
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Marta Vieira | + 3339 word(s) | 3339 | 2021-01-28 05:19:52 | | | |
| 2 | Peter Tang | Meta information modification | 3339 | 2021-04-21 04:29:08 | | |
Video Upload Options
Microalgae are microorganisms with a singular biochemical composition, including several biologically active compounds with proven pharmacological activities, such as anticancer, antioxidant and anti-inflammatory activities, among others. These properties make microalgae an interesting natural resource to be used as a functional ingredient, as well as in the prevention and treatment of diseases, or cosmetic formulations. Nevertheless, natural bioactives often possess inherent chemical instability and/or poor solubility, which are usually associated with low bioavailability. As such, their industrial potential as a health-promoting substance might be severely compromised. In this context, encapsulation systems are considered as a promising and emerging strategy to overcome these shortcomings due to the presence of a surrounding protective layer.
1. Introduction
Microalgae are a heterogeneous group of photosynthetic microorganisms, whose evolutionary and phylogenetic diversity has provided a vast assortment of biochemical compositions [1]. These microorganisms are able to biosynthesize, accumulate and secrete a great range of primary and secondary metabolites as a response to changes in the external environment, many of which are highly valuable substances with industrial applications and health benefits [2].
The use of microalgae by humans dates back thousands of years, where it was used as a food source by different populations; nevertheless, the commercial exploitation of this resource is only a few decades old, when there was an apprehension regarding a possible insufficient protein supply due to the rapid increase in the world population [3][4]. Microalgae are well-known for their high protein and nutritional content, but more recently, studies have been focused on the unique biologically active compounds produced by their species, such as polyunsaturated fatty acids, pigments, antioxidants, polyphenols, polysaccharides, and other equally important substances [3][4].
Lately, there has been a growing trend towards using natural ingredients in food, pharmaceutical, and cosmetic industries due to the increasing concerns regarding consumer safety, environmental sustainability, and regulatory issues over the introduction of synthetic chemicals in human nutrition, healthcare, and beauty products [5][6][7]. Several microalgae bioactives possess significant biological activities, including anticancer, antioxidant, anti-inflammatory, antimicrobial, and immunomodulatory activities, among others [8][9][10]. Therefore, the use of such compounds seems to be a promising and innovative approach to the development of healthier, functional, and sustainable products.
Overall, microalgae may be proposed to obtain commodities with existing market value, refined bioactives, or the whole cell could even be the target product [11]. Yet, purified compounds or bioactive extracts are usually chemically unstable and strongly susceptible to oxidative degradation, particularly when exposed to oxygen, light, moisture, extreme pH, and high temperatures. The oxidative degradation may also deteriorate the compounds, leading to the development of unpleasant tastes and off-odours in the fortified product and, subsequently, may result in a negative effect on shelf stability, sensory characteristics, and consumer acceptability of the product [12]. Moreover, the low bioavailability and poor water solubility are usually recurrent issues related to the application of microalgae bioactives; in pharmaceutical and functional food products, for instance, the absorption of these compounds may be hindered due to gastrointestinal tract conditions, as well as due to their physicochemical properties [13][14].
These developmental and technological issues address the importance of researching strategies to preserve the functionality of microalgae bioactives from processing until they reach their target site. In this context, encapsulation systems are considered a promising approach, which have been applied successfully in diverse fields. The process of encapsulating a bioactive consists of its entrapment within one or more coating materials through different techniques, resulting in nano- or microparticles [15]. This strategy is associated with several advantages, including protecting the bioactive compound during processing, storage, and distribution; promoting release control; masking off-flavours; improving solubility and bioavailability, among others [16][17].
2. Microalgae
Microalgae are single-celled, ubiquitous, prokaryotic, and eukaryotic primary photosynthetic microorganisms, which are taxonomically and phylogenetically diverse [9][18]. They are ancestral living organisms that have adapted uniquely to extreme habitats over billions of years of evolution and can be found almost anywhere on Earth; in freshwater, seawater, and hypersaline environments, but also in moist soils and rocks [19]. Their classification is based on various properties, such as pigmentation, the chemical nature of photosynthetic storage products, the organization of photosynthetic membranes, and other morphological features. The most abundant microalgal classes are Cyanophyceae (blue-green algae), Chlorophyceae (green algae), Bacillariophyceae (including the diatoms), and Chrysophyceae (including golden algae) [3][20]. A resume of the main microalgae classes, their most studied species, and associated biological activities are described in Figure 1.
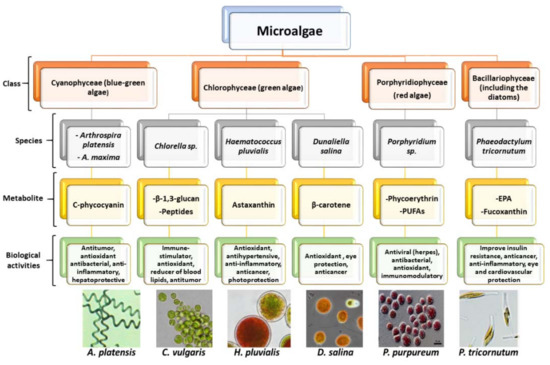
Figure 1. Main microalgae classes, their most important species, and associated biological activity (microalgae images were obtained from the “Microalgae strain catalogue”, second edition, published in the Enhance Microalgae Project, available at https://www.enhancemicroalgae.eu/wp-content/uploads/2020/05/EMA-Strain-catalogue-2nd-Edition.pdf).
Interest in microalgae cultivation has been prospering globally in recent decades for diverse reasons. There are several industrial and commercial applications associated with these microorganisms and examples of success include formulations in different sectors, such as functional foods, feed, cosmetics, pharmaceuticals, and fertilizers; as well as tools for wastewater treatment and biofuel production [21][22]. Moreover, many advantages have already been reported involving their cultivation process in comparison with other feedstocks. Firstly, microalgae reproduce themselves using photosynthesis to convert sun energy into chemical energy, completing an entire growth cycle every few days. Secondly, they can grow almost anywhere, requiring mostly sunlight and some simple nutrients; although the process can be accelerated heterotrophically by the addition of specific nutrients and changes in cultivation parameters. Accordingly, microalgae have much higher growth rates and productivity when compared to conventional forestry, crops, and other aquatic plants, demanding much less land area [23][24].
Through adaptive evolution and metabolic diversity, microalgae have developed a wide range of high value biologically active compounds, comprising pigments, antioxidants, polysaccharides, triglycerides, fatty acids, and vitamins [25]. It is estimated there are 70,000 to one million microalgae species; however, only about 44,000 have already been described. Furthermore, from those, only a limited number have been studied for commercial purposes [26]. Some of the most biotechnologically relevant microalgae are the green algae (Chlorophyceae) Chlorella vulgaris, Haematococcus pluvialis, Dunaliella salina, and the Cyanobacteria Arthrospira platensis, which are broadly commercialized, mainly as nutritional supplements for humans and as animal feed additives [27].
The multicellular filamentous Cyanobacteria from the genus Arthrospira (formerly known as “Spirulina”) occur naturally in alkaline lakes and ponds, being widely cultured around the world. The two most important species of Arthrospira, A. maxima, and A. platensis, are commonly applied both as a functional ingredient in food preparations and as a source of the blue photosynthetic pigment C-phycocyanin, which is used in cosmetics and the food industry [28]. This species has been used as a nutrient-rich (especially vitamin B12 and proteins) food source with the oldest records indicating use by the Aztecs, who harvested this microalga from Lake Texcoco in Mexico; and by the local people in Lake Chad, who used A. platensis as a nutritional supplement known as “dihe” [6][29]. Apart from its significance as a food additive, A. platensis is also recognized by the broad range of potential medical and pharmaceutical applications attributed to its metabolites. Studies have evidenced several biological activities, such as antitumor, antibacterial, anti-inflammatory and hepatoprotective activities, among others, directly related to the antioxidant capacity ascribed for C-phycocyanin and other compounds [30][31]. Similarly, the freshwater unicellular blue-green microalga Aphanizomenon flos-aquae, which grows spontaneously in Upper Klamath Lake in Oregon, USA, is also consumed as a nutrient-rich food source and for its health properties. Similar to the Arthrospira species, A. flos-aquae is an important source of the pigment C-phycocyanin; hence, demonstrating a strong antioxidant potential [32][33].
The unicellular green alga Chlorella is one of the largely studied microalgae due to their biotechnological importance as a valuable source of nutrients. Species from this genus were one of the first microalgae considered for mass cultivation and the first microalga produced commercially. Chlorella cells actively growing under normal conditions are typically rich in protein (40–60%) and are largely made up of essential amino acids, with a profile that suits human nutrition. In this context, Chlorella biomass may be considered as a desirable candidate for protein supplements or single-cell protein [34][35]. Furthermore, these species are also rich in carotenoids, vitamins and other bioactives, demonstrating potential health benefits, such as efficacy on gastric ulcers, wounds, and constipation; preventive action against both atherosclerosis and hyper-cholesterol; and antitumor activity. The suggested most important active compound is β-1,3-glucan, which is believed to be an active immune-stimulator, free radical scavenger, and a reducer of blood lipids [4][36][37].
Another important member of the green algae class is the freshwater unicellular microalga Haematococcus pluvialis. Under extreme environmental conditions, such as high-intensity light or oligotrophic circumstances, this species undergoes several morphological and biochemical modifications, including an intense biosynthesis of the carotenoid astaxanthin [38]. In the last few decades, H. pluvialis has received significant attention from the scientific and biotechnological communities for being considered as the most significant biological source of that carotenoid in nature [39][40]. Astaxanthin is a natural pigment with several applications in the nutraceutical, cosmetic, food, and feed industries [41]. Moreover, it also possesses a powerful antioxidant potential due to its unique chemical configuration, which is associated with assorted biological activities demonstrated in both animal and clinical studies [42]. Many authors have already described astaxanthin’s valuable effects in inflammatory responses and the immune system, in hypertension, cancer, ocular and cardiovascular diseases, as well as in skin ageing defence [43][44][45].
Regarding carotenoid production, the unicellular green microalga Dunaliella salina is equally important for its recognition as the richest source of natural β-carotene [46]. When exposed to specific extreme environmental conditions, such as high-intensity light, high salinity, extreme temperatures, and/or nutrient deprivation, D. salina can accumulate an exceptionally large amount of β-carotene (up to 14% of the dry algal biomass), resulting in orange-coloured cells. This great carotene productivity has led to the large-scale application of D. salina for commercial production of natural β-carotene, widely used as an antioxidant and colourant in the food, feed, cosmetics, and pharmaceutical industries [47][48]. Additionally, this species also contains other important lipid components, glycerol, proteins, and carbohydrates [49].
In the context of microalgae importance in different fields, some species have not been fully explored, but have been demonstrated to be a promising source of bioactives according to published studies. The microalga Phaeodactylum tricornutum is a marine diatom, which accumulates eicosapentaenoic acid (EPA, 20:5n-3) as a major component of its fatty acid content [50]. This species is also a rich source of the carotenoid fucoxanthin, whose intake has been suggested to improve insulin resistance and to decrease the blood glucose level, along with anticancer and anti-inflammatory effects [51][52]. Furthermore, some other microalgae genera, such as Nannochloropsis, Tetraselmis, Scenedesmus, and Isochrysis, have revealed their importance due to the production of long-chain fatty acids, i.e., docosahexaenoic acid (DHA) and EPA, representing also a source of antioxidant compounds [53][54].
Although there are a very large number of red algae (Rhodophyta) in nature, only a few species represent the microalgae group. The genus Porphyridium is the most studied one due to the particular interest in its species as a source of sulphated polysaccharides, proteins, the polyunsaturated fatty acids (PUFAs) arachidonic acid and EPA, and the phycobiliprotein phycoerythrin [35]. Studies have demonstrated that the sulphated polysaccharides of Porphyridium sp. exhibit potential antiviral activity against herpes simplex virus (HSV-1 and 2) both in vitro and in vivo [55][56]. Furthermore, it has also been reported that different-molecular-weight subunits of its polysaccharides demonstrate important antioxidant and immunomodulatory activities [57][58]. Likewise, the Cyanobacteria Phormidium sp. is a recognized source of extracellular polymeric substances (EPS), which possess applications in the pharmaceutical, cosmetic, and food industries as an emulsifier and thickening agent [59]. Additionally, species of this genus have been reported to inhibit the growth of different Gram-positive and Gram-negative bacterial strains, yeasts, and fungi [60].
3. Encapsulation
Encapsulation may be defined as a process in which a substance (active agent) is entrapped or coated by a carrier material, in order to form a particulate system. The encapsulated compounds are also designated as the core, fill, or internal phase, whereas the carrier substances can be identified as wall material, membrane, capsule, shell, matrix, or external phase [12]. This technique may be used to encapsulate compounds in the solid, liquid, or gaseous state in small particles, which can be classified as nanoparticles when the dimensions vary from 1 to 100 nm; or microparticles, when the dimensions range from 100 nm to 1000 µm [61][62].
The protective barrier provided by encapsulation offers several advantages. The primary reason to develop these particulate systems is to maintain the biological, functional, and physicochemical properties of the active agent [63]. The wall material serves as a protection from adverse environmental and processing conditions, such as the undesirable effect of light, temperature, moisture, and oxygen; therefore, contributing to an increase in stability and an extended shelf-life [64]. Another important advantage is the possibility of overcoming challenges that normally restrict the incorporation of certain substances into commercial products. Encapsulation allows the increase in solubility of a compound into a dissimilar medium, masking unpleasant flavours, enhancing the bioavailability and bioactivity, as well as controlling and targeting the release of the core material as a response to external conditions (pH, temperature, etc.) [15][63][65].
The retention of the core substance within a particle and its stability depends on several factors, comprising the desired physicochemical and functional properties of the final encapsulation system, along with the properties of the carrier material and the active agent. The major characteristics to be considered regarding the core and coating materials are their chemical nature, molecular weight, polarity, and solubility; while for the final particulate system, the entrapment efficiency, permeability, degradability, and release profile must be equally taken into account [66][67][68].
Encapsulation technology has been extensively researched and applied in diverse areas, such as the pharmaceutical, medical, food, cosmetics, chemical, and agricultural industries [67][69]. In the pharmaceutical field, for instance, encapsulation is a key strategy to assist specific drawbacks in the formulation development, as it is capable of promoting drug delivery to specific body sites, control drug release, act as diagnostic tools, and improve physicochemical properties, e.g., water solubility, which, in turn, can bring positive changes to the medical treatment, such as lowering the therapeutic dose and minimizing the side effects [70][71][72][73]. Similar advantages can be related to the application of encapsulation in the cosmetics segment, where particulate systems could lead to a sustained release of the active agent, as well as an enhanced and deeper skin penetration [74][75].
Likewise, the food industry can particularly benefit from the use of encapsulation, especially regarding the development of functional foods. The addition of biologically active compounds into food has emerged as an exciting health-promoting strategy in recent decades; however, it may present several limiting factors, including high sensitivity to processing conditions, short shelf-life, fast-release of flavour during storage, limited uptake and bioavailability, lack of compatibility and uniformity with the food matrix, or degradability through the gastrointestinal tract passage [16][76][77]. In light of this, encapsulation represents a useful tool for the suppression of the aforementioned limitations, since it enables the protection of a wide range of compounds by their entrapment into a protective matrix [78].
4. Microalgae Encapsulation
4.1. Functional Foods
Functional food, in general terms, may be defined as a natural or processed food, which contains an identified component, in qualitative and quantitative amounts, with a proven and documented health benefit [79][80]. This concept was created in recent decades, opening a new research field that is in constant expansion due to consumers’ increasing awareness of the close correlation between diet and health. Beyond providing nutrients required for the bodily metabolism, it is well-known that food may play a key role in the prevention and treatment of certain diseases, along with the improvement of physical and mental well-being [81][82]. Following this trend, safety issues regarding the consumption of processed foods have also become a concern. National authorities, such as the Food and Drug Administration (FDA) and the European Food Safety Authority (EFSA), have restricted the use of many synthetic additives in food, e.g., synthetic dyes, due to a growth in cancer development or allergic reactions [6][51].
Accordingly, there is a great interest in the investigation of natural resources and biologically active compounds with high nutritional value and functionality to be used as a food ingredient in the development of novel functional foods [83]. Among these, microalgae are emerging as a valuable and economically viable alternative, as they represent a rich source of food-grade compounds and almost an unlimited field of exploration due to their abundant taxonomic diversity [83][84]. A variety of microalgae biomass has already been successfully applied in the fortification of assorted food products, such as cookies, bread, pasta, and some dairy goods [85].
On the other hand, the incorporation of nutraceutical compounds into food is more of a challenging approach. The effectiveness of a bioactive as a health-promoting substance within the food matrix depends on keeping its functionality intact during food processing and storage; conserving the characteristics (taste, texture, colour, smell, etc.) and acceptability of the original food; and lastly, assuring the bioavailability of the active ingredients, which includes sustaining sufficient time of gastric residence without degradation and appropriate gut permeability [64]. Due to the inherent instability of most bioactive compounds present in microalgae, the efficacy of this process may be compromised. Consequently, their incorporation into encapsulation systems seems to be a promising strategy to deliver microalgae health benefits at boosted levels through functional foods [65].
One of the most explored microalgae concerning encapsulation systems for food applications is the species Haematococcus pluvialis. Several research groups have investigated the encapsulation of its extract obtained by different methods or purified compounds, essentially the carotenoid astaxanthin.
4.2. Pharmaceutical
Naturally derived products have served as a vital source of drugs since ancient times. Nowadays, approximately one-third of the top-selling pharmaceuticals are of natural origins or their derivatives. Plants and microorganisms represent a practically unlimited source of biochemical molecules, which may have promising pharmacological activities and therapeutic benefits in the treatment of diverse diseases [86]. In particular, microalgae have shown their importance in the discovery of new therapeutic molecules, as well as in the isolation and characterization of already acknowledged ones [87].
As previously mentioned, the application of natural compounds in therapeutics faces significant shortcomings and developmental challenges, highlighting their usually poor aqueous solubility, inherent instability, and low bioavailability [88]. The use of micro/nanoencapsulation has been shown as a solution by the pharmaceutical industry to address the issues associated with these drawbacks, where the therapeutic value of biologically active compounds can be drastically improved [86]. Microalgae, as a rich and valuable universe of natural products with proven pharmacological properties, are assumed to benefit from this strategy. However, the application of these microorganisms in drug delivery systems for pharmaceutical purposes is still a field to be explored.
4.3. Cosmetics
Cosmetics are a class of products aimed at improving the structure, morphology, and appearance of skin or external parts of the body. A large section of this segment comprises skin topical formulations, which are composed of excipients and one or more active ingredients. Following the current global trend for products derived from natural sources, there is a demand for the development of environmentally sustainable cosmetic products, with less chemical compounds, which could act as cosmeceuticals [89].
The interest in microalgae regarding cosmetics application is relatively recent; these microorganisms produce metabolites in response to changes in the environment, whose main function is linked to the cell’s ability to regenerate and self-protect against external adverse conditions. In this context, it is assumed these compounds could instigate the equivalent effect when applied on the skin. Among the bioactives extracted from microalgae that can be potentially used in cosmetics formulation are the ones with pronounced antioxidant activity, such as astaxanthin and C-phycocyanin [90][91].
The skin is the outer organ of the body and therefore acts as the primary barrier against the loss of endogenous substances, as well as the penetration of external agents into the human body. As it constitutes an interface with the environment, the skin is considered a target of several exogenous factors, such as UV radiation, pathogens, pollution, and other toxic compounds. Such factors are usually associated with excessive production of reactive oxygen species and other free radicals, which are pro-inflammatory mediators and may induce many deleterious effects, including DNA damage, oxidative stress, photoaging, and carcinogenesis [91][92]. As such, microalgae bioactives could play an advantageous role in maintaining the skin health status and in the treatment of some dermatological issues, such as hyperpigmentation, dehydration, photo-oxidation, photoaging, as well as protection against skin cancer [89][93].
References
- Borowitzka, M.A. High-value products from microalgae-their development and commercialisation. J. Appl. Phycol. 2013, 25, 743–756.
- De Morais, M.G.; Vaz, B.D.S.; De Morais, E.G.; Costa, J.A.V. Biologically Active Metabolites Synthesized by Microalgae. BioMed Res. Int. 2015, 15, 1–15.
- Chacón-Lee, T.L.; González-Mariño, G.E. Microalgae for “Healthy” Foods-Possibilities and Challenges. Compr. Rev. Food Sci. Food Saf. 2010, 9, 655–675.
- Milledge, J.J. Commercial application of microalgae other than as biofuels: A brief review. Rev. Environ. Sci. Biotechnol. 2011, 10, 31–41.
- Gouveia, L.; Batista, A.P.; Sousa, I.; Raymundo, A.; Bandarra, N.M. Microalgae in novel food products. In Food Chemistry Research Developments; Papadopoulos, K.N., Ed.; Nova Science: New York, NY, USA, 2008; p. 36. ISBN 9781604562620.
- Vaz, B.D.S.; Moreira, J.B.; De Morais, M.G.; Costa, J.A.V. Microalgae as a new source of bioactive compounds in food supplements. Curr. Opin. Food Sci. 2016, 7, 73–77.
- Tang, D.Y.Y.; Khoo, K.S.; Chew, K.W.; Tao, Y.; Ho, S.H.; Show, P.L. Potential utilization of bioproducts from microalgae for the quality enhancement of natural products. Bioresour. Technol. 2020, 304, 122997.
- Lauritano, C.; Andersen, J.H.; Hansen, E.; Albrigtsen, M.; Escalera, L.; Esposito, F.; Helland, K.; Hanssen, K.; Romano, G.; Ianora, A. Bioactivity screening of microalgae for antioxidant, anti-inflammatory, anticancer, anti-diabetes, and antibacterial activities. Front. Mar. Sci. 2016, 3, 1–2.
- Singh, S.; Kate, B.N.; Banecjee, U.C. Bioactive compounds from cyanobacteria and microalgae: An overview. Crit. Rev. Biotechnol. 2005, 25, 73–95.
- Christaki, E.; Florou-Paneri, P.; Bonos, E. Microalgae: A novel ingredient in nutrition. Int. J. Food Sci. Nutr. 2011, 62, 794–799.
- Dominguez, H. Functional Ingredients from Algae for Foods and Nutraceuticals, 1st ed.; Woodhead Publishing Limited: Cambridge, MA, USA, 2013.
- Shishir, M.R.I.; Xie, L.; Sun, C.; Zheng, X.; Chen, W. Advances in micro and nano-encapsulation of bioactive compounds using biopolymer and lipid-based transporters. Trends Food Sci. Technol. 2018, 78, 34–60.
- Rein, M.J.; Renouf, M.; Cruz-Hernandez, C.; Actis-Goretta, L.; Thakkar, S.K.; Pinto, M.D.S. Bioavailability of bioactive food compounds: A challenging journey to bioefficacy. Br. J. Clin. Pharmacol. 2012, 75, 588–602.
- Singh, H. Nanotechnology applications in functional foods; Opportunities and challenges. Prev. Nutr. Food Sci. 2016, 21, 1–8.
- Ye, Q.; Georges, N.; Selomulya, C. Microencapsulation of active ingredients in functional foods: From research stage to commercial food products. Trends Food Sci. Technol. 2018, 78, 167–179.
- Kuang, S.S.; Oliveira, J.C.; Crean, A.M. Microencapsulation as a tool for incorporating bioactive ingredients into food. Crit. Rev. Food Sci. Nutr. 2010, 50, 951–968.
- Siddiqui, I.A.; Sanna, V. Impact of nanotechnology on the delivery of natural products for cancer prevention and therapy. Mol. Nutr. Food Res. 2016, 60, 1330–1341.
- Bux, F. Biotechnological Applications of Microalgae: Biodiesel and Value-Added Products, 1st ed.; Taylor & Francis: Boca Raton, FL, USA, 2013.
- Camacho, F.; Macedo, A.; Malcata, F. Potential industrial applications and commercialization of microalgae in the functional food and feed industries: A short review. Mar. Drugs 2019, 17, 312.
- Hemaiswarya, S.; Raja, R.; Ravikumar, R.; Carvalho, I.S. Microalgae taxonomy and breeding. Biofuel Crop. Prod. Physiol. Genet. 2013, 44–53.
- Raja, R.; Hemaiswarya, S.; Kumar, N.A.; Sridhar, S.; Rengasamy, R. A perspective on the biotechnological potential of microalgae. Crit. Rev. Microbiol. 2008, 34, 77–88.
- Rizwan, M.; Mujtaba, G.; Memon, S.A.; Lee, K.; Rashid, N. Exploring the potential of microalgae for new biotechnology applications and beyond: A review. Renew. Sustain. Energy Rev. 2018, 92, 394–404.
- Mata, T.M.; Martins, A.A.; Caetano, N.S. Microalgae for biodiesel production and other applications: A review. Renew. Sustain. Energy Rev. 2010, 14, 217–232.
- Chen, Y.; Chen, J.; Chang, C.; Chen, J.; Cao, F.; Zhao, J.; Zheng, Y.; Zhu, J. Physicochemical and functional properties of proteins extracted from three microalgal species. Food Hydrocoll. 2019, 96, 510–517.
- Safi, C.; Zebib, B.; Merah, O.; Pontalier, P.Y.; Vaca-Garcia, C. Morphology, composition, production, processing and applications of Chlorella vulgaris: A review. Renew. Sustain. Energy Rev. 2014, 35, 265–278.
- Neofotis, P.; Huang, A.; Sury, K.; Chang, W.; Joseph, F.; Gabr, A.; Twary, S.; Qiu, W.; Holguin, O.; Polle, J.E.W. Characterization and classification of highly productive microalgae strains discovered for biofuel and bioproduct generation. Algal Res. 2016, 15, 164–178.
- Koyande, A.K.; Chew, K.W.; Rambabu, K.; Tao, Y.; Chu, D.-T.; Show, P.-L. Microalgae: A potential alternative to health supplementation for humans. Food Sci. Hum. Wellness 2019, 8, 16–24.
- Soni, R.A.; Sudhakar, K.; Rana, R.S. Spirulina—From growth to nutritional product: A review. Trends Food Sci. Technol. 2017, 69, 157–171.
- Colla, L.M.; Oliveira Reinehr, C.; Reichert, C.; Costa, J.A.V. Production of biomass and nutraceutical compounds by Spirulina platensis under different temperature and nitrogen regimes. Bioresour. Technol. 2007, 98, 1489–1493.
- Nuhu, A.A. Spirulina (Arthrospira): An Important Source of Nutritional and Medicinal Compounds. J. Mar. Biol. 2013, 2013, 1–8.
- Zaid, A.A.A.; Hammad, D.M.; Sharaf, E.M. Antioxidant and anticancer activity of Spirulina platensis water extracts. Int. J. Pharmacol. 2015, 11, 846–851.
- Benedetti, S.; Benvenuti, F.; Pagliarani, S.; Francogli, S.; Scoglio, S.; Canestrari, F. Antioxidant properties of a novel phycocyanin extract from the blue-green alga Aphanizomenon flos-aquae. Life Sci. 2004, 75, 2353–2362.
- Scoglio, S.; Lo Curcio, V.; Catalani, S.; Palma, F.; Battistelli, S.; Benedetti, S. Inhibitory effects of Aphanizomenon flos-aquae constituents on human UDP-glucose dehydrogenase activity. J. Enzym. Inhib. Med. Chem. 2016, 31, 1492–1497.
- Kim, D.Y.; Vijayan, D.; Praveenkumar, R.; Han, J.I.; Lee, K.; Park, J.Y.; Chang, W.S.; Lee, J.S.; Oh, Y.K. Cell-wall disruption and lipid/astaxanthin extraction from microalgae: Chlorella and Haematococcus. Bioresour. Technol. 2016, 199, 300–310.
- Borowitzka, M.A. Biology of microalgae. In Microalgae in Health and Disease Prevention; Levine, I., Fleurence, J., Eds.; Elsevier: London, UK, 2018; pp. 23–72. ISBN 9780128114056.
- Fradique, M.; Batista, A.P.; Nunes, M.C.; Gouveia, L.; Bandarra, N.M.; Raymundo, A. Incorporation of Chlorella vulgaris and Spirulina maxima biomass in pasta products. Part 1: Preparation and evaluation. J. Sci. Food Agric. 2010, 90, 1656–1664.
- Liu, J.; Chen, F. Biology and industrial applications of Chlorella: Advances and prospects. In Microalgae Biotechnology; Posten, C., Chen, S.F., Eds.; Springer: Cham, Swizerland, 2015; pp. 1–35.
- Saha, S.K.; McHugh, E.; Hayes, J.; Moane, S.; Walsh, D.; Murray, P. Effect of various stress-regulatory factors on biomass and lipid production in microalga Haematococcus pluvialis. Bioresour. Technol. 2013, 128, 118–124.
- Shah, M.M.R.; Liang, Y.; Cheng, J.J.; Daroch, M. Astaxanthin-producing green microalga Haematococcus pluvialis: From single cell to high value commercial products. Front. Plant Sci. 2016, 7, 531.
- Jaime, L.; Rodríguez-Meizoso, I.; Cifuentes, A.; Santoyo, S.; Suarez, S.; Ibáñez, E.; Señorans, F.J. Pressurized liquids as an alternative process to antioxidant carotenoids’ extraction from Haematococcus pluvialis microalgae. LWT Food Sci. Technol. 2010, 43, 105–112.
- Guerin, M.; Huntley, M.E.; Olaizola, M. Haematococcus astaxanthin: Applications for human health and nutrition. Trends Biotechnol. 2003, 21, 210–216.
- Hussein, G.; Sankawa, U.; Goto, H.; Matsumoto, K.; Watanabe, H. Astaxanthin, a Carotenoid with Potential in Human Health and Nutrition. J. Nat. Prod. 2006, 69, 443–449.
- Higuera-Ciapara, I.; Félix-Valenzuela, L.; Goycoolea, F.M. Astaxanthin: A review of its chemistry and applications. Crit. Rev. Food Sci. Nutr. 2006, 46, 185–196.
- Fakhri, S.; Abbaszadeh, F.; Dargahi, L.; Jorjani, M. Astaxanthin: A mechanistic review on its biological activities and health benefits. Pharmacol. Res. 2018, 136, 1–20.
- Yuan, J.P.; Peng, J.; Yin, K.; Wang, J.H. Potential health-promoting effects of astaxanthin: A high-value carotenoid mostly from microalgae. Mol. Nutr. Food Res. 2011, 55, 150–165.
- Xu, Y.; Ibrahim, I.M.; Wosu, C.I.; Ben-Amotz, A.; Harvey, P.J. Potential of new isolates of Dunaliella salina for natural β-carotene production. Biology 2018, 7, 1–18.
- Lamers, P.P.; Janssen, M.; De Vos, R.C.H.; Bino, R.J.; Wijffels, R.H. Exploring and exploiting carotenoid accumulation in Dunaliella salina for cell-factory applications. Trends Biotechnol. 2008, 26, 631–638.
- Hosseini Tafreshi, A.; Shariati, M. Dunaliella biotechnology: Methods and applications. J. Appl. Microbiol. 2009, 107, 14–35.
- Monte, J.; Ribeiro, C.; Parreira, C.; Costa, L.; Brive, L.; Casal, S.; Brazinha, C.; Crespo, J.G. Biorefinery of Dunaliella salina: Sustainable recovery of carotenoids, polar lipids and glycerol. Bioresour. Technol. 2020, 297, 10.
- Peng, K.T.; Zheng, C.N.; Xue, J.; Chen, X.Y.; Yang, W.D.; Liu, J.S.; Bai, W.; Li, H.Y. Delta 5 fatty acid desaturase upregulates the synthesis of polyunsaturated fatty acids in the marine diatom Phaeodactylum tricornutum. J. Agric. Food Chem. 2014, 62, 8773–8776.
- Papadaki, S.; Kyriakopoulou, K.; Krokida, M. Recovery and Encapsualtion of Bioactive Extracts from Haematococcus Pluvialis and Phaedodactylum Tricornutum for food Applications. IOSR J. Environ. Sci. Toxicol. Food Technol. 2016, 10, 2319–2399.
- Zhao, C.; Wu, Y.; Yang, C.; Liu, B.; Huang, Y. Hypotensive, hypoglycaemic and hypolipidaemic effects of bioactive compounds from microalgae and marine micro-organisms. Int. J. Food Sci. Technol. 2015, 50, 1705–1717.
- Chew, K.W.; Yap, J.Y.; Show, P.L.; Suan, N.H.; Juan, J.C.; Ling, T.C.; Lee, D.J.; Chang, J.S. Microalgae biorefinery: High value products perspectives. Bioresour. Technol. 2017, 229, 53–62.
- Custódio, L.; Soares, F.; Pereira, H.; Barreira, L.; Vizetto-Duarte, C.; Rodrigues, M.J.; Rauter, A.P.; Alberício, F.; Varela, J. Fatty acid composition and biological activities of Isochrysis galbana T-ISO, Tetraselmis sp. and Scenedesmus sp.: Possible application in the pharmaceutical and functional food industries. J. Appl. Phycol. 2014, 26, 151–161.
- Priyadarshani, I.; Rath, B. Bioactive Compounds From Microalgae And Cyanobacteria: Utility and Applications. Int. J. Pharm. Sci. Res. 2012, 3, 4123–4130.
- Huheihel, M.; Ishanu, V.; Tal, J.; Arad, S. Activity of Porphyridium sp. polysaccharide against herpes simplex viruses in vitro and in vivo. J. Biochem. Biophys. Methods 2002, 50, 189–200.
- Sun, L.; Wang, C.; Shi, Q.; Ma, C. Preparation of different molecular weight polysaccharides from Porphyridium cruentum and their antioxidant activities. Int. J. Biol. Macromol. 2009, 45, 42–47.
- Sun, L.; Wang, L.; Zhou, Y. Immunomodulation and antitumor activities of different-molecular-weight polysaccharides from Porphyridium cruentum. Carbohydr. Polym. 2012, 87, 1206–1210.
- Vicente-García, V.; Rios-Leal, E.; Calderón-Domínguez, G.; Cañizares-Villanueva, R.O.; Olvera-Ramírez, R. Detection, Isolation, and Characterization of Exopolysaccharide Produced by a Strain of Phormidium 94a Isolated from an Arid Zone of Mexico. Biotechnol. Bioeng. 2004, 85, 306–310.
- Abed, R.M.M.; Dobretsov, S.; Sudesh, K. Applications of cyanobacteria in biotechnology. J. Appl. Microbiol. 2009, 106, 1–12.
- Nedovic, V.; Kalusevic, A.; Manojlovic, V.; Levic, S.; Bugarski, B. An overview of encapsulation technologies for food applications. Procedia Food Sci. 2011, 1, 1806–1815.
- McClements, D.J. Delivery by Design (DbD): A Standardized Approach to the Development of Efficacious Nanoparticle- and Microparticle-Based Delivery Systems. Compr. Rev. Food Sci. Food Saf. 2018, 17, 200–219.
- Bakry, A.M.; Abbas, S.; Ali, B.; Majeed, H.; Abouelwafa, M.Y.; Mousa, A.; Liang, L. Microencapsulation of Oils: A Comprehensive Review of Benefits, Techniques, and Applications. Compr. Rev. Food Sci. Food Saf. 2016, 15, 143–182.
- Fang, Z.; Bhesh, B. Encapsulation of polyphenols—a review. Trends Food Sci. Technol. 2010, 21, 510–523.
- Onwulata, C.I. Encapsulation of New Active Ingredients. Annu. Rev. Food Sci. Technol. 2012, 3, 183–204.
- Dias, D.R.; Botrel, D.A.; de Fernandes, R.V.B.; Borges, S.V. Encapsulation as a tool for bioprocessing of functional foods. Curr. Opin. Food Sci. 2017, 13, 31–37.
- Augustin, M.A.; Hemar, Y. Nano- and micro-structured assemblies for encapsulation of food ingredients. Chem. Soc. Rev. 2009, 38, 902–912.
- Joye, I.J.; McClements, D.J. Biopolymer-based nanoparticles and microparticles: Fabrication, characterization, and application. Curr. Opin. Colloid Interface Sci. 2014, 19, 417–427.
- Champagne, C.P.; Fustier, P. Microencapsulation for the improved delivery of bioactive compounds into foods. Curr. Opin. Biotechnol. 2007, 18, 184–190.
- Sonawane, S.H.; Bhanvase, B.A.; Sivakumar, M. Encapsulation of Active Molecules and Their Delivery System, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2020.
- De Jong, W.H.; Borm, P.J.A. Drug delivery and nanoparticles: Applications and hazards. Int. J. Nanomed. 2008, 3, 133–149.
- Alexander, A.; Ajazuddin; Patel, R.J.; Saraf, S.; Saraf, S. Recent expansion of pharmaceutical nanotechnologies and targeting strategies in the field of phytopharmaceuticals for the delivery of herbal extracts and bioactives. J. Control. Release 2016, 241, 110–124.
- Gao, G.H.; Li, Y.; Lee, D.S. Environmental pH-sensitive polymeric micelles for cancer diagnosis and targeted therapy. J. Control. Release 2013, 169, 180–184.
- Van Tran, V.; Moon, J.Y.; Lee, Y.C. Liposomes for delivery of antioxidants in cosmeceuticals: Challenges and development strategies. J. Control. Release 2019, 300, 114–140.
- Montenegro, L.; Lai, F.; Offerta, A.; Sarpietro, M.G.; Micicchè, L.; Maccioni, A.M.; Valenti, D.; Fadda, A.M. From nanoemulsions to nanostructured lipid carriers: A relevant development in dermal delivery of drugs and cosmetics. J. Drug Deliv. Sci. Technol. 2016, 32, 100–112.
- Katouzian, I.; Jafari, S.M. Nano-encapsulation as a promising approach for targeted delivery and controlled release of vitamins. Trends Food Sci. Technol. 2016, 53, 34–48.
- Rashidi, L.; Khosravi-Darani, K. The applications of nanotechnology in food industry. Crit. Rev. Food Sci. Nutr. 2011, 51, 723–730.
- Đorđević, V.; Balanč, B.; Belščak-Cvitanović, A.; Lević, S.; Trifković, K.; Kalušević, A.; Kostić, I.; Komes, D.; Bugarski, B.; Nedović, V. Trends in Encapsulation Technologies for Delivery of Food Bioactive Compounds. Food Eng. Rev. 2015, 7, 452–490.
- Vieira, M.V.; Oliveira, S.M.; Amado, I.R.; Fasolin, L.H.; Vicente, A.A.; Pastrana, L.M.; Fuciños, P. 3D printed functional cookies fortified with Arthrospira platensis: Evaluation of its antioxidant potential and physical-chemical characterization. Food Hydrocoll. 2020, 107, 105893.
- Kaur, S.; Das, M. Functional foods: An overview. Food Sci. Biotechnol. 2011, 20, 861–875.
- Betoret, E.; Betoret, N.; Vidal, D.; Fito, P. Functional foods development: Trends and technologies. Trends Food Sci. Technol. 2011, 22, 498–508.
- Freitas, A.C.; Rodrigues, D.; Rocha-Santos, T.A.P.; Gomes, A.M.P.; Duarte, A.C. Marine biotechnology advances towards applications in new functional foods. Biotechnol. Adv. 2012, 30, 1506–1515.
- Plaza, M.; Cifuentes, A.; Ibáñez, E. In the search of new functional food ingredients from algae. Trends Food Sci. Technol. 2008, 19, 31–39.
- Dewapriya, P.; Kim, S. Marine microorganisms: An emerging avenue in modern nutraceuticals and functional foods. Food Res. Int. 2014, 56, 115–125.
- Caporgno, M.P.; Mathys, A. Trends in Microalgae Incorporation Into Innovative Food Products With Potential Health Benefits. Front. Nutr. 2018, 5, 1–10.
- Watkins, R.; Wu, L.; Zhang, C.; Davis, R.M.; Xu, B. Natural product-based nanomedicine: Recent advances and issues. Int. J. Nanomed. 2015, 10, 6055–6074.
- Bajpai, V.K.; Shukla, S.; Kang, S.M.; Hwang, S.K.; Song, X.; Huh, Y.S.; Han, Y.K. Developments of cyanobacteria for nano-marine drugs: Relevance of nanoformulations in cancer therapies. Mar. Drugs 2018, 16, 1–23.
- Kumari, A.; Kumar, V.; Yadav, S.K. Nanotechnology: A tool to enhance therapeutic values of natural plant products. Trends Med. Res. 2012, 7, 34–42.
- Wang, H.M.D.; Chen, C.C.; Huynh, P.; Chang, J.S. Exploring the potential of using algae in cosmetics. Bioresour. Technol. 2015, 184, 355–362.
- Mourelle, M.L.; Gómez, C.P.; Legido, J.L. The potential use of marine microalgae and cyanobacteria in cosmetics and thalassotherapy. Cosmetics 2017, 4, 2–14.
- Ariede, M.B.; Candido, T.M.; Jacome, A.L.M.; Velasco, M.V.R.; de Carvalho, J.C.M.; Baby, A.R. Cosmetic attributes of algae A review. Algal Res. 2017, 25, 483–487.
- Vinardell, M.P.; Mitjans, M. Nanocarriers for delivery of antioxidants on the skin. Cosmetics 2015, 2, 342–354.
- Hu, F.; Liu, W.; Yan, L.; Kong, F.; Wei, K. Optimization and characterization of poly(lactic-co-glycolic acid) nanoparticles loaded with astaxanthin and evaluation of anti-photodamage effect in vitro. R. Soc. Open Sci. 2019, 6, 191184.




