
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Sophie Wedekind | + 1386 word(s) | 1386 | 2021-04-13 05:18:05 | | | |
| 2 | Dean Liu | Meta information modification | 1386 | 2021-04-21 02:48:17 | | |
Video Upload Options
Human milk oligosaccharides (HMOs) are a family of free oligosaccharides which are diverse in structure and unique to human milk. Their diversity enables multiple paths of interference against viral entry, which include norovirus, rotavirus and human immunodeficiency virus (HIV).
1. Introduction
Human milk is a complex biofluid, containing not just tailored nutrition to satisfy growth requirements, but developmental cues and protection of the relatively immunocompromised infant against pathogens. As an evolutionary strategy, lactation enables the infant to transition from placental protection to an environment full of microbes. With minimally developed adaptive immune systems, infants rely on an immature innate immune system in a predominant Th2 state as the first line of their defence [1][2]. Human milk contains various mechanisms and bioactive molecules that compensate for these limitations and support normal immune development throughout a natural term of lactation up to several years postnatally. Milk contains an abundance of antimicrobial and immunomodulatory molecules which aid in antiviral defence to aid the immature infant immune system [3][4].
Along with the transfer of maternal antibodies, other immunological and bioactive activity is provided via human milk. Cellular immunity is provided by macrophages and leukocytes, which are highly abundant at the beginning of lactation and particularly in colostrum–the specialised fluid produced for several days postnatally before the onset of the second stage of lactogenesis [5]. Multiple maternal characteristics influence the cellular composition of human milk, such as maternal age and certain aspects of diet [6][7]. However, a lack of studies makes it difficult to elucidate the effects these changes in composition have on the infant and their health, both in the short and long term.
2. Human Milk Oligosaccharides (HMOs)
As the third largest class of components within human milk after lactose and lipids, HMOs are a family of free oligosaccharides which are diverse in structure and unique to human milk [8][9]. HMO composition varies between women and over the course of the lactation period, as well as by season of the year [10]. The concentration of HMOs in early milk can be as much as 20–25 g/L, and is thought to decline as the milk matures, although scant research on full lactation durations (beyond 2 years) exists [11][12]. Lactose serves as the template for all HMOs at the reducing end and can be elongated, fucosylated or sialylated, causing variations which creates over a hundred different HMOs [13].
Of the multiple functions of HMOs, immunomodulation is one of the most significant [14][15]. Viruses invade and colonise by binding to host cells, utilising cell surface glycoconjugates as receptors, inducing oxidative stress and anti-inflammatory signals [16]. Some HMOs express glycans that can bind onto surface lectins on the host cells which prevents viral molecules binding and invading these cells, acting as receptor decoys Figure 1 [17]. HMOs may indirectly reduce immune responses by altering the composition of the infant gut microbiota [18]. A study which investigated this looked at infant formula supplemented with 2′FL (1 g/L) and LNnT (0.5 g/L) compared to those fed without supplemented formula [19]. Those that were supplemented had a significantly different microbiota composition in comparison, where Bifidobacterium was more abundant. However, this study focussed on formula fed infants rather than exclusively breastfed infants, and therefore it is not able to show the difference in effect between formula supplemented and exclusively breastfed. A number of studies indicated the relationship between the gut microbiota composition and inflammation [20][21]. While there is evidence supporting HMOs indirect changes to aid in the infant’s immune response, there are also results from in vitro studies which show their ability to directly modulate the immune response [22].
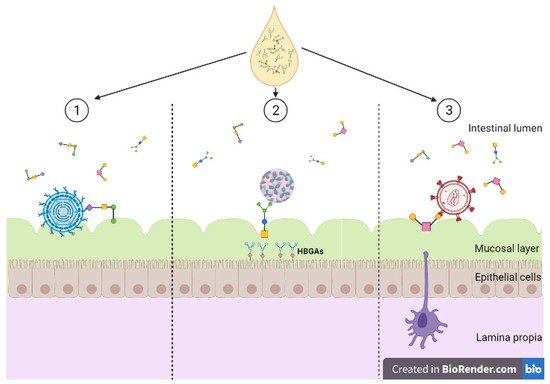
Figure 1. Diversity of human milk oligosaccharide (HMO) interference against viral entry. (1) HMOs act as soluble decoy receptors for rotavirus, preventing direct binding to host cells; (2) HMOs mimic histo-blood group antigens (HBGAs), binding to both GI and GII HBGA pockets; (3) HMOs bind to glycoprotein gp120 interfering and preventing viral binding to dendritic cell-SIGN. Created with BioRender.com.
Specific research has identified the role of HMOs in preventing infection with several viruses across different viral classes. For example, rotaviruses constitute the most common cause of diarrhoea in infants and children worldwide and is the main agent towards gastroenteritis [23]. Prevention can be via vaccination, but this is not indicated before the first two months postnatally. Viral entry is a complex multi-step process where different rotavirus surface proteins interact with different cell surface receptors [24]. Viral proteins VP7 and VP4 are involved with the receptor binding and permeabilise membranes [16][25]. Rotavirus entry may occur via calcium-dependent endocytosis, where an observed decrease in calcium concentration solubilises the surface protein from the virus [26]. HMOs have been observed to directly intervene with rotavirus acting as soluble decoy receptors for the rotavirus reducing infectivity [27]. Specific HMOs were shown to reduce infectivity of human rotavirus strains, as well as impacts on colonic microbiota and cytokine responses. However, it was noted that there were differences in effect between rotavirus strains, but research is lacking on the effects of different HMO concentrations against different rotavirus species [28].
Noroviruses are small non-enveloped RNA viruses. Susceptibility to norovirus infection depends on a genetic trait caused by the expression of histo-blood group antigens (HBGAs) [29]. The host mucosal surface in the gastrointestinal tract contains HBGAs that facilitate the binding of noroviruses [30][31]. HGBAs are carbohydrate-based antigens which include the Lewis and ABH antigens expressed on the cell surface of red blood cells and mucosal surface epithelial cells. Binding is strain-dependent and occurs by the viral capsid protein via the P domain [30]. X-ray crystallography has shown that HMOs interact with norovirus by mimicking HBGAs [32]. Specifically, 2′-fucosyllactose (2′FL) HMOs able to block multiple strains of norovirus, by being able to bind to both GI and GII HBGA pockets. An investigation focussing on high-molecular mass HMOs and their binding ability with a specific strain of norovirus (GII.4, Sydney, Australia, 2012, JX459908) showed these types of HMOs have a higher affinity for binding compared to monovalent HMOs as a result of the higher avidity of α-fucose [33]. While these studies have highlighted the ability of certain HMOs to bind sufficiently to norovirus strains, most HMOs are bound to a lipid or protein carrier rather than present as free oligosaccharides, such as 2′FL or 3FL. There is a need for more research to understand the efficacy of HMOs in their various forms against multiple strains of noroviruses.
Human immunodeficiency virus (HIV) comprises two species of the Lentivirus family, named HIV-1 and HIV-2. These are single-stranded enveloped RNA viruses which integrate with the host cellular DNA. HIV can be latent for 2 to 10 years and avoids detection from the immune system; once active the pre-virus DNA is transcribed into RNA and ultimately leads to the expression of new virus particles which are readily able to attack CD4 T lymphocytes [27]. Eventually this leads to the individual being at high risk of diseases and other infection [34]. With modern anti-retroviral therapy, the risk of transmission for a mother with undetectable viral load if the infant is exclusively human milk fed is low (<0.5%). However, if the mother has detectable levels of virus during pregnancy or postnatally, HIV-1 can be transmitted from the mother to infant via breastfeeding only around 10–15% of infants become ill from an HIV-infected mother when breastfed exclusively [35]. Interestingly, if the infant is mixed fed and receives some infant formula alongside breastfeeding, the risk again rises to 6–10%, suggesting even small doses of formula override the protective effects of breastfeeding.
HIV viruses interact with the cell surface receptor dendritic cell-specific intercellular adhesion molecule-3-grabbing non-integrin (DC-SIGN) to enter the cell [36]. These receptors are on the surface of macrophages and dendritic cells, allowing HIV to self-replicate and transform CD4+ T lymphocytes [37]. HMOs isolated in an ELISA assay show prevention of glycoprotein gp120 on the surface of HIV-1 from binding on the DC-SIGN [38]. HIV-infected mothers with HMOs above the median average concentration were less likely to transmit HIV via breastfeeding [8][39]. Higher concentrations of 3′-sialyllactose (3-SL) HMOs were associated with protection against postnatal HIV transmission, but this type of HMO is significantly higher in HIV-infected women compared to uninfected women [8]. A follow-up study investigated the differences in HMO profiles between HIV-infected and HIV-uninfected mothers, confirming higher quantities of 3′-SL in HIV-infected mothers [39]. The authors proposed that the difference could be due to HIV infection changing the glycosylation process in the mammary epithelial gland epithelial cell and therefore changing the composition of HMOs, or that HIV-infected individuals differentially express glycosylation-related genes, either greater or fewer.
References
- Ghazal, P.; Dickinson, P.; Smith, C.L. Early life response to infection. Curr. Opin. Infect. Dis. 2013, 26, 213–218.
- Yu, J.C.; Khodadadi, H.; Malik, A.; Davidson, B.; Salles, É.D.S.L.; Bhatia, J.; Hale, V.L.; Baban, B. Innate immunity of neonates and infants. Front. Immunol. 2018, 9, 1759.
- Witkowska-Zimny, M.; Kaminska-El-Hassan, E. Cells of human breast milk. Cell. Mol. Biol. Lett. 2017, 22, 11.
- Eriksen, K.G.; Christensen, S.H.; Lind, M.V.; Michaelsen, K.F. Human milk composition and infant growth. Curr. Opin. Clin. Nutr. Metab. 2018, 21, 200–206.
- Hamosh, M. Bioactive factors in human milk. Pediatr. Clin. N. Am. 2001, 48, 69–86.
- Lubetzky, R.; Sever, O.; Mimouni, F.B.; Mandel, D. Human milk macronutrients content: Effect of advanced maternal age. Breastfeed. Med. 2015, 10, 433–436.
- Keikha, M.; Bahreynian, M.; Saleki, M.; Kelishadi, R. Macro-and micronutrients of human milk composition: Are they related to maternal diet? A comprehensive systematic review. Breastfeed. Med. 2017, 12, 517–527.
- Bode, L.; Kuhn, L.; Kim, H.Y.; Hsiao, L.; Nissan, C.; Sinkala, M.; Kankasa, C.; Mwiya, M.; Thea, D.M.; Aldrovandi, G.M. Human milk oligosaccharide concentration and risk of postnatal transmission of HIV through breastfeeding. Am. J. Clin. Nutr. 2012, 96, 831–839.
- Smilowitz, J.T.; Lebrilla, C.B.; Mills, D.A.; German, J.B.; Freeman, S.L. Breast Milk Oligosaccharides: Structure-Function Relationships in the Neonate. Annu. Rev. Nutr. 2014, 34, 143–169.
- Azad, M.B.; Robertson, B.; Atakora, F.; Becker, A.B.; Subbarao, P.; Moraes, T.J.; Mandhane, P.J.; Turvey, S.E.; Lefebvre, D.L.; Sears, M.R.; et al. Human Milk Oligosaccharide Concentrations Are Associated with Multiple Fixed and Modifiable Maternal Characteristics, Environmental Factors, and Feeding Practices. J. Nutr. 2018, 148, 1733–1742.
- Bode, L. Human milk oligosaccharides: Every baby needs a sugar mama. Glycobiology 2012, 22, 1147–1162.
- Albrecht, S.; Schols, H.; van den Heuvel, E.; Voragen, A.; Gruppen, H. Occurrence of oligosaccharides in feces of breast-fed babies in their first six months of life and the corresponding breast milk. Carbohydr. Res. 2011, 346, 2540–2550.
- Wiciński, M.; Sawicka, E.; Gębalski, J.; Kubiak, K.; Malinowski, B. Human milk oligosaccharides: Health benefits, potential applications in infant formulas, and pharmacology. Nutrients 2020, 12, 266.
- Eiwegger, T.; Stahl, B.; Haidl, P.; Schmitt, J.; Boehm, G.; Dehlink, E.; Urbanek, R.; Szepfalusi, Z. Prebiotic oligosaccharides: In vitro evidence for gastrointestinal epithelial transfer and immunomodulatory properties. Pediatr. Allergy Immunol. 2010, 21, 1179–1188.
- Morozov, V.; Hansman, G.; Hanisch, F.G.; Schroten, H.; Kunz, C. Human milk oligosaccharides as promising antivirals. Mol. Nutr. Food Res. 2018, 62, 1700679.
- Guerrero, C.A.; Acosta, O. Inflammatory and oxidative stress in rotavirus infection. World J. Virol. 2016, 5, 38–62.
- Etzold, S.; Bode, L. Glycan-dependent viral infection in infants and the role of human milk oligosaccharides. Curr. Opin. Virol. 2014, 7, 101–107.
- Pannaraj, P.S.; Li, F.; Cerini, C.; Bender, J.M.; Yang, S.; Rollie, A.; Adisetiyo, H.; Zabih, S.; Lincez, P.J.; Bittinger, K.; et al. Association between breast milk bacterial communities and establishment and development of the infant gut microbiome. JAMA Pediatr. 2017, 171, 647–654.
- Steenhout, P.; Sperisen, P.; Martin, F.P.; Sprenger, N.; Wernimont, S.; Pecquet, S.; Berger, B. Term Infant Formula Supplemented with Human Milk Oligosaccharides (2′ Fucosyllactose and Lacto-N-neotetraose) Shifts Stool Microbiota and Metabolic Signatures Closer to that of Breastfed Infants. FASEB J. 2016, 30, 275–277.
- Roessler, A.; Friedrich, U.; Vogelsang, H.; Bauer, A.; Kaatz, M.; Hipler, U.C.; Schmidt, I.; Jahreis, G. The immune system in healthy adults and patients with atopic dermatitis seems to be affected differently by a probiotic intervention. Clin. Exp. Allergy 2008, 38, 93–102.
- Viljanen, M.; Kuitunen, M.; Haahtela, T.; Juntunen-Backman, K.; Korpela, R.; Savilahti, E. Probiotic effects on faecal inflammatory markers and on faecal IgA in food allergic atopic eczema/dermatitis syndrome infants. Pediatr. Allergy Immunol. 2005, 16, 65–71.
- Donovan, S.M.; Comstock, S.S. Human milk oligosaccharides influence neonatal mucosal and systemic immunity. Ann. Nutr. Metab. 2016, 69, 41–51.
- Parashar, U.D.; Hummelman, E.G.; Bresee, J.S.; Miller, M.A.; Glass, R.I. Global illness and deaths caused by rotavirus disease in children. Emerg. Infect. Dis. 2003, 9, 565–572.
- López, S.; Arias, C.F. Multistep entry of rotavirus into cells: A Versaillesque dance. Trends Microbiol. 2004, 12, 271–278.
- Charpilienne, A.; Abad, M.J.; Michelangeli, F.; Alvarado, F.; Vasseur, M.; Cohen, J.; Ruiz, M.C. Solubilized and cleaved VP7, the outer glycoprotein of rotavirus, induces permeabilization of cell membrane vesicles. J. Gen. Virol. 1997, 78, 1367–1371.
- Chemello, M.E.; Aristimuño, O.C.; Michelangeli, F.; Ruiz, M.C. Requirement for vacuolar H+-ATPase activity and Ca2+ gradient during entry of rotavirus into MA104 cells. J. Virol. 2002, 76, 13083–13087.
- Laucirica, D.R.; Triantis, V.; Schoemaker, R.; Estes, M.K.; Ramani, S. Milk oligosaccharides inhibit human rotavirus infectivity in MA104 cells. J. Nutr. 2017, 147, 1709–1714.
- Ramani, S.; Stewart, C.J.; Laucirica, D.R.; Ajami, N.J.; Robertson, B.; Autran, C.A.; Shinge, D.; Rani, S.; Anandan, S.; Hu, L.; et al. Human milk oligosaccharides, milk microbiome and infant gut microbiome modulate neonatal rotavirus infection. Nat. Commun. 2018, 9, 1–12.
- Chassaing, M.; Boudaud, N.; Belliot, G.; Estienney, M.; Majou, D.; de Rougemont, A.; Gantzer, C. Interaction between norovirus and Histo-Blood Group Antigens: A key to understanding virus transmission and inactivation through treatments? Food Microbiol. 2020, 92, 103594.
- Tan, M.; Jiang, X. Norovirus–host interaction: Multi-selections by human histo-blood group antigens. Trends Microbiol. 2011, 19, 382–388.
- Marionneau, S.; Ruvoën, N.; Le Moullac-Vaidye, B.; Clement, M.; Cailleau-Thomas, A.; Ruiz-Palacois, G.; Huang, P.; Jiang, X.; Le Pendu, J. Norwalk virus binds to histo-blood group antigens present on gastroduodenal epithelial cells of secretor individuals. Gastroenterology 2002, 122, 1967–1977.
- Schroten, H.; Hanisch, F.G.; Hansman, G.S. Human norovirus interactions with histo-blood group antigens and human milk oligosaccharides. J. Virol. 2016, 90, 5855–5859.
- Hanisch, F.G.; Hansman, G.S.; Morozov, V.; Kunz, C.; Schroten, H. Avidity of α-fucose on human milk oligosaccharides and blood group-unrelated oligo/polyfucoses is essential for potent norovirus-binding targets. J. Biol. Chem. 2018, 293, 11955–11965.
- Eldholm, V.; Rieux, A.; Monteserin, J.; Lopez, J.M.; Palmero, D.; Lopez, B.; Ritacco, V.; Didelot, X.; Balloux, F. Impact of HIV co-infection on the evolution and transmission of multidrug-resistant tuberculosis. eLife 2016, 5, e16644.
- Little, K.M.; Kilmarx, P.H.; Taylor, A.W.; Rose, C.E.; Rivadeneira, E.D.; Nesheim, S.R. A review of evidence for transmission of HIV from children to breastfeeding women and implications for prevention. Pediatr. Infect. Dis. J. 2012, 31, 938–942.
- Granelli-Piperno, A.; Pritsker, A.; Pack, M.; Shimeliovich, I.; Arrighi, J.F.; Park, C.G.; Trumpfheller, C.; Piguet, V.; Moran, T.M.; Steinman, R.M. Dendritic cell-specific intercellular adhesion molecule 3-grabbing nonintegrin/CD209 is abundant on macrophages in the normal human lymph node and is not required for dendritic cell stimulation of the mixed leukocyte reaction. J. Immunol. 2005, 175, 4265–4273.
- Woodham, A.W.; Skeate, J.G.; Sanna, A.M.; Taylor, J.R.; Da Silva, D.M.; Cannon, P.M.; Kast, W.M. Human Immunodeficiency Virus Immune Cell Receptors, Coreceptors, and Cofactors: Implications for Prevention and Treatment. AIDS Patient Care STDs 2016, 30, 291–306.
- Hong, P.; Ninonuevo, M.R.; Lee, B.; Lebrilla, C.; Bode, L. Human milk oligosaccharides reduce HIV-1-gp120 binding to dendritic cell-specific ICAM3-grabbing non-integrin (DC-SIGN). Br. J. Nutr. 2008, 101, 482–486.
- Van Niekerk, E.; Autran, C.A.; Nel, D.G.; Kirsten, G.F.; Blaauw, R.; Bode, L. Human milk oligosaccharides differ between HIV-infected and HIV-uninfected mothers and are related to necrotizing enterocolitis incidence in their preterm very-low-birth-weight infants. J. Nutr. 2014, 144, 1227–1233.




