Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Soraya Mezouar | + 1327 word(s) | 1327 | 2021-05-25 04:56:53 | | | |
| 2 | Karina Chen | Meta information modification | 1327 | 2021-07-01 04:19:03 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Mezouar, S. Coxiella burnetii. Encyclopedia. Available online: https://encyclopedia.pub/entry/10042 (accessed on 08 February 2026).
Mezouar S. Coxiella burnetii. Encyclopedia. Available at: https://encyclopedia.pub/entry/10042. Accessed February 08, 2026.
Mezouar, Soraya. "Coxiella burnetii" Encyclopedia, https://encyclopedia.pub/entry/10042 (accessed February 08, 2026).
Mezouar, S. (2021, May 25). Coxiella burnetii. In Encyclopedia. https://encyclopedia.pub/entry/10042
Mezouar, Soraya. "Coxiella burnetii." Encyclopedia. Web. 25 May, 2021.
Copy Citation
Coxiella burnetii is a Gram-negative bacterium that infects numerous animals, including mammals, birds, and arthropods.
Coxiella burnetii
pregnancy
trophoblasts
macrophages
dendritic cells
mast cells
1. Natural History of C. burnetii Infection in Pregnancy
In 1958, Syrucek et al. isolated C. burnetii organisms from aborted human placentas [1]. It is now known that placental infection by C. burnetii mostly occurs during the first and second trimesters of pregnancy [2]. Women who are infected during pregnancy are at risk of miscarriage, stillbirth, pre-term delivery, low infant birth weight, and foetal death or malformations (omphalocele, hypospadias, Potter syndrome, congenital hydronephrosis, and syndactyly) and growth retardation [3][4]. It has been shown that women who contract acute Q fever just before pregnancy do not present an increased risk of abortion or premature delivery. In contrast, infection that occurs during the first trimester of pregnancy leads to abortion and during the second trimester is more likely to result in prematurity [5]. C. burnetii infection in pregnant women increases the risk of maternal chronic Q fever endocarditis [6][7]. Q fever during a first pregnancy favours spontaneous abortions of future pregnancies [8], suggesting Q fever reactivation during latter pregnancies [5]. In women, a silenced immune response and the production of antibodies have been reported in response to infection [9].
In recent decades, outbreaks of Q fever have been observed around the world, including in the United Kingdom in 1989, France in 1998, Germany in 2005, and the Netherlands in 2007 [10]. Seroprevalence studies in pregnant women show highly variable rates in areas of endemicity: 0.15% in southeastern France, 3.8% in Canada, and 4.6% in the United Kingdom. In Denmark, a seroprevalence rate of up to 47% was reported in pregnant women who were occupationally exposed to livestock versus 4.8% in unexposed women [3]. More specifically, a seroprevalence of acute Q fever of 1.2% was found among women who experienced spontaneous abortion in the first semester of pregnancy in Denmark, with a significant proportion of asymptomatic patients [11]. In France, the seroprevalence in women who experienced spontaneous abortion was 0.27% [11][12].
C. burnetii infection is particularly deleterious in goats. C. burnetii infection is responsible for metritis, abortion, stillbirth and the delivery of weak offspring, which are the most frequent clinical signs of disease [13]. The placenta of goats is very rich in bacteria, and it is often through the aerosol route that farmers are contaminated. C. burnetii organisms are present in different organs such as the liver, spleen, lung [14][15][16], kidney, heart [14][15], and muscles [15] of goat foetuses and their mothers. However, to date, the vertical transmission of infection remains unclear. Bacteria are found in the stomach of the foetus, indicating that they contaminate amniotic liquid [16][17]. Alternatively, the inhalation of contaminated aerosols during parturition or lactation may be another source of contamination [18][19].
2. Coxiella burnetii
Several C. burnetii strains isolated from animals or humans including patients with acute or persistent focalised Q fever have been used for research [20]. However, the Nine Mile strain, isolated from the Dermacentor andersoni tick in Montana in 1938, is to date the strain mostly used in host–pathogen studies [21]. The lipopolysaccharide (LPS) structure, plasmid, and genotype have been commonly related to the different disease manifestations [20][22], cytopathic effects in cell cultures [20], and immune response [23][24][25][26]. The repeated cultures of C. burnetii Nile Mile strain result in a truncated LPS (O-antigen modification), which is associated with virulence decrease. This transition from phase I LPS to phase II LPS is related to a ≈26 Kb chromosomal deletion of C. burnetii genome [27]. Despite their frequent use in studies, phase II C. burnetii are not suitable for pathophysiopathological studies.
Generally speaking, placental C. burnetii isolates appear to be more virulent than other isolates. It has been proposed that the virulence found in the different strains could be due to the variation of LPS but also to the genomic content. Correlations have been found between C. burnetii genome variations or LPS chemotype and clinical presentations of Q fever [28][29]. The C. burnetii RSA493 strain genome was the first strain sequenced in 2003, which led to significant progress in understanding of C. burnetii pathogenicity [30]. The genome contains a 1,995,275 bp chromosome and a QpH1 plasmid with 37,393 bp. C. burnetii virulence appears to be correlated with the expression of certain plasmids. Four different plasmids have been identified among C. burnetii isolates, including QpH1 (Nine Mile strain), QpRS (Priscilla strain), QpDG (wild rodents), and QpDV [23]. C. burnetii Nine Mile strain presents the plasmid QpH1, MST16, and GGI [23]. It was reported that C. burnetti Nine Mile strain with QpH1 plasmid is the cause of severe C. burnetii infection in a guinea pig model [22][24]. Moreover, QpRS and QpDG plasmids have been associated with moderate infection and lack of virulence, respectively [22][24]. Three of the plasmids identified among C. burnetii isolates have been found in placental isolates from France and Spain (QpH1, QpRS, and QpDV) [23]. Obstetric complications are likely to be related to the QpDV plasmid [23][31]. Indeed, this plasmid has been found in three of six placentas and in placental bacterial strains from abortive women [23][32]. However, the presence of the QpDV plasmid has also been reported in a healthy woman, and other placental strains of C. burnetii harbour the QpH1 plasmid (Dutch strains) [23][33][34].
Despite the links between C. burnetii genome and virulence factors, there is an urgent need to develop genomic studies to better understand the pathogenicity of this bacterium during pregnancy.
3. The Placenta, a Target Tissue for C. burnetii
The placenta is a complex tissue formed by the chorion and the decidua, corresponding to the foetal and maternal tissues, respectively [33]. Due to its intrinsic structure and plasticity during pregnancy [34], the placenta is essential for foetal growth. Chorionic villus units are composed of mesenchymal cells, decidual macrophages (maternal macrophages), Hofbauer cells (foetal macrophages), foetal vascular cells, and trophoblast cells (Figure 1). The decidua is rich in immune cells, including NK cells, macrophages, T and B lymphocytes, DCs and mast cells, with an over-representation of NK cells and macrophages [35][36].
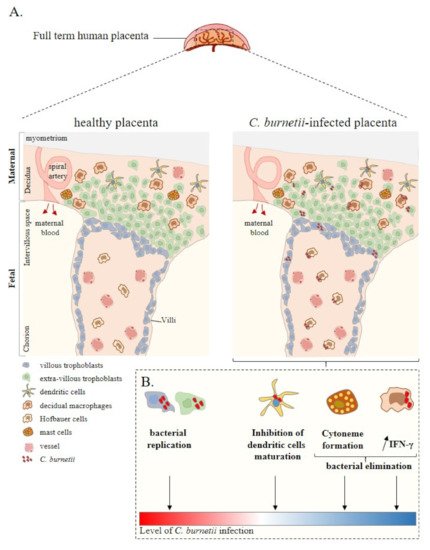
Figure 1. Placenta cell responses against C. burnetii infection. (A) Schematic representation of a full-term human placenta showing the maternal (decidua, myometrium) and foetal (intervillous space, chorion) parts. Placental cells are represented including extra-villous and villous trophoblasts and immune placental cells such as dendritic cells, macrophages (decidual and Hofbauer), and mast cells. During infection, C. burnetii was found in the placental tissue. (B) Ex vivo experiments based on in vitro infection of isolated primary cells from healthy at term human placentas reported the infectious capability of trophoblasts, dendritic cells, and macrophages by C. burnetii. In this context, although trophoblast infection leads to bacterial replication, the other cell lines present a specific anti-bacterial response promoting bacterial elimination. Thus, ex vivo experiments allow evaluating the level of C. burnetii infection at the placental level. Adapted from [37].
The examination of infected placentas from goats, cows, or mice using immunochemistry reveals the presence of C. burnetii organisms in this tissue (Table 1). A direct detection of C. burnetii using the polymerase chain reaction technique has confirmed the presence of bacteria in the placental tissue of goats, cows, ewes, calves, and mice [18][15][16][37][38][39]. The bacterium is found at the extracellular level, mainly in necrotic areas, or in placental cells through immunohistochemistry [38][39].
The anatomopathological examination of animal placentas reveals significant damage to the tissue (Table 1). Inflammatory response and some injury as a consequence are identified in C. burnetii-positive placenta from goats, ewes and cows [37][40][41]. Necrosis yellow/brownish exudates are found in C. burnetii-positive placentas from abortions and non-abortive pregnancies [14]. Necrotic lesions are observed in chorioallantoic membrane (cotyledonary and intercotyledonary), chorionic epithelium, chorionic villi, and ulcerated chorioallantoic membrane. Leucocyte infiltration is also detected within interplacentomal areas, chorionic and allantoic blood vessel walls at the base of the villi, and in allantochorionic stroma: leucocytes include neutrophils, lymphocytes, and occasional macrophages [14][15][39][40][42].
In women infected with C. burnetii, two main histological profiles are observed. In symptomatic pregnant women, the placental tissue shows necrosis, villitis, and perivillitis with abundant nuclear debris, necrosis intermixed with numerous disintegrating immune cells, neutrophils, and plasma cells in the decidua [43]. In asymptomatic pregnant women, no foci of necrosis or active inflammation are found in the placenta, but the placental tissue presents fibrotic chorion villi, loss of capillaries, stromal karyorrhexis and haemorrhages [40].
References
- Syrucek, L.; Sobeslavsky, O.; Gutvirth, I. Isolation of Coxiella Burneti from Human Placentas. J. Hyg. Epidemiol. Microbiol. Immunol. 1958, 2, 29–35.
- Melenotte, C.; Protopopescu, C.; Million, M.; Edouard, S.; Carrieri, M.P.; Eldin, C.; Angelakis, E.; Djossou, F.; Bardin, N.; Fournier, P.-E.; et al. Clinical Features and Complications of Coxiella Burnetii Infections from the French National Reference Center for Q Fever. JAMA Netw. Open 2018, 1, e181580.
- Eldin, C.; Mélenotte, C.; Mediannikov, O.; Ghigo, E.; Million, M.; Edouard, S.; Mege, J.-L.; Maurin, M.; Raoult, D. From Q Fever to Coxiella Burnetii Infection: A Paradigm Change. Clin. Microbiol. Rev. 2017, 30, 115–190.
- Ghaoui, H.; Bitam, I.; Ait-Oudhia, K.; Achour, N.; Saad-Djaballah, A.; Saadnia, F.Z.; Kedjour, S.; Fournier, P.-E. Coxiella Burnetii Infection with Women’s Febrile Spontaneous Abortion Reported in Algiers. New Microbes New Infect. 2018, 26, 8–14.
- Raoult, D.; Fenollar, F.; Stein, A. Q Fever during Pregnancy: Diagnosis, Treatment, and Follow-Up. Arch. Intern. Med. 2002, 162, 701–704.
- Ghanem-Zoubi, N.; Paul, M. Q Fever during Pregnancy: A Narrative Review. Clin. Microbiol. Infect. Off. Publ. Eur. Soc. Clin. Microbiol. Infect. Dis. 2020, 26, 864–870.
- Boden, K.; Brueckmann, A.; Wagner-Wiening, C.; Hermann, B.; Henning, K.; Junghanss, T.; Seidel, T.; Baier, M.; Straube, E.; Theegarten, D. Maternofetal Consequences of Coxiella Burnetii Infection in Pregnancy: A Case Series of Two Outbreaks. BMC Infect. Dis. 2012, 12, 359.
- Stein, A.; Raoult, D. Q Fever during Pregnancy: A Public Health Problem in Southern France. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 1998, 27, 592–596.
- Nielsen, S.Y.; Mølbak, K.; Henriksen, T.B.; Krogfelt, K.A.; Larsen, C.S.; Villumsen, S. Adverse Pregnancy Outcomes and Coxiella Burnetii Antibodies in Pregnant Women, Denmark. Emerg. Infect. Dis. 2014, 20, 925–931.
- Alemneh, T.; Melaku, A. Q Fever (Coxiellosis) in Animals and Humans. Biology 2018, 5, 4.
- Nielsen, S.Y.; Andersen, A.-M.N.; Mølbak, K.; Hjøllund, N.H.; Kantsø, B.; Krogfelt, K.A.; Henriksen, T.B. No Excess Risk of Adverse Pregnancy Outcomes among Women with Serological Markers of Previous Infection with Coxiella Burnetii: Evidence from the Danish National Birth Cohort. BMC Infect. Dis. 2013, 13, 87.
- Quijada, S.G.; Terán, B.M.; Murias, P.S.; Anitua, A.A.; Cermeño, J.L.B.; Frías, A.B. Q Fever and Spontaneous Abortion. Clin. Microbiol. Infect. 2012, 18, 533–538.
- Arricau-Bouvery, N.; Souriau, A.; Bodier, C.; Dufour, P.; Rousset, E.; Rodolakis, A. Effect of Vaccination with Phase I and Phase II Coxiella Burnetii Vaccines in Pregnant Goats. Vaccine 2005, 23, 4392–4402.
- Roest, H.-J.; van Gelderen, B.; Dinkla, A.; Frangoulidis, D.; van Zijderveld, F.; Rebel, J.; van Keulen, L. Q Fever in Pregnant Goats: Pathogenesis and Excretion of Coxiella Burnetii. PLoS ONE 2012, 7, e48949.
- Oporto, B.; Barandika, J.F.; Hurtado, A.; Aduriz, G.; Moreno, B.; Garcia-Perez, A.L. Incidence of Ovine Abortion by Coxiella Burnetii in Northern Spain. Ann. N. Y. Acad. Sci. 2006, 1078, 498–501.
- Bouvery, N.A.; Souriau, A.; Lechopier, P.; Rodolakis, A. Experimental Coxiella Burnetii Infection in Pregnant Goats: Excretion Routes. Vet. Res. 2003, 34, 423–433.
- Abinanti, F.R.; Lennette, E.H.; Winn, J.F.; Welsh, H.H. Q Fever Studies. XVIII. Presence of Coxiella Burnetii in the Birth Fluids of Naturally Infected Sheep. Am. J. Hyg. 1953, 58, 385–388.
- Stein, A.; Lepidi, H.; Mege, J.L.; Marrie, T.J.; Raoult, D. Repeated Pregnancies in BALB/c Mice Infected with Coxiella Burnetii Cause Disseminated Infection, Resulting in Stillbirth and Endocarditis. J. Infect. Dis. 2000, 181, 188–194.
- Rodolakis, A.; Berri, M.; Héchard, C.; Caudron, C.; Souriau, A.; Bodier, C.C.; Blanchard, B.; Camuset, P.; Devillechaise, P.; Natorp, J.C.; et al. Comparison of Coxiella Burnetii Shedding in Milk of Dairy Bovine, Caprine, and Ovine Herds. J. Dairy Sci. 2007, 90, 5352–5360.
- Stein, A.; Louveau, C.; Lepidi, H.; Ricci, F.; Baylac, P.; Davoust, B.; Raoult, D. Q Fever Pneumonia: Virulence of Coxiella Burnetii Pathovars in a Murine Model of Aerosol Infection. Infect. Immun. 2005, 73, 2469–2477.
- Public Health Weekly Reports for DECEMBER 30, 1938. Public Health Rep. Wash. DC 1896 1938, 53, 2259–2309.
- Long, C.M.; Beare, P.A.; Cockrell, D.C.; Larson, C.L.; Heinzen, R.A. Comparative Virulence of Diverse Coxiella Burnetii Strains. Virulence 2019, 10, 133–150.
- Glazunova, O.; Roux, V.; Freylikman, O.; Sekeyova, Z.; Fournous, G.; Tyczka, J.; Tokarevich, N.; Kovacava, E.; Marrie, T.J.; Raoult, D. Coxiella Burnetii Genotyping. Emerg. Infect. Dis. 2005, 11, 1211–1217.
- Russell-Lodrigue, K.E.; Andoh, M.; Poels, M.W.J.; Shive, H.R.; Weeks, B.R.; Zhang, G.Q.; Tersteeg, C.; Masegi, T.; Hotta, A.; Yamaguchi, T.; et al. Coxiella Burnetii Isolates Cause Genogroup-Specific Virulence in Mouse and Guinea Pig Models of Acute Q Fever. Infect. Immun. 2009, 77, 5640–5650.
- Abnave, P.; Muracciole, X.; Ghigo, E. Coxiella Burnetii Lipopolysaccharide: What Do We Know? Int. J. Mol. Sci. 2017, 18, 2509.
- Honstettre, A.; Ghigo, E.; Moynault, A.; Capo, C.; Toman, R.; Akira, S.; Takeuchi, O.; Lepidi, H.; Raoult, D.; Mege, J.-L. Lipopolysaccharide from Coxiella Burnetii Is Involved in Bacterial Phagocytosis, Filamentous Actin Reorganization, and Inflammatory Responses through Toll-like Receptor 4. J. Immunol. Baltim. Md 1950 2004, 172, 3695–3703.
- Hackstadt, T.; Peacock, M.G.; Hitchcock, P.J.; Cole, R.L. Lipopolysaccharide Variation in Coxiella Burnetti: Intrastrain Heterogeneity in Structure and Antigenicity. Infect. Immun. 1985, 48, 359–365.
- Hendrix, L.R.; Samuel, J.E.; Mallavia, L.P. Differentiation of Coxiella Burnetii Isolates by Analysis of Restriction-Endonuclease-Digested DNA Separated by SDS-PAGE. J. Gen. Microbiol. 1991, 137, 269–276.
- Hackstadt, T. Antigenic Variation in the Phase I Lipopolysaccharide of Coxiella Burnetii Isolates. Infect. Immun. 1986, 52, 337–340.
- Seshadri, R.; Paulsen, I.T.; Eisen, J.A.; Read, T.D.; Nelson, K.E.; Nelson, W.C.; Ward, N.L.; Tettelin, H.; Davidsen, T.M.; Beanan, M.J.; et al. Complete Genome Sequence of the Q-Fever Pathogen Coxiella Burnetii. Proc. Natl. Acad. Sci. USA 2003, 100, 5455–5460.
- Téllez, A.; Sanz Moreno, J.; Valkova, D.; Domingo, C.; Anda, P.; de Ory, F.; Albarrán, F.; Raoult, D. Q Fever in Pregnancy: Case Report after a 2-Year Follow-Up. J. Infect. 1998, 37, 79–81.
- Angelakis, E.; Million, M.; D’Amato, F.; Rouli, L.; Richet, H.; Stein, A.; Rolain, J.-M.; Raoult, D. Q Fever and Pregnancy: Disease, Prevention, and Strain Specificity. Eur. J. Clin. Microbiol. Infect. Dis. Off. Publ. Eur. Soc. Clin. Microbiol. 2013, 32, 361–368.
- Pinhal-Enfield, G.; Leibovich, J.; Vas, N. The Role of Macrophages in the Placenta. In Embryology—Updates and Highlights on Classic Topics; Violin Pereira, L., Ed.; InTech: London, UK, 2012; ISBN 978-953-51-0465-0.
- Mezouar, S.; Mege, J.-L. Gene Expression Profiling of Placenta from Normal to Pathological Pregnancies. In Placenta; Ahmed, R.G., Ed.; IntechOpen: London, UK, 2018; ISBN 978-1-78984-598-3.
- Hansen, M.S.; Rodolakis, A.; Cochonneau, D.; Agger, J.F.; Christoffersen, A.-B.; Jensen, T.K.; Agerholm, J.S. Coxiella Burnetii Associated Placental Lesions and Infection Level in Parturient Cows. Vet. J. Lond. Engl. 1997 2011, 190, e135–e139.
- Muskens, J.; Wouda, W.; von Bannisseht-Wijsmuller, T.; van Maanen, C. Prevalence of Coxiella Burnetii Infections in Aborted Fetuses and Stillborn Calves. Vet. Rec. 2012, 170, 260.
- Mezouar, S.; Katsogiannou, M.; Ben Amara, A.; Bretelle, F.; Mege, J.-L. Placental Macrophages: Origin, Heterogeneity, Function and Role in Pregnancy-Associated Infections. Placenta 2021, 103, 94–103.
- Bildfell, R.J.; Thomson, G.W.; Haines, D.M.; McEwen, B.J.; Smart, N. Coxiella Burnetii Infection Is Associated with Placentitis in Cases of Bovine Abortion. J. Vet. Diagn. Investig. Off. Publ. Am. Assoc. Vet. Lab. Diagn. Inc 2000, 12, 419–425.
- van Moll, P.; Baumgärtner, W.; Eskens, U.; Hänichen, T. Immunocytochemical Demonstration of Coxiella Burnetii Antigen in the Fetal Placenta of Naturally Infected Sheep and Cattle. J. Comp. Pathol. 1993, 109, 295–301.
- Munster, J.M.; Leenders, A.C.A.P.; Hamilton, C.J.C.M.; Hak, E.; Aarnoudse, J.G.; Timmer, A. Placental Histopathology after Coxiella Burnetii Infection during Pregnancy. Placenta 2012, 33, 128–131.
- Sobotta, K.; Bonkowski, K.; Liebler-Tenorio, E.; Germon, P.; Rainard, P.; Hambruch, N.; Pfarrer, C.; Jacobsen, I.D.; Menge, C. Permissiveness of Bovine Epithelial Cells from Lung, Intestine, Placenta and Udder for Infection with Coxiella Burnetii. Vet. Res. 2017, 48, 23.
- Baumgärtner, W.; Bachmann, S. Histological and Immunocytochemical Characterization of Coxiella Burnetii-Associated Lesions in the Murine Uterus and Placenta. Infect. Immun. 1992, 60, 5232–5241.
- Eitan, K.; Howard, A.; Danny, A. Q Fever in Pregnancy—Case Presentation and Literature Review. Int. J. Clin. Med. 2013, 04, 364–368.
More
Information
Subjects:
Pathology
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
877
Revisions:
2 times
(View History)
Update Date:
01 Jul 2021
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




