Glioblastoma (GBM) is a tumor of the brain or spinal cord with poor clinical prognosis. Current interventions, such as chemotherapy and surgical tumor resection, are constrained by tumor invasion and cancer drug resistance. Dietary natural substances are therefore evaluated for their potential as agents in GBM treatment. Various substances found in fruits, vegetables, and other natural products restrict tumor growth and induce GBM cell death. These preclinical effects are promising but remain constrained by natural substances’ varying pharmacological properties. While many of the reviewed substances are available as over-the-counter supplements, their anti-GBM efficacy should be corroborated by clinical trials moving forward.
Glioblastoma (GBM) is an aggressive, often fatal astrocyte-derived tumor of the central nervous system. Conventional medical and surgical interventions have greatly improved survival rates; however, tumor heterogeneity, invasiveness, and chemotherapeutic resistance continue to pose clinical challenges. As such, dietary natural substances—an integral component of the lifestyle medicine approach to chronic diseases—are examined as potential chemotherapeutic agents. These heterogenous substances exert anti-GBM effects by upregulating apoptosis and autophagy, inducing cell cycle arrest, interfering with tumor metabolism, and inhibiting proliferation, neuroinflammation, chemoresistance, angiogenesis, and metastasis. Although these beneficial effects are promising, natural substances’ efficacy in GBM is constrained by their bioavailability and blood–brain barrier permeability; various chemical formulations are proposed to improve their pharmacological properties. Many of the reviewed substances are available as over-the-counter dietary supplements, underscoring their viability as lifestyle interventions. However, clinical trials remain necessary to substantiate the in vitro and in vivo properties of natural substances.
1. Glioblastoma: Occurrence, Mechanisms, Treatments, and Challenges
Glioblastoma (GBM) is a malignant tumor of the central nervous system (brain or spinal cord) that arises from astrocytes. It is the most common type of primary brain tumor, with occurrence rates of 3.19 cases per 100,000 patients in the United States, and 2.05 per 100,000 in the United Kingdom
[1]. While the prognosis of GBM is often poor, two-year survival rates have improved in recent years, rising from 7% for cases diagnosed from 1993–1995 to 17% for cases diagnosed from 2005–2007 in the USA. Survival rates are also age-related: 39% of patients diagnosed between ages 20 and 44 survive, compared to only 1% of those diagnosed past age 80
[2].
While the efficacy of GBM treatment has improved, numerous challenges remain—especially concerning conventional therapeutic modalities. For instance, surgical tumor resection improves survival rates but is hindered by the extensive invasion and ill-defined tumor boundaries of GBM
[3][4]. The efficacy of chemotherapeutic drugs may be reduced by the development of (multi-)drug resistance
[5]. Moreover, extracranial metastasis—though rare—can greatly complicate treatment
[6].
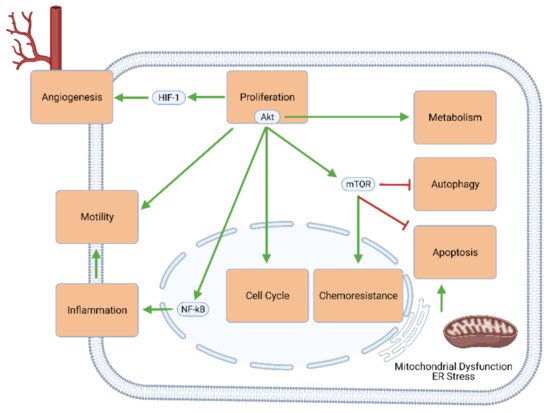
The challenges posed by GBM stem mainly from the genetic and molecular signaling pathways through which this type of tumor occurs. Genetic alterations in GBM include the amplification of the epidermal growth factor receptor (EGFR) and cyclin-dependent kinase (e.g., CDK4) genes, the deletion of the genes for cyclin-dependent kinase inhibitors (e.g., CDK2NA), and the silencing of the O-6-methylguanine-DNA methyltransferase (MGMT) gene
[7]. These and other genetic changes upregulate cellular mechanisms that favor proliferation (e.g., through Akt/mTOR signaling), cell cycle progression, excessive and self-perpetuating inflammation, tumor metastasis, angiogenesis, metabolic changes (known as the Warburg effect), and chemoresistance. Simultaneously, the effectors of apoptosis and autophagy are largely downregulated or inhibited (). As such, conventional oncologic therapies mostly aim to reverse this imbalance between growth and death by inhibiting proliferation and upregulating apoptosis.
Figure 1. Intracellular signaling mechanisms involved in GBM development and progression. Elements of proliferative signaling pathways—especially Akt and mTOR—promote angiogenesis, motility and migratory potential, neuroinflammation, cell cycle progression, chemoresistance, and tumor metabolism, and concurrently inhibit GBM cell death through apoptosis and autophagy.
The molecular complexity and difficulties posed by chronic diseases such as brain cancers have encouraged some clinicians to take a holistic approach to their treatment. Lifestyle medicine focuses on lifestyle factors (e.g., diet, physical activity, and the environment) and overall health maintenance to minimize risk factors associated with chronic diseases
[8]. Dietary natural substances are an essential component of lifestyle medicine and can suppress cancer or overcome challenges associated with conventional therapies. Intake of these compounds may occur through the daily diet or over-the-counter supplements. While in vitro studies are promising, they are yet to be tangibly replicated in clinical trials.
2. Natural Compounds Modulating Glioblastoma
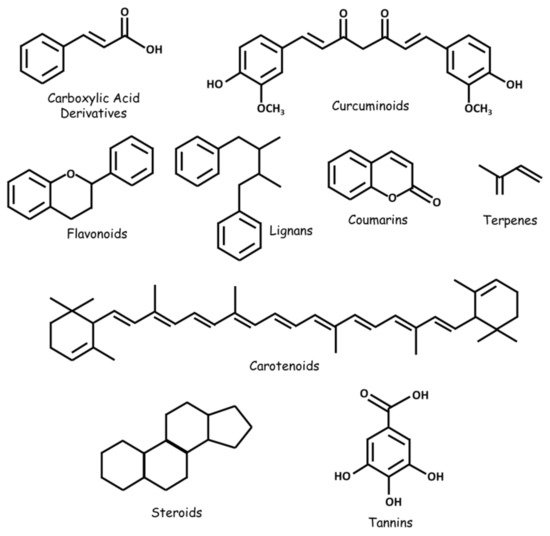
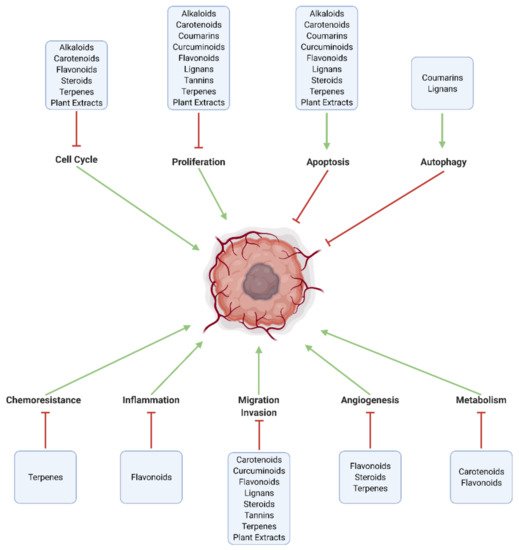
Numerous natural substances—with established biological benefits—exert oncologic effects on GBM in vitro and/or in vivo. These include alkaloids, carboxylic acid derivatives, carotenoids, flavonoids, coumarins, curcuminoids, terpenes, lignans, natural steroids, tannins, and plant extracts ( and ; ).
Figure 2. Some classes of natural substances with therapeutic potential in GBM.
Figure 3. Major pathways modulated by natural substances in GBM. Effective chemotherapeutic substances increase cell death through apoptosis and autophagy, and inhibit intracellular mechanisms related to proliferation, cell cycle progression, tumor metabolism (Warburg effect), angiogenesis, invasion and metastasis, neuroinflammation, and chemoresistance.
Table 1. Classes and sources of natural substances with anti-GBM efficacy demonstrated in recent preclinical studies. Many of the listed compounds occur in multiple natural sources.
| Substance |
Class/Type |
Primary Source(s) |
| Alkaloids |
| Berberine |
Quaternary Ammonium Salt |
Barberry (Berberis) |
| Carboxylic Acid Derivatives |
| Cinnamic Acid |
Monocarboxylic Acid |
Cinnamon (Cinnamomum) |
| Ferulic Acid |
Hydroxycinnamic Acid |
Giant fennel (Ferula communis) |
| Carotenoids |
| Adonixanthin |
Carotenone |
Derivative of astaxanthin |
| Astaxanthin |
Xanthophyll |
Chlorophyte (Haematococcus pluvialis) |
| Crocetin |
Apocarotenoid |
Saffron (Crocus sativus) |
| Coumarins |
| Galbanic Acid |
Sesquiterpene Coumarin |
Celery/carrot/parsley family (Umbelliferae) |
| Osthole |
Coumarin |
Monnier’s snowparsley (Cnidium monnieri) |
| Curcuminoids |
| Curcumin |
Curcumin |
Turmeric (Curcuma longa) |
| Flavonoids |
| Chrysin |
Dihydroxyflavone |
Blue passion flower (Passiflora caerulea) |
| Diosmin |
Flavone Glycoside |
Germander (Teucrium gnaphalodes) |
| EGCG |
Catechin |
Green tea (Camellia sinensis) |
| Galangin |
Trihydroxyflavone |
Galangal (Alpinia officinarum) |
| Matteucinol |
Dihydroxyflavonone |
Naudin (Miconia chamissois) |
| Naringin |
Flavanone Glycoside |
Grapefruit (Citrus × paradisi) |
| Quercetin |
Flavonol |
Oak (Quercetus) |
| Resveratrol |
Stilbenoid |
Grape (Vitis) |
| Rutin |
Flavonol Glycoside |
Rue (Ruta graveolens) |
| Silymarin (Silibinin) |
Flavonolignan |
Milk thistle (Silybum marianum) |
| Tectorigenin |
Methylated Isoflavone |
Leopard lily (Iris domestica) |
| Xanthohumol |
Prenylated Chalconoid |
Hops (Humulus lupulus) |
| Lignans |
| Arctigenin |
Lignan/Polyphenol |
Greater burdock (Arctium lappa) |
| Magnolol |
Biphenyl |
Houpu magnolia (Magnolia officinalis) |
| Steroids |
| Diosgenin |
Phytosteroid Sapogenin |
Fenugreek (Trigonella foenum-graecum) |
| Gamabufotalin |
Steroidal Lactone |
Toad (Bufo) |
| N45 |
Steroidal Saponin |
Nan chong lou (Paris vietnamensis) |
| Withaferin A |
Steroidal Lactone |
Ashwa-gandha (Withania somnifera) |
| Tannins |
| Tannic Acid |
Hydrolysable Tannin |
Oak (Quercetus) |
| Terpenes |
| AM01-06 |
Sesquiterpene Lactone |
Sunflower (Eremanthus spp.) |
| Betulinic Acid |
Triterpenoid |
White birch (Betula pubescens) |
| Cedrol |
Sesquiterpene Alcohol |
Cypress (Cupressus); Juniper (Juniperus) |
| Coronarin D |
Diterpene |
White ginger lily (Hedychium coronarium) |
| Eucalyptal A |
Monoterpenoid |
Southern blue gum (Eucalyptus globulus) |
| Gossypol |
Terpenoid Aldehyde |
Cotton (Gossypium) |
| Paeoniflorin |
Terpene Glycoside |
Chinese peony (Paeonia lactiflora) |
| Paris saponin H |
Triterpenoid Saponin |
Chong Lou (Rhizoma paridis) |
| Pisosterol |
Triterpene |
Dead man’s foot (Pisolithus tinctorius) |
| Rupesin E |
Iridoid (Monoterpenoid) |
Indian valerian (Valeriana jatamansi) |
| Tubeimoside-1 |
Triterpenoid Saponin |
Tu bei mu (Rhizoma bolbostemmae) |
| Crude/Purified Plant Extracts |
| BcH, BcS |
Extract-Food Supplement |
Water hyssop (Bacopa monnieri) |
| CE70, CE95 |
Ethanol Extract |
Shaggy ink cap (Coprinus comatus) |
| CP |
Chloroform Partition |
Johnnyberry (Miconia chamissois) |
| CW |
Aqueous Extract |
Shaggy ink cap (Coprinus comatus) |
| KE70, KE95 |
Ethanol Extract |
Golden chanterelle (Cantherellus cibarius) |
| KW |
Aqueous Extract |
Golden chanterelle (Cantherellus cibarius) |
| PE70, PE95 |
Ethanol Extract |
Puffball (Lycoperdon perlatum) |
| PPE |
Ethanol Extract |
Polish propolis (bee glue) |
| PW |
Aqueous Extract |
Puffball (Lycoperdon perlatum) |
| RE70, RE95 |
Ethanol Extract |
Saffron milk cap (Lactarius delicious) |
| RW |
Aqueous Extract |
Saffron milk cap (Lactarius delicious) |
| Other |
| Carnosine |
Dipeptide |
Liebig’s meat extract |
| CrataBL |
Protein: Lectin + Serine Protease Inhibitor |
Beach block (Crataeva tapia) |
| GL-PP |
Polysaccharide Peptide |
Lingzhi (Ganoderma lucidum)
|
3. Mechanistic Effects of Natural Compounds on Glioblastoma
3.1. Generalized Anti-Cancer Markers
Several generalizable effects can demonstrate the anti-GBM potential of natural compounds and highlight promising substances for further mechanistic studies (). Nearly all the substances discussed in this review decrease GBM cell viability in vitro. Cell viability assays are useful in (1) differentiating cytotoxic from biologically inert compounds and (2) identifying effective treatment concentrations to be used in further experiments. For example, decreased intracellular ATP is a marker of cell death; this effect was observed in GBM cells after treatment with curcumin, BBR, gossypol, and carnosine
[9][10][11]. Several other substances, including xanthohumol and rupesin E, decreased cloning and colony formation—further indicators of cancer cell viability and malignancy—in GBM cultures.
Table 2. Generalized downstream effects of natural compounds on GBM. Many of the reviewed substances exert measurable cytotoxic effects in vitro. Moreover, several substances reduce tumor size and improve survival in-animal models of GBM.
| Effect |
Substance |
Cell Line |
Source |
| Increases survival |
Eucalyptal A |
U87MG orthotopic implants, nude mice |
[12] |
| Cedrol |
DBTRG-05MG subcutaneous xenografts, nude mice |
[13] |
| Crocetin |
Luc-U251MG orthotopic implants, CD1 mice |
[14] |
| Decreases tumor area/perimeter |
Astaxanthin |
GL261 orthotopic implants, C57BL/6J mice |
[15] |
| Adonixanthin |
GL261 orthotopic implants, C57BL/6J mice |
[15] |
| McC1 |
U251 heterotopic xenograft, fertilized chicken eggs |
[16] |
| Decreases tumor volume |
Astaxanthin |
GL261 orthotopic implants, C57BL/6J mice |
[15] |
| Adonixanthin |
GL261 orthotopic implants, C57BL/6J mice |
[15] |
| Naringin |
U87 subcutaneous xenograft, athymic mice |
[17] |
| Xanthohumol |
U87, LN229 |
[18] |
| Tannic Acid |
C6 orthotopic implants, Wistar rats |
[19] |
| Withaferin A |
U87 subcutaneous xenografts, nude mice |
[20] |
| TBMS1 |
U87 subcutaneous xenografts, NOD/SCID mice |
[21] |
| Decreases tumor weight |
Xanthohumol |
U87, LN229 |
[18] |
| TBMS1 |
U87 subcutaneous xenografts, nude mice |
[21] |
| Increases cell death/dec. viability |
EGCG |
U251, MO59J |
[22] |
| Cinnamic Acid |
LN-229 |
[23] |
| Ferulic Acid |
LN-229 |
[23] |
| Astaxanthin |
GL261, U251MG |
[15] |
| Adonixanthin |
GL261, U251MG |
[15] |
| Cedrol |
DBTRG-05MG, RG2 |
[13] |
| AM02 |
U87MG, T98G |
[24] |
| AM04 |
U87MG, T98G |
[24] |
| AM05 |
U87MG, T98G |
[24] |
| AM06 |
U87MG, T98G |
[24] |
| Naringin |
U87 |
[17] |
| Xanthohumol |
U87, T98G, LN229 |
[18] |
| Rupesin E |
GSC-3#, GSC-12#, GSC-18# |
[25] |
| Diosmin |
U87, GBM02, GBM95 |
[26] |
| Coronarin D |
U251 |
[27] |
| CP |
GAMG, U251 |
[16] |
| McC1 |
GAMG, U251 |
[16] |
| SLCP |
U87, U251 |
[9] |
| BBR |
U87, U251 |
[9] |
| Tannic Acid |
C6 |
[19] |
| Withaferin A |
U87, U251 |
[20] |
| Betulinic Acid |
U251, LN229 |
[28] |
| TBMS1 |
U87, LN229 |
[21] |
| Carnosine |
U87, T98G |
[11] |
| CrataBL |
U87 |
[29] |
| Tectorigenin |
GBM-8401, GBM-8901 |
[30] |
| Resveratrol |
U87 |
[31] |
| Quercetin |
U87 |
[31] |
| Curcumin |
U87 |
[32] |
| Paeoniflorin |
U251, T98G |
[33] |
| Diosgenin |
C6, T98G |
[34] |
| CW |
LN-18 |
[35] |
| CE70 |
U87, LN-18 |
[35] |
| CE95 |
U87, LN-18 |
[35] |
| KW |
U87, LN-18 |
[35] |
| KE70 |
U87, LN-18 |
[35] |
| KE95 |
U87, LN-18 |
[35] |
| RW |
U87, LN-18 |
[35] |
| RE70 |
U87, LN-18 |
[35] |
| RE95 |
U87, LN-18 |
[35] |
| PW |
U87, LN-18 |
[35] |
| PE70 |
U87, LN-18 |
[35] |
| PE95 |
U87, LN-18 |
[35] |
| Silymarin |
U118 |
[36] |
| BcS |
U87, T98G, LN-18 |
[37] |
| BcH |
U87, T98G, LN-19 |
[37] |
| BBR |
U87 |
[38] |
| GL-PP |
U251 |
[39] |
| Pisosterol |
U87, U343, AHOL1, 1231N1 |
[40] |
| Decreases colony formation |
Xanthohumol |
U87, T98G, LN229 |
[18] |
| Rupesin E |
GSC-3#, GSC-18# |
[25] |
| CP |
GAMG, U251 |
[16] |
| McC1 |
U251, GAMG |
[16] |
| Tannic Acid |
C6 |
[19] |
| Decreases cloning |
Arctigenin |
U87MG, T98G |
[41] |
| AM01 |
U87MG, T98G |
[24] |
| AM02 |
U87MG, T98G |
[24] |
| AM03 |
U87MG, T98G |
[24] |
| AM04 |
U87MG, T98G |
[24] |
| AM05 |
U87MG, T98G |
[24] |
| AM06 |
U87MG, T98G |
[24] |
| TBMS1 |
U87, LN229 |
[21] |
| Decreases sphere formation |
Gossypol |
TS13-20, TS13-18 |
[10] |
| Decreases intracellular ATP |
SLCP |
U87, U251 |
[9] |
| BBR |
U87, U251 |
[9] |
| Gossypol |
Diff13-20 |
[10] |
| Carnosine |
U87, T98G |
[11] |
| Upregulates p53 (mRNA) |
Pisosterol |
U87, U343, AHOL1, 1231N1 |
[40] |
| Upregulates p53 (protein) |
BBR |
U87, U251 |
[9] |
| SLCP |
U251 |
[9] |
| Pisosterol |
U87, U343, AHOL1, 1231N1 |
[40] |
The effects of some natural substances on GBM cells in culture are replicable in vivo, underscoring their therapeutic potential. Specific terpenes, carotenoids, flavonoids, and steroids inhibit tumor growth (measured through tumor area, perimeter, weight, and volume) in murine and rat xenograft models. Interestingly, the flavonoid matteucinol also reduces the area of GBM implants in fertilized chicken eggs. These effects may improve survival rates and times in tumor-bearing animals, as is the case for eucalyptal A, cedrol, and crocetin (see ).
3.2. Proliferation, Apoptosis, and Autophagy
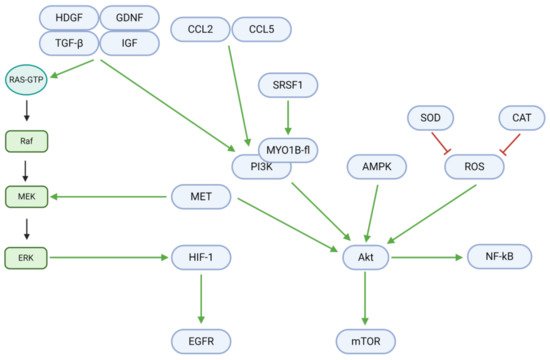
Cell fate is regulated by a delicate balance between proliferation and death. In GBM and other tumor cells, growth factors, chemokine ligands, and other upstream signals mediate a shift towards excessive growth and proliferation (; )
[42]. Growth factors, including tumor growth factor βeta (TGF-β), insulin-like growth factor (IGF), hepatoma-derived growth factor (HDGF), and glial cell-derived neurotrophic factor (GDNF), are upregulated in GBM and contribute to downstream Ras/Raf/MEK/ERK and PI3K/Akt signaling. The upregulated chemokine (C-C motif) ligands 2 (CCL2) and 5 (CCL5) further contribute to the PI3K/Akt pathway. However, the flavonoids rutin and quercetin downregulate these proliferative signals in vitro and in vivo
[43]. In the absence of natural inhibitory substances, the described growth factors and ligands bind to cell membrane receptors and activate Ras-GTP to begin the proliferative Ras/Raf/MEK/ERK pathway. In the first step, Ras-GTP activates Raf (a third degree MAPK, or MAP3K). Raf consequently activates the MAPK/ERK kinase (MEK; a second degree MAPK, or MAP2K)—an enzyme also activated by the MET proto-oncogene. MEK activates extracellular signal-regulated kinases (ERK) and their associated MAPKs in the third mechanistic step. Finally, ERK MAPKs upregulate hypoxia-inducible factor 1 αlpha (HIF-1α), whose downstream target is the epidermal growth factor receptor (EGFR). Osthole, a coumarin, may inhibit MEK activation in the second step through the downregulation of Raf
[44]. TBMS1 may have a similar inhibitory function, as it downregulates MET
[21]. Moreover, TBMS1, astaxanthin, and adonixanthin downregulate ERK/p-ERK to inhibit the final step of HIF-1α upregulation
[15][21].
Figure 4. Intracellular mechanisms promoting proliferation in GBM. Growth factors, chemokine ligands, and other upstream signals upregulate the Ras/Raf/MEK/ERK and PI3K/Akt pathways. Downstream effectors, including HIF-1, EGFR, NF-κB, and mTOR, promote DNA synthesis, transcription, and tumor cell proliferation. Proliferative effectors notably engage in crosstalk with other signals in GBM, including those for angiogenesis (HIF-1), cell cycle progression (Akt), metabolism (Akt), motility (PI3K), apoptosis (Akt/mTOR), and autophagy (Akt/mTOR/Beclin-1).
Table 3. Natural substances decrease proliferation in GBM by downregulating upstream growth factors and chemokine ligands, components of the Ras/Raf/MEK/ERK and PI3K/Akt pathways, and downstream effectors.
| Effect |
Substance |
Cell Line |
Source |
| Decreases proliferation/growth |
Rutin |
C6 |
[43] |
| Quercetin |
C6 |
[43] |
| Eucalyptal A |
U87MG, LN229 |
[12] |
| Rupesin E |
GSC-3#, GSC-18# |
[25] |
| Crocetin |
U87, U138, U251, U373 |
[14] |
| Coronarin D |
U251 |
[27] |
| SLCP |
U87, U251 |
[9] |
| BBR |
U87, U251 |
[9] |
| Tannic Acid |
C6 |
[19] |
| Gossypol |
Diff13-20, Diff13-18 |
[10] |
| Betulinic Acid |
U251, LN229 |
[28] |
| CrataBL |
U87 |
[29] |
| Galbanic Acid |
U87 |
[45] |
| N45 |
U87 |
[46] |
| Pisosterol |
U87, U343, AHOL1, 1231N1 |
[40] |
| Decreases DNA synthesis |
CE95 |
U87 |
[35] |
| CE70 |
U87, LN-18 |
[35] |
| KW |
U87, LN-18 |
[35] |
| KE95 |
U87, LN-18 |
[35] |
| KE70 |
U87, LN-18 |
[35] |
| PW |
U87 |
[35] |
| PE70 |
U87 |
[35] |
| RW |
U87, LN-18 |
[35] |
| PPE |
U87, T98G, LN-18 |
[37] |
| BcH |
U87, T98G, LN-18 |
[37] |
| Downregulates SRSF1 (mRNA) |
Eucalyptal A |
U87MG, LN229 |
[12] |
| Downregulates SRSF1 (protein) |
Eucalyptal A |
U87MG, LN229 |
[12] |
| Downregulates MYO1B-fl (protein) |
Eucalyptal A |
U87MG, LN229 |
[12] |
| Downregulates p-PDK1 (protein) |
Eucalyptal A |
U87MG, LN229 |
[12] |
| Downregulates TGF (mRNA) |
Rutin |
U251 orthotopic implants, WR |
[43] |
| Quercetin |
U251 orthotopic implants, WR |
[43] |
| Downregulates TGF-β (mRNA) |
Rutin |
C6 |
[43] |
| Quercetin |
C6 |
[43] |
| Downregulates IGF (mRNA) |
Rutin |
C6, WR-U251 orthotopic implants |
[43] |
| Quercetin |
C6, WR-U251 orthotopic implants |
[43] |
| Downregulates CCL2 (mRNA) |
Rutin |
U251 orthotopic implants, WR |
[43] |
| Quercetin |
U251 orthotopic implants, WR |
[43] |
| Upregulates CCL5 (mRNA) |
Rutin |
C6, WR-U251 orthotopic implants |
[43] |
| Quercetin |
C6, WR-U251 orthotopic implants |
[43] |
| Downregulates HDGF (mRNA) |
Rutin |
C6, WR-U251 orthotopic implants |
[43] |
| Quercetin |
C6, WR-U251 orthotopic implants |
[43] |
| Downregulates GDNF (mRNA) |
Rutin |
C6, WR-U251 orthotopic implants |
[43] |
| Quercetin |
U251 orthotopic implants, WR |
[43] |
| Downregulates PI3K (protein) |
SLCP |
U87 |
[9] |
| BBR |
U87 |
[9] |
| Diosgenin |
C6 |
[34] |
| Downregulates (p-)PI3K (protein) |
Osthole |
MOGGCCM, T98 |
[44] |
| SLCP |
U87, U251 |
[9] |
| BBR |
U87, U251 |
[9] |
| Upregulates AMPK (protein) |
Metformin |
U87 |
[47] |
| Downregulates Akt (mRNA) |
Arctigenin |
U87MG |
[41] |
| Downregulates Akt (protein) |
Cedrol |
RG2 |
[13] |
| Metformin |
U87, U251 |
[48] |
| SLCP |
U87 |
[9] |
| BBR |
U87 |
[9] |
| Downregulates p-Akt (mRNA) |
Arctigenin |
U87MG, T98G |
[41] |
| Downregulates p-Akt (protein) |
Eucalyptal A |
U87MG, LN229 |
[12] |
| Astaxanthin |
GL261 |
[15] |
| Adonixanthin |
GL261 |
[15] |
| Cedrol |
DBTRG-05MG, RG2 |
[13] |
| Arctigenin |
U87MG, T98G |
[41] |
| Xanthohumol |
U87 |
[18] |
| CP |
GAMG |
[16] |
| McC1 |
GAMG, U251 |
[16] |
| SLCP |
U87, U251 |
[9] |
| BBR |
U87, U251 |
[9] |
| Diosgenin |
C6 |
[34] |
| Downregulates mTOR (protein) |
Metformin |
U87 |
[47] |
| SLCP |
U87 |
[9] |
| BBR |
U87, U251 |
[9] |
| Downregulates p-mTOR (mRNA) |
Arctigenin |
U87MG, T98G |
[41] |
| Downregulates p-mTOR (protein) |
Arctigenin |
U87MG, T98G |
[41] |
| SLCP |
U87 |
[9] |
| BBR |
U87, U251 |
[9] |
| Diosgenin |
T98G |
[34] |
| Downregulates Raf (protein) |
Osthole |
MOGGCCM, T98 |
[44] |
| Downregulates c-Myc |
Eucalyptal A |
U87MG, LN229 |
[12] |
| Xanthohumol |
U87, T98G, LN229 |
[18] |
| SLCP |
U87 |
[9] |
| BBR |
U87 |
[9] |
| Downregulates ROS |
Astaxanthin |
GL261 |
[15] |
| Adonixanthin |
GL261 |
[15] |
| Tannic Acid |
C6 |
[19] |
| Upregulates CAT activity |
Tannic Acid |
C6 |
[19] |
| BBR |
U87 |
[38] |
| Upregulates SOD activity |
Tannic Acid |
C6 |
[19] |
| BBR |
U87 |
[38] |
| Downregulates JNK (protein) |
Cedrol |
DBTRG-05MG, RG2 |
[13] |
| Downregulates p-JNK (protein) |
Cedrol |
RG2 |
[13] |
| Downregulates p-MEK (protein) |
TBMS1 |
U87, LN229 |
[21] |
| Downregulates p-ERK (protein) |
Astaxanthin |
GL261 |
[15] |
| Adonixanthin |
GL261 |
[15] |
| TBMS1 |
LN229 |
[21] |
| Downregulates p38 (protein) |
Diosgenin |
T98G |
[34] |
| Upregulates p-p38 MAPK (protein) |
Astaxanthin |
GL261 |
[15] |
| Adonixanthin |
GL261 |
[15] |
| Downregulates HIF-1α activity |
Metformin |
U251 |
[48] |
| Downregulates NF-κB |
Diosgenin |
C6, T98G |
[34] |
| Downregulates MET (protein) |
TBMS1 |
U87, LN229 |
[21]
|
In addition to the Ras-GTP pathway, proliferation is also critically induced through Akt/mTOR and NF-κB signaling. Upstream of these targets, serine/arginine-rich splicing factor 1 (SRSF1) activates myosin 1B (MYO1B), which in turn upregulates the phosphoinositide-3-kinase (PI3K). PI3K, along with MET, adenosine monophosphate-activated protein kinase (AMPK), and reactive oxygen species (ROS), upregulates Akt, a central mediator of tumor cell proliferation. This step may be hindered by TBMS1, as it downregulates MET. Superoxide dismutase (SOD) and catalase (CAT) downregulate ROS levels and could therefore also inhibit Akt activation when upregulated by tannic acid and berberine
[19][38]. Finally, Akt activity can be reduced through the downregulation of PI3K. Eucalyptal A downregulates PI3K by inhibiting SRSF1 and MYO1B, while curcumin, osthole, diosgenin, and berberine downregulate PI3K directly
[9][12][34][44].
Downstream, Akt upregulates the mammalian target of rapamycin (mTOR) and nuclear factor κappa of activated B cells (NF-κB), which induce proliferation. However, arctigenin, curcumin, diosgenin, and berberine downregulate (p-)mTOR, while diosgenin downregulates NF-κB
[9][34][41]. Moreover, galbanic acid exerts antiproliferative, anti-metastatic, and pro-apoptotic effects via PI3K/Akt/mTOR signaling, while N45, a natural steroidal saponin, upregulates apoptosis through ROS/PI3K/Akt signaling in TMZ-resistant GBM cells
[45][46].
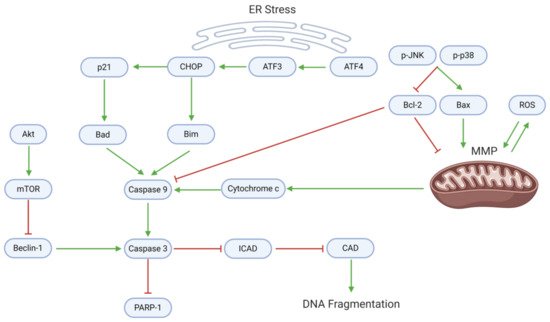
Many natural substances’ anti-cancer properties arise from the activation of cell death through apoptosis and/or autophagy. Endoplasmic reticulum (ER) stress, mitochondrial dysfunction, and downstream caspase activity mediate the apoptotic death of GBM cells (, ). Withaferin A and EGCG upregulate activating transcription factor 4 (ATF4), an upstream effector of ER stress
[20][22]. ATF4 targets ATF3, which consequently activates the C-homologous protein (CHOP). CHOP, which is also activated by withaferin A, upregulates p21 and the apoptotic proteins Bad and Bim
[20].
Figure 5. Proapoptotic mechanisms, which involve mitochondrial dysfunction, ER stress, and caspase activation, are suppressed in GBM. Dysregulation of mitochondrial homeostasis (often through oxidative imbalance) leads to the release of cytochrome c, a caspase activator. ER stress upregulates activating transcription factors; in turn, ATFs activate CHOP, p21, and proapoptotic proteins that enhance caspase activation. Active caspase 9 (along with Beclin-1) cleaves caspase 3, which enforces apoptosis and DNA fragmentation. In proliferating GBM cells, however, the anti-apoptotic protein Bcl-2 directly inhibits caspase 9, while mTOR inhibits Beclin-1.
Table 4. Natural substances increase apoptotic cell death in GBM by downregulating apoptotic inhibitors and upregulating active caspases, which cleave PARP-1 and induce DNA fragmentation.
| Effect |
Substance |
Cell Line |
Source |
| Causes apoptosis |
Arctigenin |
U87MG, T98G |
[41] |
| Osthole |
MOGGCCM, T98 |
[44] |
| Xanthohumol |
U87 |
[18] |
| Rupesin E |
GSC-3#, GSC-18# |
[25] |
| Diosmin |
GBM02, GBM95 |
[26] |
| SLCP |
U87, U251 |
[9] |
| BBR |
U87, U251 |
[9] |
| Gossypol |
TS13-20, Diff13-20 |
[10] |
| Withaferin A |
U87, U251 |
[20] |
| Tectorigenin |
GBM-8401, GBM-8901 |
[30] |
| Diosgenin |
C6, T98G |
[34] |
| Pisosterol |
U87, U343, AHOL1, 1231N1 |
[40] |
| Causes DNA fragmentation |
SLCP |
U87, U251 |
[9] |
| BBR |
U87, U251 |
[9] |
| Upregulates (c-)caspase 9 (protein) |
Cedrol |
RG2 |
[13] |
| Coronarin D |
U251 |
[27] |
| CP |
GAMG |
[16] |
| McC1 |
GAMG |
[16] |
| Withaferin A |
U87, U251 |
[20] |
| Upregulates caspase 3 (mRNA) |
Pisosterol |
U87, U343, AHOL1, 1231N1 |
[40] |
| Upregulates (c-)caspase 3 (protein) |
EGCG |
MO59J, U251 |
[22] |
| Cedrol |
DBTRG-05MG, RG2 |
[13] |
| Osthole |
T98 |
[44] |
| Xanthohumol |
U87, T98G, LN229 |
[18] |
| Rupesin E |
GSC-3#, GSC-18# |
[25] |
| Crocetin |
U87, U138, U251, U373 |
[14] |
| Diosmin |
GBM02, GBM95 |
[26] |
| Coronarin D |
U251 |
[27] |
| CP |
GAMG |
[16] |
| McC1 |
GAMG, U251 |
[16] |
| SLCP |
U87, U251 |
[9] |
| BBR |
U87, U251 |
[9] |
| Withaferin A |
U87, U251 |
[20] |
| Betulinic Acid |
U251, LN229 |
[28] |
| Resveratrol |
U87 |
[31] |
| Quercetin |
U87 |
[31] |
| GL-PP |
U251 |
[39] |
| Pisosterol |
U87, U343, AHOL1, 1231N1 |
[40] |
| Upregulates (c-)PARP (protein) |
Cedrol |
RG2 |
[13] |
| Xanthohumol |
U87 |
[18] |
| Coronarin D |
U251 |
[27] |
| CP |
U251 |
[16] |
| McC1 |
GAMG, U251 |
[16] |
| Gossypol |
TS13-20, Diff13-20 |
[10] |
| Withaferin A |
U87, U251 |
[20] |
| Downregulates PARP-1 (protein) |
Diosgenin |
C6, T98G |
[34] |
| Downregulates ICAD (protein) |
Diosgenin |
C6, T98G |
[34] |
| Upregulates Bax (protein) |
SLCP |
U87, U251 |
[9] |
| BBR |
U87, U251 |
[9] |
| Diosgenin |
C6, T98G |
[34] |
| Downregulates Bcl-2 (mRNA) |
Pisosterol |
U87, U343, AHOL1, 1231N1 |
[40] |
| Downregulates Bcl-2 (protein) |
Diosgenin |
C6, T98G |
[34] |
| Pisosterol |
U87, U343, AHOL1, 1231N1 |
[40] |
| Upregulates Bad (protein) |
Withaferin A |
U87, U251 |
[20] |
| Upregulates Bim (protein) |
Withaferin A |
U87, U251 |
[20] |
| Depolarizes MMP |
Coronarin D |
U251 |
[27] |
| CP |
U251 |
[16] |
| McC1 |
U251 |
[16] |
| Gossypol |
TS13-20 |
[10] |
| Withaferin A |
U87, U251 |
[20] |
| Upregulates ROS |
Coronarin D |
U251 |
[27] |
| SLCP |
U87, U251 |
[9] |
| BBR |
U87, U251 |
[9] |
| Upregulates cytochrome c (protein) |
SLCP |
U87, U251 |
[9] |
| BBR |
U87, U251 |
[9] |
| Upregulates GRP78 (mRNA) |
Withaferin A |
U87, U251 |
[20] |
| Upregulates GRP78 (protein) |
EGCG |
MO59J |
[22] |
| Upregulates ATF4 (mRNA) |
Withaferin A |
U87, U251 |
[20] |
| Upregulates ATF4 (protein) |
Withaferin A |
U87, U251 |
[20] |
| EGCG |
U251 |
[22] |
| Upregulates ATF6 (mRNA) |
Withaferin A |
U251 |
[20] |
| Upregulates XBP1 (mRNA) |
Withaferin A |
U87, U251 |
[20] |
| Upregulates XBP1 (protein) |
Withaferin A |
U87, U251 |
[20] |
| Upregulates CHOP (mRNA) |
Withaferin A |
U87, U251 |
[20] |
| Upregulates CHOP (protein) |
Withaferin A |
U87, U251 |
[20] |
| Upregulates Bax (protein) |
Cedrol |
DBTRG-05MG |
[13]
|
In conjunction with ER stress, several mitochondrial mechanisms promote GBM cell apoptosis. Astaxanthin and adonixanthin upregulate (p-)p38 and associated MAPKs, which upregulate the proapoptotic Bax and downregulate the antiapoptotic Bcl-2
[15]. Alterations in the Bax:Bcl-2 ratio, mediated also by curcumin, berberine, pisosterol, and diosgenin, contribute to the depolarization of the mitochondrial membrane potential (MMP)
[9][34]. Moreover, coronarin D, curcumin, and berberine upregulate intracellular ROS, further contributing to MMP depolarization
[9][27][38]. MMP depolarization leads to cytochrome c release, as seen after treatment with curcumin or berberine
[9].
Downstream, cytochrome c, Bad, and Bim promote the activation of caspase 9, which in turn activates caspase 3. A blockade of Akt/mTOR signaling mediated by arctigenin or osthole enhances the activity of Beclin-1, which supports caspase 3 activation
[41][44]. Caspase 3 specifically blocks the inhibitor of caspase-activated DNAse (ICAD), allowing CAD to cause DNA fragmentation—an effect observed after diosgenin application
[34]. Consequently, caspase 3 cleaves poly-ADP ribose polymerase 1 (PARP-1), activating apoptosis.
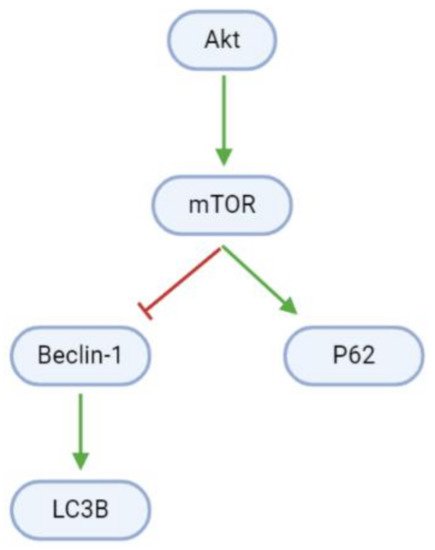
Autophagy is blocked in proliferating GBM cells by Akt/mTOR signaling (, ). However, arctigenin and osthole upregulate Beclin-1 mRNA and protein levels. Beclin-1 interestingly has dual roles in apoptosis and autophagy, and upregulates light chain 3B (LC3B), which promotes autophagosome formation
[41][44]. Moreover, arctigenin may increase autophagy through the upregulation of phosphorylated P62 (p-P62)
[41].
Figure 6. Pathways promoting cell death through autophagy are inhibited in GBM cells. mTOR inactivates the pro-autophagy Beclin-1 and upregulates the anti-autophagy P62.
Table 5. Arctigenin and osthole promote autophagy by upregulating Beclin-1 and LC3B-II and downregulating P62.
| Effect |
Substance |
Cell Line |
Source |
| Causes autophagy |
Osthole |
MOGGCCM |
[44] |
| Upregulates Beclin-1 (mRNA) |
Arctigenin |
U87MG, T98G |
[41] |
| Upregulates Beclin-1 (protein) |
Arctigenin |
U87MG, T98G |
[41] |
| Osthole |
MOGGCCM |
[44] |
| Upregulates LC3B-II (mRNA) |
Arctigenin |
U87MG, T98G |
[41] |
| Upregulates LC3B-II (protein) |
Arctigenin |
U87MG |
[41] |
| Downregulates P62 (mRNA) |
Arctigenin |
U87MG, T98G |
[41] |
| Downregulates P62 (protein) |
Arctigenin |
U87MG, T98G |
[41]
|
3.3. Cell Cycle
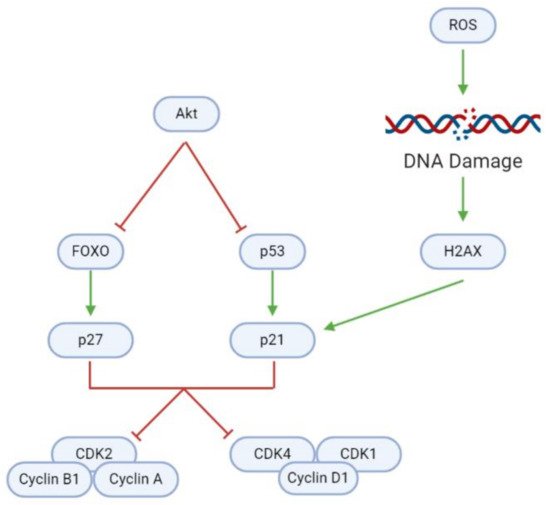
In addition to modulating cell proliferation and death, natural substances may also affect tumorigenesis through the induction of cell cycle arrest (, ). Uncontrolled cell cycle progression due to the Akt-mediated inhibition of cyclin-dependent kinase (CDK) inhibitors (CDKNs) causes rapid GBM cell division. However, a blockade of Akt activates (1) forkhead homeobox O (FOXO), which in turn activates the CDKN p27; and (2) the p53 tumor suppressor, which activates the CDKN p21. Elevated intracellular ROS levels mediate further upregulation of p21. ROS damages DNA, upregulating H2A family member X (H2AX) and consequently p21, as evidenced after Coronarin D, CP, and McC1 treatment
[16][27].
Figure 7. Inhibition of regulatory proteins allows for continuous cyclin/CDK activity and cell cycle progression in GBM cells. In healthy cells, FOXO and p53 can activate p27 and p21, respectively, and consequently induce cell cycle arrest to maintain homeostasis. DNA damage as a result of ROS accumulation is a key trigger for p21 activation. However, overactive Akt inhibits FOXO and p53, and therefore facilitates uncontrolled tumor cell growth and division.
Table 6. Natural substances induce cell cycle arrest in GBM by upregulating p53, p21, and p27, and inhibiting several cyclins and their associated CDKs.
| Effect |
Substance |
Cell Line |
Source |
| Causes G0/G1 phase cell cycle arrest |
Cedrol |
DBTRG-05MG, RG2 |
[13] |
| Coronarin D |
U251 |
[27] |
| Tannic Acid |
C6 |
[19] |
| Tectorigenin |
GBM-8401 |
[30] |
| BBR |
U87 |
[38] |
| GL-PP |
U251 |
[39] |
| Causes G2/M phase cell cycle arrest |
Eucalyptal A |
U87MG, LN229 |
[12] |
| Withaferin A |
U87, U251 |
[20] |
| TBMS1 |
U87, LN229 |
[21] |
| Pisosterol |
U87, U343, AHOL1, 1231N1 |
[40] |
| Downregulates Cyclin D1 (protein) |
Astaxanthin |
GL261 |
[15] |
| Adonixanthin |
GL261 |
[15] |
| Cedrol |
DBTRG-05MG |
[13] |
| Downregulates CDK1 (protein) |
Withaferin A |
U87, U251 |
[20] |
| TBMS1 |
U87 |
[21] |
| Downregulates CDK2 (protein) |
Cedrol |
DBTRG-05MG, RG2 |
[13] |
| Downregulates CDK4 (protein) |
Tectorigenin |
GBM-8401 |
[30] |
| Downregulates Cyclin A (protein) |
Cedrol |
DBTRG-05MG, RG2 |
[13] |
| TBMS1 |
U87, LN229 |
[21] |
| Downregulates Cyclin B1 (protein) |
Cedrol |
DBTRG-05MG, RG2 |
[13] |
| TBMS1 |
U87, LN229 |
[21] |
| Upregulates (p-)H2AX (protein) |
Coronarin D |
U251 |
[27] |
| CP |
U251 |
[16] |
| McC1 |
GAMG, U251 |
[16] |
| Downregulates (p-)RB (protein) |
Tectorigenin |
GBM-8401 |
[30] |
| Upregulates p21 (protein) |
Coronarin D |
U251 |
[27] |
| Paris saponin H |
U251 |
[49] |
| Withaferin A |
U87, U251 |
[20] |
| Tectorigenin |
GBM-8401 |
[30] |
| Upregulates p27 (protein) |
Astaxanthin |
GL261 |
[15] |
| Paris saponin H |
U251 |
[49] |
| Adonixanthin |
GL261 |
[15] |
| AM05 |
T98G |
[24] |
As CDKNs, p21 and p27 inhibit specific cyclin-CDK complexes that are necessary for cell cycle progression. Inhibition of CDK2, Cyclin A, and Cyclin B1, as seen after treatment with cedrol or TBMS1, leads to G2/M phase cell cycle arrest
[13][21]. In contrast,
Paris saponin H upregulates p21 and p27 and downregulates Cyclin D1, eventually causing G1 phase cell cycle arrest
[49]. Likewise, the inhibition of CDK1, CDK4, and Cyclin D1 by Withaferin A, TBMS1, astaxanthin, adonixanthin, and cedrol prompts G0/G1 phase arrest
[13][15][20][21].
3.4. Inflammation and Immune Cell Modulation
Neuroinflammation is an essential component of GBM tumorigenesis and interacts with various pro- and anticancer mechanisms (). Bispo da Silva et al. characterized rutin and quercetin’s pleiotropic effects on GBM-associated inflammation
[43]. These flavonoids induce the chemotaxis and activation of microglia—resident macrophages in the nervous system—as evidenced by the immune cells’ adoption of amoeboid and multipolar morphologies. Moreover, rutin and quercetin promote microglial proliferation and migration to tumor sites, where they modulate cytokine levels and thereby affect the tumor inflammatory profile. For instance, rutin and quercetin treatment upregulates interleukins 1 (IL-1), 1-βeta (IL-1β), and 18 (IL-18)—pro-inflammatory cytokines of the IL-1 family. Chemokine (C-X3-C motif) ligand 1 (CX3CL1), which promotes microglial migration, is also activated. Concurrently, interleukins 4 (IL-4), 8 (IL-8), and 10 (IL-10), which have tumorigenic properties under certain circumstances, are downregulated.
Table 7. Rutin, quercetin, and CrataBL exert pleiotropic and sometimes cell line-dependent effects on neuroinflammation.
| Effect |
Substance |
Cell Line |
Source |
| Activates microglia |
Rutin |
C6 |
[43] |
| Quercetin |
C6 |
[43] |
| Upregulates IL-1 (mRNA) |
Rutin |
U251 orthotopic implants, WR |
[43] |
| Quercetin |
U251 orthotopic implants, WR |
[43] |
| Upregulates IL-1β (mRNA) |
Rutin |
C6 |
[43] |
| Quercetin |
C6 |
[43] |
| Downregulates IL-4 (mRNA) |
Rutin |
U251 orthotopic implants, WR |
[43] |
| Quercetin |
U251 orthotopic implants, WR |
[43] |
| Upregulates IL-6 (mRNA) |
Rutin |
C6 |
[43] |
| Quercetin |
C6, TG1 |
[43] |
| Downregulates IL-6 (mRNA) |
Rutin |
U251, TG1, WR-U251 orthotopic implants |
[43] |
| Quercetin |
U251, WR-U251 orthotopic implants |
[43] |
| Downregulates IL-6 (protein) |
Rutin |
C6 |
[43] |
| CrataBL |
U87 |
[29] |
| Downregulates IL-8 (protein) |
CrataBL |
U87 |
[29] |
| Downregulates IL-10 (mRNA) |
Rutin |
C6, U251, TG1, WR-U251 orthotopic implants |
[43] |
| Quercetin |
C6, U251, TG1, WR-U251 orthotopic implants |
[43] |
| Downregulates IL-10 (protein) |
Rutin |
C6 |
[43] |
| Upregulates IL-18 (mRNA) |
Rutin |
U251 orthotopic implants, WR |
[43] |
| Quercetin |
U251 orthotopic implants, WR |
[43] |
| Upregulates TNF (mRNA) |
Rutin |
U251, TG1 |
[43] |
| Quercetin |
U251 |
[43] |
| Downregulates TNF (mRNA) |
Rutin |
U251 orthotopic implants, WR |
[43] |
| Quercetin |
U251 orthotopic implants, WR |
[43] |
| Upregulates TNF (protein) |
Rutin |
C6 |
[43] |
| Upregulates TNF-α (mRNA) |
Rutin |
C6 |
[43] |
| Quercetin |
C6 |
[43] |
| Upregulates CX3CL1 (mRNA) |
Rutin |
C6, WR-U251 orthotopic implants |
[43] |
| Quercetin |
C6 |
[43] |
| Downregulates (p-)STAT3 (protein) |
Curcumin |
U87 |
[32] |
Interestingly, the effects of natural compounds on interleukin 6 (IL-6) and tumor necrosis factor (TNF) vary between cell lines (see ). Rutin and quercetin upregulate IL-6 at the mRNA level in C6 and TG1 (quercetin only) cells. However, along with CrataBL, they downregulate IL-6 at the mRNA level in U251 and TG1 (rutin only) cells and U251 xenografts in Wistar rats. They also downregulate IL-6 at the protein level in C6 and U87 cells. Similar pleiotropic effects are observed with TNF, which is upregulated at the mRNA and protein levels in U251, C6, and TG1 cells, but downregulated at the mRNA level in U251-Wistar rat xenograft models. These varying data underscore the need for further investigation into the immuno-modulatory properties of natural substances in GBM.
3.5. Migration, Invasion, and Metastasis
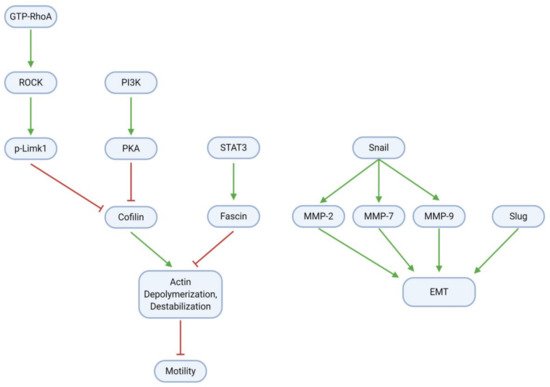
GBM cell migration, invasion, and metastasis are mainly mediated through the epithelial-mesenchymal transition (EMT) and modulation of the cytoskeletal actin framework (; ). To promote cell motility through actin, RhoA, a small GTPase, activates the Rho-associated protein kinase (ROCK); ROCK, in turn, activates the Lim kinase (Limk) through phosphorylation. Concurrently with RhoA/ROCK/Limk signaling, PI3K activates protein kinase A (PKA). Both Limk and PKA inhibit the activity of Cofilin (actin depolymerization factor), which ordinarily destabilizes cytoskeletal actin filaments and thereby impairs cell motility. Cofilin is active in the dephosphorylated form; as such, Limk and PKA may inhibit its activity through phosphorylation to produce p-Cofilin. Cofilin activity may be restored by paeoniflorin, which downregulates all components of the RhoA/ROCK/Limk pathway
[33]. Eucalyptal A may also promote Cofilin activity, as it downregulates PKA
[12]. Finally, the signal transducer and activator of transcription 3 (STAT3), a transcription factor commonly associated with inflammation, activates the actin bundling protein Fascin. Fascin acts antagonistically to Cofilin to stabilize the cytoskeleton and enhance cell motility; however, curcumin suppresses (p-)STAT3 and thereby downregulates Fascin activity
[32].
Figure 8. GBM cells gain migration and invasion abilities through EMT and modulation of the cytoskeletal actin framework. Regularization of actin filaments by STAT3/Fascin enhances cell motility; this process is reversible by Cofilin, which in tumor cells is inhibited by RhoA/ROCK/Limk and PI3K/PKA signaling. Upregulation of Snail, Slug, and MMPs further increases motility through EMT induction.
Tumor cell adhesion and motility are further influenced by EMT, a process in which tumor cells become less adhesive and more migratory, and therefore more invasive. The Snail protein is upregulated in glioblastoma and activates the matrix metalloproteinases (MMP) 2, 7, and 9, which together with Slug contribute to the EMT. Several natural compounds have anti-EMT properties in GBM. These include TBMS1 and galangin, which directly downregulate Snail (and therefore the MMPs) and Slug
[21][50]. Astaxanthin, adonixanthin, and diosgenin also downregulate MMPs; however, it remains unclear whether these effects are Snail-dependent
[15]. Moreover, magnolol suppresses GBM cell migration by regulating focal adhesions and N-cadherin, while gamabufotalin demonstrates antimetastatic effects by downregulating urokinase plasminogen activator (uPA) and carbonic anhydrase 9 (CA9) and upregulating tissue inhibitor of metalloproteinases 3 (TIMP-3)
[51][52].
Table 8. Natural substances decrease GBM cell migration and invasion by downregulating EMT modulators (Snail, Slug, and MMPs), Cofilin inhibitors (RhoA/ROCK/Limk and PKA), and actin polymerizers (STAT3/Fascin).
| Effect |
Substance |
Cell Line |
Source |
| Reduces cell migration |
Eucalyptal A |
U87MG, LN229 |
[12] |
| Astaxanthin |
GL261, U251MG |
[15] |
| Adonixanthin |
GL261, U251MG |
[15] |
| Arctigenin |
U87MG, T98G |
[41] |
| Crocetin |
U87, U251 |
[14] |
| CP |
GAMG |
[16] |
| McC1 |
U251, GAMG |
[16] |
| Tannic Acid |
C6 |
[19] |
| TBMS1 |
U87, LN229 |
[21] |
| Curcumin |
U87 |
[32] |
| Paeoniflorin |
U251, T98G |
[33] |
| Diosgenin |
C6, T98G |
[34] |
| Rutin |
C6 |
[43] |
| Magnolol |
LN229, U87MG |
[51] |
| Gamabufotalin |
U87 |
[52] |
| Quercetin |
C6 |
[43] |
| Reduces cell invasion |
Eucalyptal A |
U87MG, LN229 |
[12] |
| Arctigenin |
U87MG, T98G |
[41] |
| McC1 |
GAMG, U251 |
[16] |
| CrataBL |
U87 |
[29] |
| Curcumin |
U87 |
[32] |
| Paeoniflorin |
U251, T98G |
[33] |
| Diosgenin |
C6, T98G |
[34] |
| Downregulates MMP-2 (protein) |
Astaxanthin |
GL261 |
[15] |
| Adonixanthin |
GL261 |
[15] |
| Arctigenin |
U87MG |
[41] |
| TBMS1 |
U87, LN229 |
[21] |
| Diosgenin |
T98G |
[34] |
| Downregulates MMP-7 (protein) |
TBMS1 |
U87, LN229 |
[21] |
| Downregulates MMP-9 (protein) |
Arctigenin |
U87MG |
[41] |
| Diosgenin |
C6 |
[34] |
| Downregulates p-PKA 1/2/3 (prot.) |
Eucalyptal A |
U87MG, LN229 |
[12] |
| Downregulates p-Cofilin (protein) |
Eucalyptal A |
U87MG, LN229 |
[12] |
| Downregulates fibronectin (protein) |
Adonixanthin |
GL261 |
[15] |
| Downregulates laminin (protein) |
CrataBL |
U87 |
[29] |
| Downregulates Snail (protein) |
TBMS1 |
U87, LN229 |
[21] |
| Galangin |
U87, U251 |
[50] |
| Downregulates Snail (mRNA) |
Galangin |
U87, U251 |
[50] |
| Downregulates Slug (protein) |
TBMS1 |
U87, LN229 |
[21] |
| Downregulates Fascin (protein) |
Curcumin |
U87 |
[32] |
| Reduces actin filament number |
Paeoniflorin |
T98G, U251 |
[33] |
| Downregulates GTP-RhoA (protein) |
Paeoniflorin |
T98G, U251 |
[33] |
| Downregulates ROCK (protein) |
Paeoniflorin |
T98G, U251 |
[33] |
| Downregulates (p-)Limk1 (protein) |
Paeoniflorin |
T98G, U251 |
[33] |
3.6. Angiogenesis
Angiogenesis—the development of active blood vessels in and around tumor sites—is a key element of GBM progression (). Vascular endothelial growth factor (VEGF) mediates this process; it is upregulated by HIF-1 and downregulated by A disintegrin and metalloproteinase with thrombospondin motifs 1 (ADAMTS1). As such, substances such as
Paris saponin H that inhibit HIF-1 will consequently downregulate VEGF
[49]. The sesquiterpene lactone AM04 upregulates ADAMTS1 and thereby downregulates VEGF in U87 and T98G cells
[24].
Table 9. Natural substances reduce angiogenesis and neovascularization primarily by downregulating VEGF.
| Effect |
Substance |
Cell Line |
Source |
| Decreases angiogenesis area |
McC1 |
U251 heterotopic xenograft, fertilized chicken eggs |
[16] |
| Decreases blood vessel junctions |
McC1 |
U251 heterotopic xenograft, fertilized chicken eggs |
[16] |
| Decreases tube formation |
Diosgenin |
C6, T98G |
[34] |
| Upregulates ADAMTS1 (protein) |
AM04 |
U87MG, T98G |
[24] |
| Downregulates CD31 (mRNA) |
Naringin |
U87 subcutaneous xenograft, athymic mice |
[17] |
| Downregulates CD105 (mRNA) |
Naringin |
U87 subcutaneous xenograft, athymic mice |
[17] |
| Downregulates tumor hemoglobin |
Naringin |
U87 subcutaneous xenograft, athymic mice |
[17] |
| Downregulates VEGF (protein) |
Metformin |
U251 |
[48] |
| Paris saponin H |
U251 |
[49] |
| CrataBL |
U87 |
[29] |
| Diosgenin |
C6 |
[34] |
Reduced VEGF activity decreases tumor neovascularization; importantly, this is observable in vivo. Treatment of U87 xenografts in athymic mice with the flavonoid naringin downregulates tumor hemoglobin and the angiogenic markers cluster of differentiation 31 (CD31) and 105 (CD105)
[17]. Moreover, matteucinol decreases the angiogenic area and the number of blood vessel junctions in a U251 xenograft-fertilized chicken egg model
[16]. These in vivo effects demonstrate the potential applicability of specific natural substances as angiogenic modulators that inhibit GBM.
3.7. Metabolism
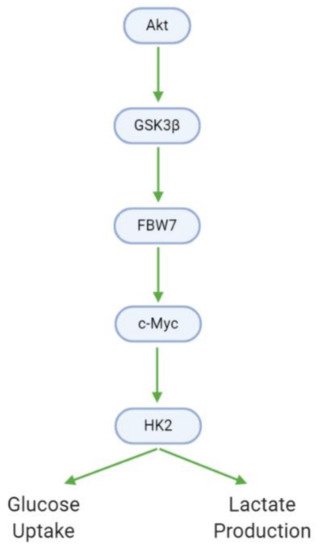
Cancer cells exhibit modified metabolic processes that meet the extensive energy demands of growth, proliferation, and metastasis—a phenomenon known as the Warburg effect
[53]. In GBM cells, Akt promotes glucose metabolism through the upregulation of glycogen synthase kinase 3 βeta (GSK3β). GSK3β, in turn, upregulates F-box and WD-40 domain-containing protein 7 (FBW7) and c-Myc, leading to the activation of hexokinase 2 (HK2). HK2 is a major metabolic enzyme that contributes to the aerobic glycolysis observed in tumor cells by increasing glucose uptake and lactate production (, ). Xanthohumol downregulates GSK3β, and as such decreases downstream HK2 activity, glucose consumption, and lactate production
[18]. In contrast, carnosine upregulates pyruvate dehydrogenase kinase 4 (PDK4), which downregulates metabolism, while crocetin downregulates fatty acid synthase (FASN), which catalyzes metabolically relevant fatty acid synthesis
[11][14].
Figure 9. GBM cells utilize altered metabolic processes (Warburg effect) characterized by increased glucose uptake and lactate generation. Akt, via GSK3β, mediates the transition between the healthy and Warburg phenotypes.
Table 10. Xanthohumol, carnosine, and crocetin interfere with key enzymes in GBM cell metabolism.
| Effect |
Substance |
Cell Line(s) |
Source |
| Downregulates HK2 (protein) |
Xanthohumol |
U87, T98G, LN229 |
[18] |
| Decreases glucose consumption |
Xanthohumol |
U87, T98G, LN229 |
[18] |
| Decreases lactate production |
Xanthohumol |
U87, T98G, LN229 |
[18] |
| Downregulates (p-)GSK3β (protein) |
Xanthohumol |
U87 |
[18] |
| Upregulates PDK4 (mRNA) |
Carnosine |
U87, T98G |
[11] |
| Downregulates FASN (protein) |
Crocetin |
U87, U138, U251, U373 |
[14] |
3.8. Chemoresistance
The effects of natural substances on GBM chemoresistance remain largely uncharacterized in the recent literature. Chang et al. reported that cedrol downregulates O6-alkylguanine DNA alkyltransferase (MGMT) at the protein level in DBTRG-05MG and RG2 cells
[13]. MGMT, a DNA repair protein, confers resistance to alkylating agents (e.g., temozolomide) by reversing guanine alkylation
[54]. Studies from 2014 moreover indicate that pine needle extract, chrysin, and quercetin sensitize GBM cells to TMZ
[55][56]. At any rate, further data are necessary to substantiate the potential of natural substances in overcoming GBM drug resistance.