
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Cesare Gargioli | + 1169 word(s) | 1169 | 2021-05-25 11:31:37 | | | |
| 2 | Vicky Zhou | Meta information modification | 1169 | 2021-05-26 04:36:45 | | | | |
| 3 | Vicky Zhou | -5 word(s) | 1164 | 2021-05-26 04:38:32 | | |
Video Upload Options
Volumetric muscle loss (VML) is the massive wasting of skeletal muscle tissue due to traumatic events or surgical ablation. This pathological condition exceeds the physiological healing process carried out by the muscle itself, which owns remarkable capacity to restore damages but only when limited in dimensions. Upon VML occurring, the affected area is severely compromised, heavily influencing the affected person’s quality of life. Overall, this condition is often associated with chronic disability, which makes the return to duty of highly specialized professional figures (e.g., military personnel or athletes) almost impossible. The actual treatment for VML is based on surgical conservative treatment followed by physical exercise; nevertheless, the results, in terms of either lost mass and/or functionality recovery, are still poor. On the other hand, the efforts of the scientific community are focusing on reconstructive therapy aiming at muscular tissue void volume replenishment by exploiting biomimetic matrix or artificial tissue implantation. Reconstructing strategies represent a valid option to build new muscular tissue not only to recover damaged muscles, but also to better socket prosthesis in terms of anchorage surfaces and reinnervation substrates for reconstructed mass.
1. Introduction
2. Emerging Reconstructive Strategies
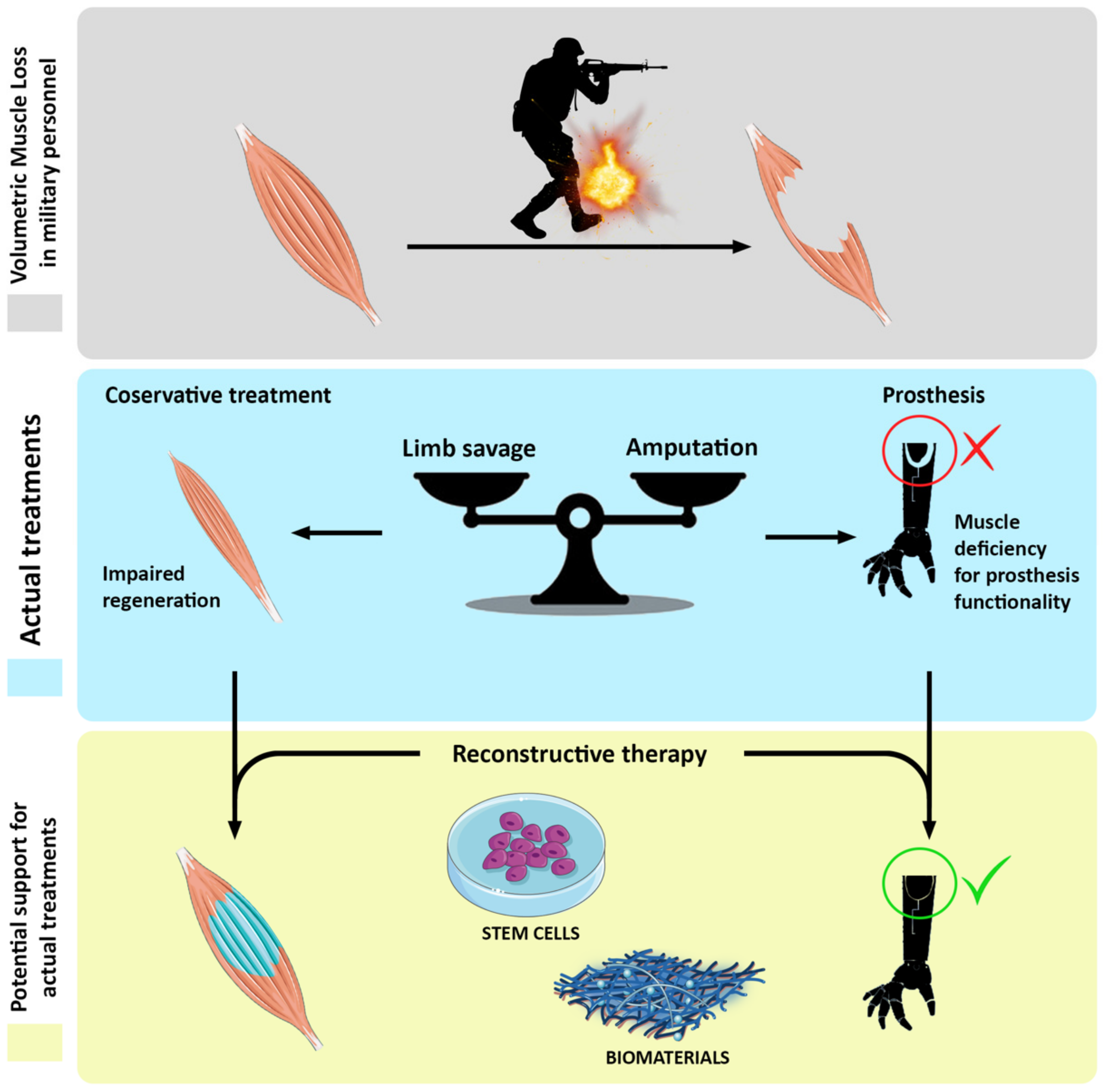 Figure 1. Schematic representation of cell-based reconstructive approach to volumetric muscle loss (VML) recovery.
Figure 1. Schematic representation of cell-based reconstructive approach to volumetric muscle loss (VML) recovery.References
- Costantini, M.; Testa, S.; Mozetic, P.; Barbetta, A.; Fuoco, C.; Fornetti, E.; Tamiro, F.; Bernardini, S.; Jaroszewicz, J.; Święszkowski, W.; et al. Microfluidic-enhanced 3D bioprinting of aligned myoblast-laden hydrogels leads to functionally organized myofibers in vitro and in vivo. Biomaterials 2017, 131, 98–110.
- Patel, K.H.; Talovic, M.; Dunn, A.J.; Patel, A.; Vendrell, S.; Schwartz, M.; Garg, K. Aligned nanofibers of decellularized muscle extracellular matrix for volumetric muscle loss. J. Biomed. Mater. Res. Part B Appl. Biomater. 2020, 108, 2528–2537.
- Corona, B.T.; Garg, K.; Ward, C.L.; McDaniel, J.S.; Walters, T.J.; Rathbone, C.R. Autologous minced muscle grafts: A tissue engineering therapy for the volumetric loss of skeletal muscle. Am. J. Physiol. Cell Physiol. 2013, 305, C761–C775.
- Corona, B.T.; Wenke, J.C.; Ward, C.L. Pathophysiology of Volumetric Muscle Loss Injury. Cells Tissues Organs 2016, 202, 180–188.
- Belmont, P.J.; McCriskin, B.J.; Hsiao, M.S.; Burks, R.; Nelson, K.J.; Schoenfeld, A.J. The Nature and Incidence of Musculoskeletal Combat Wounds in Iraq and Afghanistan (2005–2009). J. Orthop. Trauma 2013, 27, e107–e113.
- Corona, B.T.; Rivera, J.C.; Owens, J.G.; Wenke, J.C.; Rathbone, C.R. Volumetric muscle loss leads to permanent disability following extremity trauma. J. Rehabil. Res. Dev. 2015, 52, 785–792.
- Akpoto, Y.M.; Abalo, A.; Adam, S.; Sama, H.D.; Dellanh, Y.Y.; Amavi, K.A.; Bakriga, B.; Walla, A.; Dossim, A. Extremity injuries in soldiers during the conflict in Mali: Experience of Togo Level two Hospital. Int. Orthop. 2015, 39, 1895–1899.
- Forsberg, J.A.; Pepek, J.M.; Wagner, S.; Wilson, K.; Flint, J.; Andersen, R.C.; Tadaki, D.; Gage, F.A.; Stojadinovic, A.; Elster, E.A. Heterotopic Ossification in High-Energy Wartime Extremity Injuries: Prevalence and Risk Factors. J. Bone Jt. Surg. Am. Vol. 2009, 91, 1084–1091.
- Kaplan, F.S.; Glaser, D.L.; Hebela, N.; Shore, E.M. Heterotopic Ossification. J. Am. Acad. Orthop. Surg. 2004, 12, 116–125.
- Rosina, M.; Langone, F.; Giuliani, G.; Perpetuini, A.C.; Reggio, A.; Calderone, A.; Fuoco, C.; Castagnoli, L.; Gargioli, C.; Cesareni, G. Osteogenic differentiation of skeletal muscle progenitor cells is activated by the DNA damage response. Sci. Rep. 2019, 9, 5447.
- Dey, D.; Wheatley, B.M.; Cholok, D.; Agarwal, S.; Yu, P.B.; Levi, B.; Davis, T.A. The traumatic bone: Trauma-induced heterotopic ossification. Transl. Res. 2017, 186, 95–111.
- Steinberg, G.G.; Hubbard, C. Heterotopic Ossification After Femoral Intramedullary Rodding. J. Orthop. Trauma 1993, 7, 536–542.
- Potter, B.K.; Burns, T.C.; Lacap, A.P.; Granville, R.R.; Gajewski, D.A. Heterotopic Ossification Following Traumatic and Combat-Related Amputations. J. Bone Jt. Surg. Am. Vol. 2007, 89, 476–486.
- Crane, N.J.; Polfer, E.; Elster, E.A.; Potter, B.K.; Forsberg, J.A. Raman spectroscopic analysis of combat-related heterotopic ossification development. Bone 2013, 57, 335–342.
- Hoyt, B.W.; Pavey, G.J.; Potter, B.K.; Forsberg, J.A. Heterotopic ossification and lessons learned from fifteen years at war: A review of therapy, novel research, and future directions for military and civilian orthopaedic trauma. Bone 2018, 109, 3–11.
- Alfieri, K.A.; Forsberg, J.A.; Potter, B.K. Blast injuries and heterotopic ossification. Bone Jt. Res. 2012, 1, 174–179.
- Court-Brown, C.; McBirnie, J. The epidemiology of tibial fractures. J. Bone Jt. Surge. Br. Vol. 1995, 77, 417–421.
- Järvinen, T.A.H.; Järvinen, T.L.N.; Kääriäinen, M.; Kalimo, H.; Järvinen, M. Muscle Injuries. Am. J. Sports Med. 2005, 33, 745–764.
- Bosse, M.J.; MacKenzie, E.J.; Kellam, J.F.; Burgess, A.R.; Webb, L.X.; Swiontkowski, M.F.; Sanders, R.W.; Jones, A.L.; McAndrew, M.P.; Patterson, B.M.; et al. An Analysis of Outcomes of Reconstruction or Amputation after Leg-Threatening Injuries. N. Engl. J. Med. 2002, 347, 1924–1931.
- Singh, J.; Dhillon, M.S.; Dhatt, S.S. Single-stage “Fix and Flap” gives Good Outcomes in Grade 3B/C Open Tibial Fractures: A Prospective Study. Malays. Orthop. J. 2020, 14, 61–73.
- Garg, K.; Ward, C.L.; Hurtgen, B.J.; Wilken, J.M.; Stinner, D.J.; Wenke, J.C.; Owens, J.G.; Corona, B.T. Volumetric muscle loss: Persistent functional deficits beyond frank loss of tissue. J. Orthop. Res. 2014, 33, 40–46.
- Costantini et al., Biofabricating murine and human myo-substitutes for rapid volumetric muscle loss restoration 2021, doi: 10.15252/emmm.202012778
- Han, N.; Yabroudi, M.A.; Stearns-Reider, K.; Helkowski, W.; Sicari, B.M.; Rubin, J.P.; Badylak, S.F.; Boninger, M.L.; Ambrosio, F. Electrodiagnostic Evaluation of Individuals Implanted with Extracellular Matrix for the Treatment of Volumetric Muscle Injury: Case Series. Phys. Ther. 2016, 96, 540–549.
- Sarrafian, T.L.; Bodine, S.C.; Murphy, B.; Grayson, J.K.; Stover, S.M. Extracellular matrix scaffolds for treatment of large volume muscle injuries: A review. Vet. Surg. 2018, 47, 524–535.
- Sicari, B.M.; Rubin, J.P.; Dearth, C.L.; Wolf, M.T.; Ambrosio, F.; Boninger, M.; Turner, N.J.; Weber, D.J.; Simpson, T.W.; Wyse, A.; et al. An Acellular Biologic Scaffold Promotes Skeletal Muscle Formation in Mice and Humans with Volumetric Muscle Loss. Sci. Transl. Med. 2014, 6, 234ra58.
- Dziki, J.; Badylak, S.; Yabroudi, M.; Sicari, B.; Ambrosio, F.; Stearns, K.; Turner, N.; Wyse, A.; Boninger, M.L.; Brown, E.H.P.; et al. An acellular biologic scaffold treatment for volumetric muscle loss: Results of a 13-patient cohort study. NPJ Regen. Med. 2016, 1, 16008.
- Ward, C.L.; Pollot, B.E.; Goldman, S.M.; Greising, S.M.; Wenke, J.C.; Corona, B.T. Autologous Minced Muscle Grafts Improve Muscle Strength in a Porcine Model of Volumetric Muscle Loss Injury. J. Orthop. Trauma 2016, 30, e396–e403.
- Corona, B.T.; Rivera, J.C.; Wenke, J.C.; Greising, S.M. Tacrolimus as an adjunct to autologous minced muscle grafts for the repair of a volumetric muscle loss injury. J. Exp. Orthop. 2017, 4, 36.
- Novakova, S.S.; Rodriguez, B.L.; Vega-Soto, E.E.; Nutter, G.P.; Armstrong, R.E.; MacPherson, P.C.; Larkin, L.M. Repairing Volumetric Muscle Loss in the Ovine Peroneus Tertius Following a 3-Month Recovery. Tissue Eng. Part A 2020, 26, 837–851.
- Bian, W.; Bursac, N. Cellular/Tissue Engineering. IEEE Eng. Med. Boil. Mag. 2008, 27, 109–113.




