleaching, etc. | [28] |
| Manganese peroxidases (MnPs) | Oxidation of one electron donor Mn2+ to Mn3+ and subsequent oxidation of phenolic substrates | EC 1.11.1.13 | Treatment of dye wastewater | [24] |
| Versatile peroxidases (VPs) | Combined oxidation-active sites | EC 1.11.1.16 | In combination with LiPs and MnPs | [24][29] |
| Laccases | multicopper oxidases with one electron oxidation | EC 1.10.3.2 | Food, paper, and textile industries | [24][30] |
| Heme peroxidases (DyPs) | Heme peroxidases under low pH | EC 1.11.1.19 | Degradation of anthraquinone dyes in textile industry | [24][31] |
| Oxidoreductases | glyoxal oxidase aryl alcohol oxidase pyranose 2-oxidase and many others |
EC 1.2.3.5, EC 1.1.3.7, EC 1.1.3.4, etc. |
In combination with other enzymes | [24][28] |
Bacterial lignin-degrading enzymes are not studied extensively compared to that of fungal enzymes. However, many different bacterial strains with the ability to produce LMEs were isolated from soil and other natural habitats. Among the various identified LMEs, LiPs and DyPs are reported extensively in the literature [27]. Most of the studies describe different homologs of DyPs that were found in the bacterial strains with the ability to degrade lignin in a different capacity compared to that of fungal strains. Bacterial species are usually slower at degrading lignin than fungal species. Bacterial laccases are another set of enzymes with the capacity to effectively degrade nonphenolic substrates [32]. Among various strategies to use bacterial laccases in the detoxification of dyes, the genes are cloned and overexpressed in various research setups [22][23]. The research on the isolation and characterization of different bacterial species, which can produce a high amount of LMEs, has been conducted and published in the last decade [33][34].
In the last two decades, there was evidence that fungal strains, especially white and brown rot fungi, can degrade plant cell wall components through the production of free hydroxyl ions (OH−). Firstly, hydrogen peroxide (H2O2) is produced, which assists with oxidization and production of OH-. These free radicals then attack different cell wall components along with lignin and cleave the bonds between these structures. As a result, the plant cell wall becomes more susceptible to the action of LMEs and other lignocellulolytic enzymes (as illustrated in Figure 2) [35]. There are three different pathways through which fungi create hydroxyl ions. These are Fenton reactions catalyzed by glycopeptides, quinone redox cycling, and cellobiose dehydrogenase catalyzed reactions [36].
Cellobiose dehydrogenase, or CDH, is a monomeric enzyme that helps in the oxidation of oligosaccharides in the plant cellulose and hemicellulose, thus helping the enzymes that degrade these two components. CDH helps with the production of H2O2 in the Fenton reactions, which then help the heme peroxidases to degrade lignin. CDH is known to take part in the depolymerization of all three plant cell wall components. Low molecular weight peptides are also produced by some fungal species, such as Gloeophyllum trabeum, which can help with cellulose degradation into smaller molecules by a set of oxidative reactions, such as quinones generation [37]. Similarly, glycopeptides were also found in white-rot fungi, and they assist lignin degradation by using hydroxyl ions. All these oxidative reactions create a complex cascade that ultimately helps the lignin-degrading enzymes to penetrate plant cell walls more effectively and with the hydrolysis of lignocellulosic structures [38].
The lignin degradation pathways described above are usually done by fungal and bacterial species in the natural environment where these species use plant cell wall martial for growth and survival. However, these pathways can be manipulated and adopted on an industrial scale to produce LMEs and other value-added products from technical lignin (byproducts of pulping, etc.). The microbial fermentation process after microbial lignin degradation can be used to enhance the production of such value-added products. Many research articles were published with the possibility of using biological lignin degradation pathways to produce value-added products from lignin, and thus, enhance the industrial value of lignin byproducts. Such literature reports are described in subsequent sections of this review.
References
- Chatterjee, S.; Saito, T. Solvent fractionation of lignin. In Polymer Precursor-Derived Carbon; ACS Publications: Columbus, OH, USA, 2014; pp. 153–168. ISBN 1947-5918.
- Chio, C.; Sain, M.; Qin, W. Lignin utilization: A review of lignin depolymerization from various aspects. Renew. Sustain. Energy Rev. 2019, 107, 232–249.
- Qi, Y.; Volmer, D.A. Chemical diversity of lignin degradation products revealed by matrix-optimized MALDI mass spectrometry. Anal. Bioanal. Chem. 2019, 411, 6031–6037.
- Luo, H.; Abu-Omar, M.M. Chemicals from lignin. In Encyclopedia of Sustainable Technologies; Elsevier: Amsterdam, The Netherlands, 2017; pp. 573–585.
- Tobimatsu, Y.; Schuetz, M. Lignin polymerization: How do plants manage the chemistry so well? Curr. Opin. Biotechnol. 2019, 56, 75–81.
- Christopher, L. Integrated Forest Biorefineries: Challenges and Opportunities; Royal Society of Chemistry: London, UK, 2012; ISBN 1849735069.
- Lourenço, A.; Pereira, H. Compositional variability of lignin in biomass. Lignin Trends Appl. 2018, 10, 65–98.
- Abu-Omar, M.M.; Barta, K.; Beckham, G.T.; Luterbacher, J.S.; Ralph, J.; Rinaldi, R.; Román-Leshkov, Y.; Samec, J.S.M.; Sels, B.F.; Wang, F. Guidelines for performing lignin-first biorefining. Energy Environ. Sci. 2021, 14, 262–292.
- Li, X.; Zheng, Y. Biotransformation of lignin: Mechanisms, applications and future work. Biotechnol. Prog. 2020, 36, e2922.
- Berlin, A.; Balakshin, M. Chapter 18—Industrial Lignins: Analysis, Properties, and Applications; Gupta, V.K., Tuohy, M.G., Kubicek, C.P., Saddler, J., Xu, F., Eds.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 315–336. ISBN 978-0-444-59561-4.
- Vishtal, A.G.; Kraslawski, A. Challenges in industrial applications of technical lignins. BioResources 2011, 6, 3547–3568.
- Xu, A.; Guo, X.; Zhang, Y.; Li, Z.; Wang, J. Efficient and sustainable solvents for lignin dissolution: Aqueous choline carboxylate solutions. Green Chem. 2017, 19, 4067–4073.
- Demuner, I.F.; Colodette, J.L.; Demuner, A.J.; Jardim, C.M. Biorefinery review: Wide-reaching products through kraft lignin. BioResources 2019, 14, 7543–7581.
- Sun, R. Lignin Source and Structural Characterization. ChemSusChem 2020, 13, 4385–4393.
- Domínguez-Robles, J.; Sánchez, R.; Espinosa, E.; Savy, D.; Mazzei, P.; Piccolo, A.; Rodríguez, A. Isolation and characterization of gramineae and fabaceae soda lignins. Int. J. Mol. Sci. 2017, 18, 327.
- Paananen, M.; Liitiä, T.; Sixta, H. Further insight into carbohydrate degradation and dissolution behavior during kraft cooking under elevated alkalinity without and in the presence of anthraquinone. Ind. Eng. Chem. Res. 2013, 52, 12777–12784.
- Bajwa, D.S.; Pourhashem, G.; Ullah, A.H.; Bajwa, S.G. A concise review of current lignin production, applications, products and their environmental impact. Ind. Crops Prod. 2019, 139, 111526.
- Jung, H.G.; Fahey, G.C., Jr. Nutritional implications of phenolic monomers and lignin: A review. J. Anim. Sci. 1983, 57, 206–219.
- Myrvold, B.O. A new model for the structure of lignosulphonates: Part 1. Behaviour in dilute solutions. Ind. Crops Prod. 2008, 27, 214–219.
- Jönsson, A.-S. Membranes for lignin and hemicellulose recovery in pulp mills. In Membrane Technologies for Biorefining; Elsevier: Amsterdam, The Netherlands, 2016; pp. 105–133.
- Li, X.; Zheng, Y. Lignin-enzyme interaction: Mechanism, mitigation approach, modeling, and research prospects. Biotechnol. Adv. 2017, 35, 466–489.
- Ahmad, M.; Taylor, C.R.; Pink, D.; Burton, K.; Eastwood, D.; Bending, G.D.; Bugg, T.D.H. Development of novel assays for lignin degradation: Comparative analysis of bacterial and fungal lignin degraders. Mol. Biosyst. 2010, 6, 815–821.
- Cagide, C.; Castro-Sowinski, S. Technological and biochemical features of lignin-degrading enzymes: A brief review. Environ. Sustain. 2020, 10, 1–19.
- Abdel-Hamid, A.M.; Solbiati, J.O.; Cann, I.K.O. Insights into lignin degradation and its potential industrial applications. Adv. Appl. Microbiol. 2013, 82, 1–28.
- Imran, M.; Asad, M.J.; Hadri, S.H.; Mehmood, S. Production and industrial applications of laccase enzyme. J. cell Mol. Biol. 2012, 10, 1.
- Ander, P.; Marzullo, L. Sugar oxidoreductases and veratryl alcohol oxidase as related to lignin degradation. J. Biotechnol. 1997, 53, 115–131.
- Kamimura, N.; Sakamoto, S.; Mitsuda, N.; Masai, E.; Kajita, S. Advances in microbial lignin degradation and its applications. Curr. Opin. Biotechnol. 2019, 56, 179–186.
- Pollegioni, L.; Tonin, F.; Rosini, E. Lignin-degrading enzymes. FEBS J. 2015, 282, 1190–1213.
- Knop, D.; Yarden, O.; Hadar, Y. The ligninolytic peroxidases in the genus Pleurotus: Divergence in activities, expression, and potential applications. Appl. Microbiol. Biotechnol. 2015, 99, 1025–1038.
- Riva, S. Laccases: Blue enzymes for green chemistry. TRENDS Biotechnol. 2006, 24, 219–226.
- Sugano, Y. DyP-type peroxidases comprise a novel heme peroxidase family. Cell. Mol. Life Sci. 2009, 66, 1387–1403.
- Arias, M.E.; Arenas, M.; Rodríguez, J.; Soliveri, J.; Ball, A.S.; Hernández, M. Kraft pulp biobleaching and mediated oxidation of a nonphenolic substrate by laccase from Streptomyces cyaneus CECT 3335. Appl. Environ. Microbiol. 2003, 69, 1953–1958.
- Molina-Guijarro, J.M.; Pérez Torres, J.; Muñoz-Dorado, J.; Guillén Carretero, F.; Moya Lobo, R.; Hernández Cutuli, M.; Arias Fernández, M.E. Detoxification of azo dyes by a novel pH-versatile, salt-resistant laccase from Streptomyces ipomoea. Int. Microbiol. 2009, 12, 13–21.
- Machczynski, M.C.; Vijgenboom, E.; Samyn, B.; Canters, G.W. Characterization of SLAC: A small laccase from Streptomyces coelicolor with unprecedented activity. Protein Sci. 2004, 13, 2388–2397.
- Kantharaj, P.; Boobalan, B.; Sooriamuthu, S.; Mani, R. Lignocellulose degrading enzymes from fungi and their industrial applications. Int. J. Curr. Res. Rev. 2017, 9, 1–13.
- Dashtban, M.; Schraft, H.; Syed, T.A.; Qin, W. Fungal biodegradation and enzymatic modification of lignin. Int. J. Biochem. Mol. Biol. 2010, 1, 36.
- Wang, W.; Huang, F.; Mei Lu, X.; Ji Gao, P. Lignin degradation by a novel peptide, Gt factor, from brown rot fungus Gloeophyllum trabeum. Biotechnol. J. Healthc. Nutr. Technol. 2006, 1, 447–453.
- Tanaka, H.; Itakura, S.; Enoki, A. Hydroxyl radical generation by an extracellular low-molecular-weight substance and phenol oxidase activity during wood degradation by the white-rot basidiomycete Trametes versicolor. J. Biotechnol. 1999, 75, 57–70.




