1000/1000
Hot
Most Recent

Transcriptomics studies are available to evaluate the potential toxicity of nanomaterials in plants, and many highlight their effect on stress-responsive genes. However, a comparative analysis of overall expression changes suggests a low impact on the transcriptome. Environmental challenges like pathogens, saline, or drought stress induce stronger transcriptional responses than nanoparticles. Clearly, plants did not have the chance to evolve specific gene regulation in response to novel nanomaterials; but they use common regulatory circuits with other stress responses.
Recent advances in nanoscience have expanded the range of applications for novel nanomaterials and driven their production to the industrial scale. In parallel, their emissions to the environment have increased up to the limits where environmental impact needs to be evaluated. Global estimations indicate that landfills and soils receive the largest share of the production volumes, followed by emissions into the aquatic environment and air [1][2]. Fate and transport studies further suggest that disposed nanomaterials end up in natural habitats at concentrations that might pose a risk for living organisms.Since plants represent by far the largest interface between the environment and the biosphere, they will be the first barrier for nanoimpact. Consequently, there is a need to evaluate the toxicological effects of nanomaterials in photosynthetic species as a way to assess for their ecological impact [3][4][5]. Together with standard toxicological methods, omics technologies are also available to quantify nanoimpact in plants. This review is focused on transcriptomics efforts carried out to approach the phenomenon of nanoimpact in plants and the global conclusions that can be drawn from these studies (Figure 1).
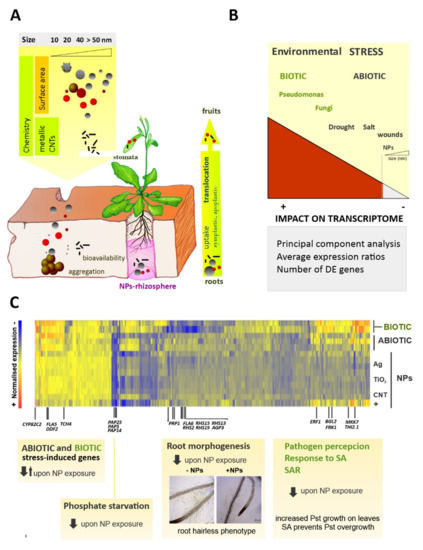
Figure 1. Transcriptomics approaches to evaluate nanoimpact in planta (A) Nanoparticles (NPs) released to the environment can enter into the biosphere and interact with plants, the primary producers in the food chain. NPs uptake occurs through the plant roots. These potentially toxic compounds can be translocated from the roots to the leaves and eventually reach the fruits. (B) The impact of NP exposure on plant transcriptome can be compared with other environmental challenges. Arabidopsis microarrays provided expression data for a large number of plant genes under different biotic or abiotic stress conditions, including early exposure to NPs. Unsupervised techniques like principal component analysis (PCA) summarize gene expression data and show that biotic stress is the main source of variation, whereas exposure to NPs causes little changes in the transcriptome. Comparative analyses of average expression ratios and the number of differentially expressed (DE) genes also support the low impact of NPs on the plant transcriptome. (C) DE genes were classified according to gene ontology (GO) categories, and enrichment was studied. The heat map shows the under-expression of genes involved in phosphate starvation, root morphogenesis, defense response to pathogens, and salicylic acid (SA) signaling. The expression of several genes that modulate systemic acquired resistance (SAR) is decreased upon NP treatment. Related phenotypes under NP exposure include a reduction in the number and length of root hairs and increased survival of Pseudomonas aeruginosa (Pst) in plant distal leaves.
Nanomaterials are defined as materials with at least one dimension in the nanoscale (1–100 nm). If all their external dimensions do not differ significantly within the nanoscale, the term nanoparticle (NP) is preferred to distinguish these objects from others like nanofibers or nanoplates. At the nanoscale, materials behave very differently compared to larger scales, and they often display unique chemical and physical properties. This makes the nanotechnological transformation of matter a promising field to develop new products and processes. On the counterpart, their unique properties allow novel materials to interact unexpectedly with biological systems [6].
The main distinction can be established between two different kinds of NPs: (1) naturally occurring, which are mostly amorphous and chemically and physically highly variable particles of nanometer dimensions, and (2) custom man-made artificial nanoparticles with highly reproducible physico-chemical properties. Natural NPs are usually generated in an uncontrolled way by natural processes (i.e., volcanic activity), or they are undesirable by-products of human activities processes. On the other hand, synthetic NPs are engineered materials with well-defined dimensions and controlled physical and chemical properties, which are the major factors driving their industrial applications.
Among the fundamental physical properties of NPs that have to be considered are overall size, surface area, and mechanical properties [6]. The overall size of NPs impacts the mechanism by which NPs can spread through the plant vascular system. Uptake of NPs by plant roots and translocation to upper tissues has been demonstrated for different species when fed with a suspension of NPs in synthetic media. Both apoplastic and symplastic transport of NPs may also occur under natural growth conditions, with a size limit for symplastic transport in most plants [4][7][8]. NPs with a diameter below 5 nm can translocate through the pores of the cell wall, 8–20 nm particles can move preferably between cells through plasmodesmata, and larger >50 nm particles can be internalized by the endocytosis [3]. Other factors affecting the amplitude of nanotoxicity for plants are the shape and surface area of the NP, which have been studied using crop plant models [9].
Regarding chemical properties, a large variety of chemical compositions and surface coating exist [6]. Metallic NPs are prepared from silver (Ag), gold (Au), copper (Cu), or metal oxides like TiO2. Carbonaceous nanofibers include single-well and multi-well carbon nanotubes (CNTs). Semiconductors such as silicon and ceramic are also used to make NPs. Polymeric NPs, on the other hand, are mostly colloid solids produced from polycaprolactone, polyacrylate, alginate, etc. The chemical composition of NPs, as well as their coating, can further regulate the chemical stability (e.g., changes in the redox state), overall reactivity, covalent attachment, and persistent binding to biomolecules. In most toxicological studies, metallic NPs exhibit the highest toxicity for living organisms, independently of taxa, and this has been strongly connected to the release of metal ions that induce the formation of reactive oxygen species (ROS) [10][11][12]. On the other side, carbonaceous and organic NPs show very low toxicity, or moreover, they might exert positive effects on plant growth or resistance to other environmental stress conditions. Nanomaterials can be made from different chemical compositions, in order to deliver faintly available nutrients and growth-promoting compounds, exhibiting beneficial rather than toxicological effects for plants [13][14][15][16].
Aside from the physico-chemical properties of individualized NPs, the formation of NP clusters and their aggregation induced by the medium or the environment can further impact the overall nanotoxicology. An aggregation process can dramatically increase the overall size of NP clusters and show a varying biological behavior of organisms. Overall, NP aggregates have been shown to induce decreased toxicity compared to individualized NPs in plants [17][18]. NP in the environment undergoes aging processes such as chemical transformation, aggregation, and disaggregation. The interplay between these processes and the NP transport determines the fate and ultimately the phyto-toxicological potential of NPs (Figure 1A).
Engineered nanomaterials represent a fast-growing market, as they have a high potential for product adoption in a variety of industries including aerospace and vehicle production, construction, chemical catalysis, or agri-food sectors [15][16][19]. Rapid developments in biomedical technology and pharmaceutical research are also expected to augment the industrial production of NPs in the years to come [20][21]. These materials are increasing their presence in consumer products and domestic devices, mostly as coatings to improve mechanical, optical, or antibacterial properties. As examples, NPs are now used in the manufacture of crack-resistant paints, scratchproof eyeglasses, transparent sunscreens, ceramic coatings for solar cells, or self-cleaning fabrics. The use of nano-scaled particles instead of their counterpart bulk materials provides advantages and increases the competitiveness of several market products. Nanoparticles of titanium oxide used in sunscreens, for example, have the same chemical composition as the larger white titanium oxide particles used in conventional products for decades, but nanoscale titanium oxide is transparent. Silver nanoparticles continuously discharge Ag+ ions, and they are used in clothing to kill the bacteria known to cause undesirable odors. Silica (SiO2) is a part of the normal mix in conventional concrete, but the use of its nano-form improves particle packing and mechanical properties of the concrete. Cerium oxide (CeO2) nanoparticles can switch from oxidation to reduction catalysts, and they have emerged as fascinating and lucrative material in biological fields such as biomedicine, drug delivery, and bio scaffolding [22].
As a result of the increasing demand by the market, the production and consequent release of NPs to the environment is growing more and more every year. For very frequently produced NPs like CeO2-, SiO2- and Ag-NPs, recent surveys estimated the annual global production volumes over 100,000, 1000–10,000, and 100–1000 t/a, respectively [20]. Probabilistic modeling has been used to predict the flow of released NPs to environmental compartments in the next years and to quantify their amounts in different environmental sinks [20][23]. Although for most environmental compartments NPs pose a relatively low risk of toxicity, organisms residing near NP “point sources”, like production plant outfalls and waste treatment plants, may be at increased risk. The model also indicates that the concentrations of NPs in soil and sediments will be higher than those in water or air. In agreement with existing measurements, modeling of NP dispersion, transport and fate predict that soils will be the final sink and major contaminant source of NPs released into the environment. Taking into account NPs life cycle, as well as the differences in their transport and stability, predicted concentrations of NPs in agricultural soils yield up to 10 μg/kg for 2050 [20]. For sludge-treated soil areas, for example, predicted concentrations might be as much as 40-fold higher.
The investigations of NP production volumes, release, and persistence into different environmental compartments, as well as their toxicological effects, increased the perception of the risk that novel nanomaterials represent for environmental and human health [24][25]. As a consequence, more research was prompted to define ecotoxicological limits of exposure to NPs.
The investigation of potential risks for living organisms has resulted in the development of an ecotoxicological scale for nanomaterials. NPs are included in the list of chemical substances for which environmental effects are monitored and regulated by different countries. By using green algae and plant assays, nanomaterials have been classified in both aquatic and terrestrial ecotoxicity categories, where they rank from harmful to very highly toxic [26]. In aquatic environments, Ag, Au and Fe NPs result in being very highly toxic with EC50 values of <0.1 mg/L, followed by other metallic (Zn, Cu) NPs with EC50 = 0.1–1 mg/L (highly toxic), Cd and Ti NPs (moderately toxic, EC50 = 1–10 mg/L) and Graphene/Carbon NTs (slightly toxic, EC50 > 10–100 mg/L). In terrestrial ecosystems, only Ag NPs are classified as toxic in plant assays, having effects at EC50 > 10–100 mg/kg dry-weight soil.
This classification only takes into account the chemical composition of NPs, without discrimination among sizes in the nanoscale or surface properties. As expressed before, these properties are important factors that govern NP stability and mobility as a colloidal suspension, and likely they will influence interactions with algae in natural aquatic systems or with rhizosphere and plant roots in terrestrial environments [27]. Thus, the phytotoxicity of nanomaterials in natural environments could largely differ from what is expected from standard ecotoxicological assays.
An early study that stimulated further research about the genetic effects and potential toxicity of nanomaterials for plants was performed by Lin et al. [28]. These researchers used transmission electron microscopy (TEM) to demonstrate the uptake and transportation of NPs in crop plants. Rice plants were seeded in a suspension of natural organic matter to mimic freshwater ecosystems, and different concentrations of CNTs were added to the suspension. Seeds were kept in this germination medium for two weeks until they were transplanted to soil pots without further NP treatment. Tissues of plants at various developmental stages were sampled for TEM monitoring to evidence the translocation of CNTs from roots to stem, and from stem to leaves. However, more interestingly, the study showed that nanomaterials that were accumulated in the first generation of exposed plants could be transmitted to the second generation through seeds. Since rice provides food crops of over half the world’s population, this work suggested the potential impact of disposed nanomaterials on the food chain and raised important concerns about the genetic consequences of plant–NPs interactions. Subsequently, efforts to investigate these consequences using genome-wide technologies were pushed forward in other crop plants like tomato [29] or the model species Arabidopsis thaliana.
A powerful approach to determine how an organism responds to a particular environmental challenge is to determine how it changes the expression of its genome [30]. In this line, transcriptome studies to approach nanoimpact have been performed in several photosynthetic species, from unicellular green algae [31] to higher vascular plants like tomato [29], rice [32], or A. thaliana [33][34][35][36][37][38][39][40][41]. Thus, transcriptional data covering a range of species for which genomics tools are fully developed are available to assess for nanoimpact on plants. Omics technologies have the power to shift the research on plant-NP interactions from low-throughput, single end-point bioassays to high-throughput discovery [42].
While information on the transcriptional effects of NP exposure is available, the findings are somewhat contradictory. Several studies show a strong effect on the transcription of stress-related genes and suggest high toxicity for the plant, whereas some others do not find significant transcriptional changes and have concluded that NPs are unlikely to produce any adverse effect for the plant. In parallel, physiological and biochemical changes observed upon NP exposure include both significant reduction and significant promotion of plant growth, elongation or shortening of plant roots, the formation of ROS, or no indications at all for oxidative stress. One reason for these apparently contradictory conclusions is that many of these studies are focused on specific effects for a given, chemically defined type of NP, and other factors like size-dependent effects are not taken into account. Moreover, a very small fraction of plant genes is used as markers for conventional toxicological studies, and they are mostly included in predefined functional categories, such as oxidative stress response, which are not very informative at the morphological level. As a consequence, there is a missing node to relativize the toxic effects of NPs.
Unicellular, green algae have been widely used as sentinel species for ecotoxicological studies both in freshwater and soil ecosystems [43]. Thus, one of the first attempts to establish the potential impact of novel nanopollutans by using transcriptomics approaches was carried out in the model species C. reinhardtii [31]. Previous toxicological studies in this alga [44] evidenced that several types of NPs are able to penetrate the cell wall and induce the production of ROS, or cause cell damage by reacting directly with the biological membrane. The researchers used mRNA sequencing to evaluate the effects of exposure to four different (nZnO, nAg, nTiO2, and CdTe/CdS quantum dots) metal-based NPs, with diameters ranging between 20 nm for nZnO and 1–10 nm for the other three types of NPs. Transcript fold change between two conditions, NP exposure vs. no exposure, was used to determine differential expression; and genes were considered as differentially expressed (DE) if they met a ≥2 -fold change ratio between both conditions. The researchers found that NP exposure resulted in largely different transcriptomic responses. Surprisingly, only the exposure to nZnO induced the transcription of GSTS1, HSP22C, and HSP70A, genes considered as the main markers for oxidative stress, since they encode for a glutathione-S transferase and heat-shock proteins induced by excess H2O2 or singlet oxygen. The effect of the other three types of NPs was actually a down-regulation of stress-related genes. The study also revealed that TiO2 and ZnO NPs and CdTe/CdS quantum dots impact the proteasome machinery and produce proteasome inhibition. Interestingly, a consistent effect of all types of tested NPs was the transcriptional inhibition of photosynthesis-related genes, suggesting toxicological effects in the chloroplast. In this line, biochemical studies in the planktonic species Scenedesmus obliquus [45] show that photocatalytic activity of nTiO2 can damage algae by directly reacting with chloroplast photosynthetic machinery and generating ROS. The effect on other stress-related genes that was observed in transcriptional studies has been interpreted as a stimulation of the plant defense system in order to scavenge produced ROS.
An important concern about the toxicological effects reported for nanoparticles is that many of them are associated with supra-environmental exposure concentrations [46]. Most studies in green algae have been conducted through acute toxicity testing (short-time exposure to high doses), even though environmental effects are likely to be better assessed by chronic toxicity testing (long-time exposure to low doses). Thus, the impact of NPs on freshwater ecosystems at environmentally relevant concentrations is not clear from these experiments, and many questions about how surrounding biota can modify the toxicology of nanopollutants for photosynthetic organisms remain to been elucidated.
An interesting approach to determine nanotoxicity for freshwater ecosystems under more realistic environmental conditions was performed by Lu et al. [47]. In a microcosm experiment including aquatic eukaryotic algae, fungi, zooplankton, and bacteria (i.e., heterotrophic bacteria and cyanobacteria), a meta-transcriptomic analysis was used to decipher the toxic effects of Ag NPs (10 nm), at relatively low doses (10 μg/L), and upon long-term (7-days) exposure. It was found that photosynthetic eukaryotes were much more tolerant to Ag NPs than cyanobacteria and displayed a number of potential Ag NPs detoxification mechanisms, which involved increasing nitrogen and sulfur metabolism, over-expression of genes related to translation and amino acids biosynthesis, and the promotion of bacterial-eukaryotic algae interactions. Thus, transcriptomic analysis reveals that photosynthetic organisms overcome exposure to nano-pollutants by triggering a set of complex responses above the transcriptional activation of genes involved in ROS detoxification.
In model plant species, genome expression microarrays are available to profile transcriptome under different environmental challenges. Most genome databases for model organisms link gene annotation to microarray expression platforms. Microarrays provide a standard mean for normalization of gene expression data which facilitates comparisons among multiple conditions, even when data are originated from different experiments. With this regard, several efforts were put into the transcriptional characterization of nanoimpact using genome-covering microarrays for higher plants.
Khodakovskaya et al. [29] integrated imaging and genetic technologies to approach the phenomenon of nanoimpact in tomato plants. By using photothermal and photoacoustic cytometry, they first mapped CNTs in roots, leaves, and fruits of plants fed with a suspension of these NPs. Next, they profiled transcriptional changes induced by CNTs by using a preliminary microarray covering approximately 1/4 of the tomato genome.
In their experiments, total mRNA was isolated from leaves and root tips of 10-day-old tomato seedlings growing on Murashige and Skoog (MS) medium, MS medium supplemented with CNTs (50, 100, 200 mg/L), or with activated carbon as a control for the effect of bulk, non-particulated material. Using a tomato microarray containing probes to interrogate over 9200 plant transcripts, the authors identified 91 and 49 transcripts in leaves and roots, respectively, that showed significant differences between the CNT-exposed seedlings and two controls.
Within this set of DE transcripts, the researchers observed that several up-regulated genes in response to CNTs (i.e., genes encoding for several endoproteases, the heat shock protein HSF70, or the LeAqp2 gene encoding for a water-channel protein) can also be activated in response to specific biotic stress factors. This observation suggested that plants can sense the penetration of nanomaterials as a stress factor, similar to pathogens or herbivore attacks. Therefore, the authors introduced the idea that important stress-signaling pathways could be activated in response to the uptake of NPs, and accordingly, nanomaterials could have a significant impact on most of the major physiological processes in planta. Conversely, the number of DE genes extracted by statistical analysis represents a small fraction of the tomato genes covered by the microarray. More challenging are the physiological effects observed after feeding tomato plants with CNTs, including a significant increase in plant biomass production, while exposure to environmental stress in crop plants usually results in poor biomass accumulation.
Other works in tomato [13] reported the induction of pathogenesis-related (PR) genes and the enhancement of biotic stress responses after exposure to chitosan-NPs. Both chitosan and chitosan-NPs were able to inhibit the development of wilt caused by Fusarium andiyazi in tomato plants and to elicit transcriptional responses characteristic of the induced systemic resistance, although these effects were not specific to the exposure to nanosized chitosan.
The extensive genome annotation available for the model species A. thaliana and the consequent development of advanced post-genomics tools enabled a finer evaluation of nanoimpact in higher plants. Whole-genome expression microarrays were used initially to characterize gene expression changes in response to different nanomaterials, including ZnO nanopowder, a roughly characterized mixture of TiO2 NPs, and fullerene soot [39].
Plants growing in MS medium were exposed for seven days to a final concentration of 100 mg/L of NPs, and roots were harvested for microarrays analysis of gene expression. nZnO caused the most dramatic transcriptomic changes of the three nanomaterials under study, both in terms of the number of affected genes and in the magnitude of the impacts on gene expression. In addition, more genes were repressed than activated, suggesting that nZnO represented a severe stress condition for the plants. However, these experiments assayed the effects of a high concentration of NPs into a defined growth medium, and the authors themselves stated that an assessment of true environmental risk should be focused on more environmentally relevant NP concentrations. Nevertheless, the study showed that genes induced by nZnO and fullerenes include mainly ontology groups annotated as stress-responsive, including both abiotic and biotic stimuli. The down-regulated genes under nZnO exposure were involved in cell organization and biogenesis, whereas fullerenes largely repressed genes involved in electron transport and energy pathways. The counterpart effects of non-particulate, bulk materials on genome expression were not tested in this study, and thus, many of the transcriptional changes described by the authors could be non-specific of NP exposure.
Silver NPs are the most widely used nanomaterials that enter into the wastewater and potentially damage the environment. Due to their antimicrobial properties, they are used in a wide variety of processes, including disinfection of domestic water or the production of antimicrobial coatings for textiles, house appliances, or biomedical devices. Many of these products contain silver nanoparticles that continuously release a low level of silver ions to protect against bacteria. Concordantly, the ecotoxicology of Ag NPs is complex because it may be related simultaneously to silver-specific and nanoparticle-specific biological effects.
In a pioneer work by Kaveh et al. [33], transcriptome analysis offered a powerful tool to approach the complexity of plant responses to Ag nanopollutants. As the first step in this study, the potential toxicity of Ag+ or Ag NPs (20 nm) was evaluated for Arabidopsis plantlets grown during ten days in MS medium containing increasing concentrations of both factors, ranging from 0 to 20 mg/L. At low levels (1.0 and 2.5 mg/L), exposure to Ag NPs resulted in a significant increase in biomass with respect to untreated plants and respect to plants treated with Ag+, although exposure to higher concentrations resulted in a decrease in biomass for both treatments. A concentration of 5 mg/L, which resulted in moderate reductions in plant biomass, was chosen for microarray experiments.
Further analysis of microarray data was based on expression fold-change values with respect to non-exposed plants. Exposure to Ag NPs resulted in differential expression of 375 genes, with a significant overlap with DE genes that responded to Ag+ treatment. The overlap suggested that Ag NP-induced stress originates partly from silver toxicity and partly from nanoparticle-specific effects. Many genes responding to both treatments were found to be involved in plant response to various stresses: up-regulated genes were associated with the response to metals and oxidative stress (i.e., genes encoding for vacuolar cation/proton exchanger, superoxide dismutases, cytochrome P450-dependent oxidases, and peroxidases), while down-regulated genes were more associated with response to pathogens, including systemic acquired resistance (SAR) against fungi and bacteria, and hormonal stimuli (auxin or ethylene signaling pathways). Interestingly, most overlapping genes, affected both by Ag+ and Ag NPs, were down-regulated.
On the other hand, a number of genes were found as differentially expressed in response to Ag NPs only. This set of genes more likely reflected the molecular mechanisms involved in NP-specific responses. The most up-regulated genes in this set were involved in salt stress response, which established a connection with the previous work in Arabidopsis, where genes related to saline stress were found to be induced upon exposure to both nZnO and fullerenes [39]. The set also included up-regulated genes encoding for a miraculin-like protein involved in the plant response to wounding, a myrosinase-binding protein induced during defense against insects and pathogens, and more intriguingly, a cluster of genes belonging to the thalianol biosynthetic pathway. In plants, these rare clusters are believed to be involved in the biosynthesis of stress-induced secondary metabolites that are required for survival under specific conditions, such as the exploitation of new environments.
Thus, transcriptome analysis of Arabidopsis plants exposed to silver provided some clues for a better understanding of the molecular mechanism of plant response to NPs and established a connection with physiological responses involved in both abiotic and abiotic stress sensing.